Abstract
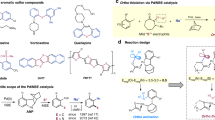
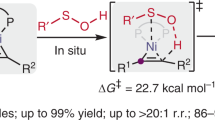
Over the past decades, many efficient methodologies have been developed that allow for the enantioselective synthesis of chiral sulfinyl compounds. However, the enantioselective deoxygenation of hexavalent sulfones for the formation of chiral sulfinyl compounds still remains one of the major challenges in the fields of asymmetric synthesis and organosulfur chemistry. Here we have demonstrated that a synergistic combination of organocatalysis and the incorporation of a cyano group into the sulfone generates a chiral sulfinic species as an active intermediate. A wide range of chiral sulfinates with high enantioselectivities could then be acquired using alcohols as nucleophiles, and the subsequent transformations allowed the collective preparation of a variety of chiral sulfinyl compounds. Density functional theory calculations revealed that the catalytic cycle involves a quinuclidine-assisted stepwise 1,2-cyano group transfer, base-assisted intermolecular substitution with alcohol and regeneration of the active catalyst. The enantioselectivity was determined by the cyano migration step.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All relevant data supporting the findings of this study, including experimental procedures and compound characterization, NMR and HPLC are available within the Article and its Supplementary Information. Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre under deposition numbers CCDC 1941245 (P3e), 2141490 ((R)-5) and 2141488 ((S)-11). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. Source data are provided with this paper.
References
Tian, S.-K. et al. Asymmetric organic catalysis with modified cinchona alkaloids. Acc. Chem. Res. 37, 621–631 (2004).
Doyle, A. G. & Jacobsen, E. N. Small-molecule H-bond donors in asymmetric catalysis. Chem. Rev. 107, 5713–5743 (2007).
MacMillan, D. W. C. The advent and development of organocatalysis. Nature 455, 304–308 (2008).
List, B. (ed) Asymmetric Organocatalysis, Topics in Current Chemistry Vol. 291 (Springer, 2009).
Bertelsen, S. & Jørgensen, K. A. Organocatalysis—after the gold rush. Chem. Soc. Rev. 38, 2178–2189 (2009).
Song, C. E. (ed.) Cinchona Alkaloids in Synthesis and Catalysis: Ligands, Immobilization and Organocatalysis (Wiley-VCH, 2009).
Yan, W., Zheng, M., Xu, C. & Chen, F.-E. Harnessing noncovalent interaction of chalcogen bond in organocatalysis: from the catalyst point of view. Green Synth. Catal. 2, 329–336 (2021).
Drabowicz, J. Stereochemistry of organic sulfur compounds: More than 100 years of history, current state and further challenges. Phosphorus, Sulfur Silicon Relat. Elem. 192, 145–148 (2017).
Wang, M. & Jiang, X. Prospects and challenges in organosulfur chemistry. ACS Sustain. Chem. Eng. 10, 671–677 (2022).
Evans, J. W., Fierman, M. B., Miller, S. J. & Ellman, J. A. Catalytic enantioselective synthesis of sulfinate esters through the dynamic resolution of tert-butanesulfinyl chloride. J. Am. Chem. Soc. 126, 8134–8135 (2004).
Shibata, N. et al. Cinchona alkaloid/sulfinyl chloride combinations: enantioselective sulfinylating agents of alcohols. J. Am. Chem. Soc. 127, 1374–1375 (2005).
Tilby, M. J., Dewez, D. F., Hall, A., Lamenca, C. M. & Willis, M. C. Exploiting configurational lability in aza-sulfur compounds for the organocatalytic enantioselective synthesis of sulfonimidamides. Angew. Chem. Int. Ed. 60, 25680–25687 (2021).
Tang, Y. & Miller, S. J. Catalytic enantioselective synthesis of pyridyl sulfoximines. J. Am. Chem. Soc. 143, 9230–9235 (2021).
Fang, S. et al. Access to S-stereogenic free sulfoximines via bifunctional phosphonium salt-catalyzed desymmetrization of bisphenols. ACS Catal. 11, 13902–13912 (2021).
Zhang, X., Ang, E. C. X., Yang, Z., Kee, C. W. & Tan, C.-H. Synthesis of chiral sulfinate esters by asymmetric condensation. Nature 604, 298–303 (2022).
Jia, T. et al. Palladium-catalyzed enantioselective arylation of aryl sulfenate anions: a combined experimental and computational study. J. Am. Chem. Soc. 139, 8337–8345 (2017).
Wang, L., Chen, M., Zhang, P., Li, W. & Zhan, J. Palladium/PC-Phos-catalyzed enantioselective arylation of general sulfenate anions: scope and synthetic applications. J. Am. Chem. Soc. 140, 3467–3473 (2018).
Mikołajczyk, M. & Drabowicz, J. in Topics in Stereochemistry Vol. 13 (eds Allinger, N. L. et al.) 333–468 (Wiley, 1982).
Mikołajczyk, M., Drabowicz, J. & Kiełbasiński, P. Chiral Sulfur Reagents: Applications in Asymmetric and Stereoselective Synthesis (CRC Press, 1997).
Page, P. C. B. (ed.) Organosulfur Chemistry: Synthetic and Stereochemical Aspects (Academic Press, 1998).
Toru, T. & Bolm, C. (eds) Organosulfur Chemistry in Asymmetric Synthesis (Wiley-VCH, 2008).
Kagan, H. B. & Luukas, T. O. in Transition Metals for Organic Synthesis (eds Beller, M. & Bolm, C.) 479–495 (Wiley-VCH, 2004).
Fernández, I. & Khiar, N. Recent developments in the synthesis and utilization of chiral sulfoxides. Chem. Rev. 103, 3651–3705 (2003).
Wojaczyńska, E. & Wojaczyński, J. Enantioselective synthesis of sulfoxides: 2000–2009. Chem. Rev. 110, 4303–4356 (2010).
Han, J., Soloshonok, V. A., Klika, K. D., Drabowicz, J. & Wzorek, A. Chiral sulfoxides: advances in asymmetric synthesis and problems with the accurate determination of the stereochemical outcome. Chem. Soc. Rev. 47, 1307–1350 (2018).
Wojaczyńska, E. & Wojaczyński, J. Modern stereoselective synthesis of chiral sulfinyl compounds. Chem. Rev. 120, 4578–4611 (2020).
Yang, L., Wang, B., Yin, X. & Zeng, Q. Advances of sulfenate anions in catalytic asymmetric synthesis of sulfoxides. Chem. Rec. 21, e202100242 (2022).
Magnus, P. D. Recent developments in sulfone chemistry. Tetrahedron 33, 2019–2045 (1977).
Patai, S. et al. (eds) The Chemistry of Sulphones and Sulphoxides (Wiley, 1988).
Whitham, G. H. (ed.) Organosulfur Chemistry (Oxford Univ. Press, 1995).
Liu, N.-W., Liang, S. & Manolikakes, G. Recent advances in the synthesis of sulfones. Synthesis 48, 1939–1973 (2016).
Jiang, X. (ed.) Sulfur Chemistry (Springer, 2018).
Firouzabadi, H. & Jamalian, A. Reduction of oxygenated organosulfur compounds. J. Sulfur Chem. 29, 53–97 (2008).
Carreño, M. C. Applications of sulfoxides to asymmetric synthesis of biologically active compounds. Chem. Rev. 95, 1717–1760 (1995).
Bentley, R. Role of sulfur chirality in the chemical processes of biology. Chem. Soc. Rev. 34, 609–624 (2005).
Legros, J., Dehli, J. R. & Bolm, C. Applications of catalytic asymmetric sulfide oxidations to the syntheses of biologically active sulfoxides. Adv. Synth. Catal. 347, 19–31 (2005).
Zeng, Q., Gao, S. & Chelashaw, A. K. Advances in titanium-catalyzed synthesis of chiral sulfoxide drugs. Mini-Rev. Org. Chem. 10, 198–206 (2013).
Mellah, M., Voituriez, A. & Schulz, E. Chiral sulfur ligands for asymmetric catalysis. Chem. Rev. 107, 5133–5209 (2007).
Sipos, G., Drinkel, E. E. & Dorta, R. The emergence of sulfoxides as efficient ligands in transition metal catalysis. Chem. Soc. Rev. 44, 3834–3860 (2015).
Trost, B. M. & Rao, M. Development of chiral sulfoxide ligands for asymmetric catalysis. Angew. Chem. Int. Ed. 54, 5026–5043 (2015).
Otocka, S., Kwiatkowska, M., Madalińska, L. & Kiełbasiński, P. Chiral organosulfur ligands/catalysts with a stereogenic sulfur atom: applications in asymmetric synthesis. Chem. Rev. 117, 4147–4181 (2017).
Marenich, A. V., Cramer, C. J. & Truhlar, D. G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 113, 6378–6396 (2009).
Krishnan, R., Binkley, J. S., Seeger, R. & Pople, J. A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 72, 650–654 (1980).
McLean, A. D. & Chandler, G. S. Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z=11–18. J. Chem. Phys. 72, 5639–5648 (1980).
Weigend, F. & Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys. Chem. Chem. Phys. 7, 3297–3305 (2005).
Zhao, Y. & Truhlar, D. G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 120, 215–241 (2008).
Acknowledgements
This study was supported by the Fundamental Research Funds for the Central Universities (grants: 2021CDJQY-035 to W.Q. and 2022CDJXY-025 to S.H.), the National Natural Science Foundation of China (grants: 21901026 to W.Q. and 21922101 to H.Y.) and the Natural Science Foundation of Chongqing (grant: cstc2021jcyj-jqX0019 to H.Y.).
Author information
Authors and Affiliations
Contributions
H.Y. and W.Q. conceived and directed the project. S.H. designed and performed the experiments. S.H. and Z.Z. prepared the Supplementary Information. S.H., Z.Z. and N.Z. analysed and interpreted the experimental data. W.Q., H.Y., Y.L. and Z.Z. wrote the paper. Z.Z. and Y.L. performed the DFT calculations. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Choon-Hong Tan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–5, Figs. 1–26, experimental data, synthesis and characterization data, NMR spectra, X-ray crystallographic data and DFT calculation data.
Supplementary Data 1
Crystallographic data for compound P3e; CCDC reference 1941245.
Supplementary Data 2
Crystallographic data for compound (R)-5; CCDC reference 2141490.
Supplementary Data 3
Crystallographic data for compound (S)-11; CCDC reference 2141488.
Supplementary Data 4
Cartesian coordinates for all optimized structures.
Source data
Source Data Fig. 2
Numerical data for panel c.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, S., Zeng, Z., Zhang, N. et al. Organocatalytic asymmetric deoxygenation of sulfones to access chiral sulfinyl compounds. Nat. Chem. 15, 185–193 (2023). https://doi.org/10.1038/s41557-022-01120-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-022-01120-x
This article is cited by
-
Enantioselective sulfur(VI) fluoride exchange reaction of iminosulfur oxydifluorides
Nature Chemistry (2024)
-
Enantioselective construction of stereogenic-at-sulfur(IV) centres via catalytic acyl transfer sulfinylation
Nature Chemistry (2024)
-
Chiral sulfinyls from sulfones
Nature Chemistry (2023)