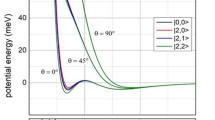
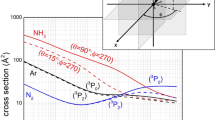
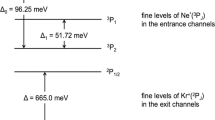
Abstract
Two quantum effects can enable reactions to take place at energies below the barrier separating reactants from products: tunnelling and intersystem crossing between coupled potential energy surfaces. Here we show that intersystem crossing in the region between the pre-reactive complex and the reaction barrier can control the rate of bimolecular reactions for weakly coupled potential energy surfaces, even in the absence of heavy atoms. For O(3P) plus pyridine, a reaction relevant to combustion, astrochemistry and biochemistry, crossed-beam experiments indicate that the dominant products are pyrrole and CO, obtained through a spin-forbidden ring-contraction mechanism. The experimental findings are interpreted—by high-level quantum-chemical calculations and statistical non-adiabatic computations of branching fractions—in terms of an efficient intersystem crossing occurring before the high entrance barrier for O-atom addition to the N-atom lone pair. At low to moderate temperatures, the computed reaction rates prove to be dominated by intersystem crossing.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Source data are provided with this paper (and can also be downloaded at https://doi.org/10.6084/m9.figshare.20423616).
References
Bao, J. L. & Truhlar, D. G. Variational transition state theory: theoretical framework and recent developments. Chem. Soc. Rev. 46, 7548–7596 (2017).
Smith, I. W. M. The temperature-dependence of elementary reaction rates: beyond Arrhenius. Chem. Soc. Rev. 37, 812–826 (2008).
Smith, I. W. M. Laboratory astrochemistry: gas-phase processes. Annu. Rev. Astron. Astrophys. 49, 29–66 (2011).
Sims, I. R. Tunnelling in space. Nat. Chem. 5, 734–736 (2013).
Tizniti, M. et al. The rate of the F + H2 reaction at very low temperatures. Nat. Chem. 6, 141–145 (2014).
Shannon, R. J., Blitz, M. A., Goddard, A. & Heard, D. E. Accelerated chemistry in the reaction between the hydroxyl radical and methanol at interstellar temperatures facilitated by tunnelling. Nat. Chem. 5, 745–749 (2013).
Heard, D. E. Rapid acceleration of hydrogen atom abstraction reactions of OH at very low temperatures through weakly bound complexes and tunneling. Acc. Chem. Res. 51, 2620–2627 (2018).
Harvey, J. N. Understanding the kinetics of spin-forbidden chemical reactions. Phys. Chem. Chem. Phys. 9, 331–343 (2007).
Ahmadvand, S., Zaari, R. R. & Varganov, S. A. Spin forbidden and spin-allowed cyclopropenone (c-H2C3O) formation in interstellar medium. Astrophys. J. 795, 173 (2014).
Jasper, A. W. Multidimensional effects in nonadiabatic statistical theories of spin-forbidden kinetics: a case study of 3O + CO → CO2. J. Phys. Chem. A 119, 7339–7351 (2015).
Domcke, W., Yarkony, D. & Köppel, H. Conical Intersections: Electronic Structure, Dynamics & Spectroscopy 15 (World Scientific, 2004).
Shen, L. et al. Role of multistate intersections in photochemistry. J. Phys. Chem. Lett. 11, 8490–8501 (2020).
Wang, J. J., Smith, D. J. & Grice, R. Role of intersystem crossing in the dynamics of the O(3P) + (CH3)2CHI, (CH3)3CI reactions. J. Phys. Chem. 100, 13603–13608 (1996).
Stevens, J. E., Cui, Q. & Morokuma, K. An ab initio investigation of spin-allowed and spin-forbidden pathways of the gas phase reactions of O(3P)+C2H5I. J. Chem. Phys. 108, 1544–1551 (1998).
Fu, B. et al. Intersystem crossing and dynamics in O(3P) + C2H4 multichannel reaction: experiment validates theory. Proc. Natl Acad. Sci. USA 109, 9733–9738 (2012).
Gimondi, I., Cavallotti, C., Vanuzzo, G., Balucani, N. & Casavecchia, P. Reaction dynamics of O(3P) + propyne: II. Primary products, branching ratios, and role of intersystem crossing from ab initio coupled triplet/singlet potential energy surfaces and statistical calculations. J. Phys. Chem. A 120, 4619–4633 (2016).
Leonori, F. et al. Experimental and theoretical studies on the dynamics of the O(3P) + propene reaction: primary products, branching ratios, and role of intersystem crossing. J. Phys. Chem. C 119, 14632–14652 (2015).
Leonori, F. et al. Crossed molecular beam dynamics studies of the O(3P) + allene reaction: primary products, branching ratios, and dominant role of intersystem crossing. J. Phys. Chem. Lett. 3, 75–80 (2012).
Savee, J. D. et al. Multiplexed photoionization mass spectrometry investigation of the O(3P) + propyne reaction. J. Phys. Chem. A 119, 7388–7403 (2015).
Cavallotti, C. et al. Theoretical study of the extent of intersystem crossing in the O(3P) + C6H6 reaction with experimental validation. J. Phys. Chem. Lett. 11, 9621–9628 (2020).
Taatjes, C. A. et al. Products of the benzene + O(3P) reaction. J. Phys. Chem. A 114, 3355–3370 (2010).
Vanuzzo, G. et al. Crossed-beam and theoretical studies of the O(3P, 1D) + benzene reactions: primary products, branching fractions, and role of intersystem crossing. J. Phys. Chem. A 125, 8434–8453 (2021).
He, C. et al. Non-adiabatic reaction dynamics in the gas-phase formation of phosphinidenesilylene, the isovalent counterpart of hydrogen isocyanide, under single-collision conditions. J. Phys. Chem. Lett. 12, 2489–2495 (2021).
Koziar, J. C. & Cowan, D. O. Photochemical heavy-atom effects. Acc. Chem. Res. 11, 334–341 (1978).
Alagia, M. et al. Crossed beam studies of the O(3P,1D)+CH3I reactions: direct evidence of intersystem crossing for bent geometries. Faraday Discuss. 113, 133–150 (1999).
Pan, H., Liu, K., Caracciolo, A. & Casavecchia, P. Crossed beam polyatomic reaction dynamics: recent advances and new insights. Chem. Soc. Rev. 46, 7517–7547 (2017).
Casavecchia, P., Leonori, F. & Balucani, N. Reaction dynamics of oxygen atoms with unsaturated hydrocarbons from crossed molecular beam studies: primary products, branching ratios and role of intersystem crossing. Int. Rev. Phys. Chem. 34, 161–204 (2015).
Mebel, A. M., Kislov, V. V. & Hayashi, M. Prediction of product branching ratios in the C(3P) + C2H2 → l-C3H + H / c-C3H + H / C3 + H2 reaction using ab initio coupled clusters calculations extrapolated to the complete basis set combined with Rice-Ramsperger-Kassel-Marcus and radiationless transition theories. J. Chem. Phys. 126, 204310 (2007).
Leonori, F. et al. Unraveling the dynamics of the C(3P,1D) + C2H2 reactions by the crossed molecular beam scattering technique. J. Phys. Chem. A 112, 1363–1379 (2008).
Li, H., Kamasah, A., Matsika, S. & Suits, A. G. Intersystem crossing in the exit channel. Nat. Chem. 11, 123–128 (2019).
Wang, B. et al. A kinetic study of NO formation during oxy-fuel combustion of pyridine. Appl. Energy 92, 361–368 (2012).
Parker, D. S. N. et al. On the formation of pyridine in the interstellar medium. Phys. Chem. Chem. Phys. 17, 32000–32008 (2015).
Puzzarini, C. & Barone, V. A never-ending story in the sky: the secrets of chemical evolution. Phys. Life Rev. 32, 59–94 (2020).
Puzzarini, C., Bloino, J., Tasinato, N. & Barone, V. Accuracy and interpretability: the devil and the holy grail. New routes across old boundaries in computational spectroscopy. Chem. Rev. 119, 8131–8191 (2019).
Klippenstein, S. J. & Cavallotti, C. in Computer Aided Chemical Engineering Vol. 45 (eds Faravelli, T., Manenti, F. & Ranzi, E.) 115–167 (Elsevier, 2019).
Alessandrini, S., Barone, V. & Puzzarini, C. Extension of the “cheap” composite approach to noncovalent interactions: the jun-ChS scheme. J. Chem. Theory Comput. 16, 988–1006 (2020).
Grimme, S. Semiempirical hybrid density functional with perturbative second-order correlation. J. Chem. Phys. 124, 034108 (2006).
Biczysko, M., Panek, P., Scalmani, G., Bloino, J. & Barone, V. Harmonic and anharmonic vibrational frequency calculations with the double-hybrid B2PLYP method: analytic second derivatives and benchmark studies. J. Chem. Theory Comput. 6, 2115–2125 (2010).
Kaliakin, D. S., Fedorov, D. G., Alexeev, Y. & Varganov, S. A. Locating minimum energy crossings of different spin states using the fragment molecular orbital method. J. Chem. Theory Comput. 15, 6074–6084 (2019).
Pulay, P. A perspective on the CASPT2 method. Int. J. Quantum Chem. 111, 3273–3279 (2011).
Frerichs, H., Schliephake, V., Tappe, M. & Wagner, H. G. Reactions of pyridine and the picolines with atomic oxygen (O3P) in the gas phase. Zeitsch. Phys. Chem. 166, 157–165 (1990).
Tabares, F. & Gonzalez Urena, A. Rate constant for the reaction of atomic oxygen (3P) with pyridine from 323 to 473 K. J. Phys. Chem. 87, 4933–4936 (1983).
El-Sayed, M. A. Triplet state. Its radiative and nonradiative properties. Acc. Chem. Res. 1, 8–16 (1968).
Alagia, M. et al. Magnetic analysis of supersonic beams of atomic oxygen, nitrogen, and chlorine generated from a radio-frequency discharge. Isr. J. Chem. 37, 329–342 (1997).
Becke, A. D. Density‐functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993).
Grimme, S., Ehrlich, S. & Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 32, 1456–1465 (2011).
Crehuet, R. & Bofill, J. M. The reaction path intrinsic reaction coordinate method and the Hamilton–Jacobi theory. J. Chem. Phys. 122, 234105 (2005).
Papajak, E., Leverentz, H. R., Zheng, J. & Truhlar, D. G. Efficient diffuse basis sets: cc-pVxZ+ and maug-cc-pVxZ. J. Chem. Theoy Comput. 5, 1197–1202 (2009).
Raghavachari, K., Trucks, G. W., Pople, J. A. & Head-Gordon, M. A fifth-order perturbation comparison of electron correlation theories. Chem. Phys. Letts. 157, 479–483 (1989).
Møller, C. & Plesset, M. S. Note on an approximation treatment for many-electron systems. Phys. Rev. 46, 618–622 (1934).
Peterson, K. A. & Dunning, T. H. Jr Accurate correlation consistent basis sets for molecular core-valence correlation effects: the second-row atoms Al-Ar, and the first row atoms B-Ne revisited. J. Chem. Phys. 117, 10548–10560 (2002).
Lupi, J., Puzzarini, C., Cavallotti, C. & Barone, V. State-of-the-art quantum chemistry meets variable reaction coordinate transition state theory to solve the puzzling case of the H2S + Cl system. J. Chem. Theory Comput. 16, 5090–5104 (2020).
Alessandrini, S., Tonolo, F. & Puzzarini, C. In search of phosphorus in astronomical environments: the reaction between the CP radical (X2∑+) and methanimine. J. Chem. Phys. 154, 054306 (2021).
Frisch, M. et al. Gaussian 16 v.C.01 (Gaussian, 2016).
Chai, J.-D. & Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 10, 6615–6620 (2008).
Krishnan, R., Binkley, J. S., Seeger, R. & Pople, J. A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 72, 650 (1980).
Cavallotti, C., Pelucchi, M., Georgievskii, Y. & Klippenstein, S. J. EStokTP: electronic structure to temperature- and pressure-dependent rate constants—a code for automatically predicting the thermal kinetics of reactions. J. Chem. Theory Comput. 15, 1122–1145 (2019).
Werner, H.-J., Knowles, P. J., Knizia, G., Manby, F. R. & Schütz, M. Molpro: a general-purpose quantum chemistry program package. WIREs Comput. Mol. Sci. 2, 242–253 (2012).
Harvey, J. N. & Aschi, M. Modelling spin-forbidden reactions: recombination of carbon monoxide with iron tetracarbonyl. Faraday Discuss. 124, 129–143 (2003).
Zener, C. Non-adiabatic crossing of energy levels. Proc. R. Soc. A 137, 696–702 (1932).
Wittig, C. The Landau−Zener formula. J. Phys. Chem. B 109, 8428–8430 (2005).
Georgievskii, Y. & Klippenstein, S. J. Transition state theory for multichannel addition reactions: multifaceted dividing surfaces. J. Phys. Chem. A 107, 9776–9781 (2003).
Barbato, A., Seghi, C. & Cavallotti, C. An ab initio Rice-Ramsperger-Kassel-Marcus/master equation investigation of SiH4 decomposition kinetics using a kinetic Monte Carlo approach. J. Chem. Phys. 130, 074108 (2009).
Miller, J. A., Klippenstein, S. J. & Raffy, C. Solution of some one-and two-dimensional master equation models for thermal dissociation: the dissociation of methane in the low-pressure limit. J. Phys. Chem. A 106, 4904–4913 (2002).
Georgievskii, Y., Miller, J. A., Burke, M. P. & Klippenstein, S. J. Reformulation and solution of the master equation for multiple-well chemical reactions. J. Phys. Chem. A 117, 12146–12154 (2013).
Acknowledgements
This work has been supported by the Italian MUR (PRIN 2017, grant 2017A4XRCA (V.B.); PRIN 2017, grant 2017PJ5XXX (P.C.); PRIN 2020, grant 202082CE3T (C.P.)) and the Italian Space Agency (‘Life in Space’ project, N.2019-3-U.0 (N.B.,V.B.)). We acknowledge the Scuola Normale Superiore (Internal Funds), the University of Bologna (RFO funds), the Italian MUR (Ministry of University and Research) and Università degli Studi di Perugia (within the programme ‘Department of Excellence−2018−2022−Project AMIS’) and the SMART@SNS Laboratory (http://smart.sns.it) for high-performance computer facilities.
Author information
Authors and Affiliations
Contributions
G.V., G.P., P.R., D.M., A.C. and V.J.M. performed the experiments and analysed the data; S.A. and A.B. performed the QC computations and analysed the results; P.C. and N.B. conceived and guided the experiments and the interpretation of the results; C.C., C.P. and V.B conceived the computational strategy and coordinated the interpretation of its results; and P.C., N.B., C.C., C.P. and V.B. cowrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–20, Tables 1–6, Results, Methods, details and references.
Supplementary Data 1
Cartesian coordinates of the equilibrium structures of all stationary points on the triplet and singlet PESs.
Supplementary Data 2
Source data for Supplementary Fig. 15.
Source data
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Recio, P., Alessandrini, S., Vanuzzo, G. et al. Intersystem crossing in the entrance channel of the reaction of O(3P) with pyridine. Nat. Chem. 14, 1405–1412 (2022). https://doi.org/10.1038/s41557-022-01047-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-022-01047-3
This article is cited by
-
Crossed molecular beam studies of bimolecular reactions of atomic oxygen with nitrogen-bearing organic molecules (nitriles and N-heterocyclic)
Rendiconti Lincei. Scienze Fisiche e Naturali (2024)
-
On the decomposition mechanism of propanal: rate constants evaluation and kinetic simulations
Theoretical Chemistry Accounts (2023)