Abstract
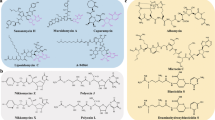
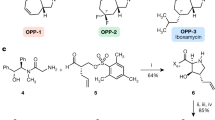
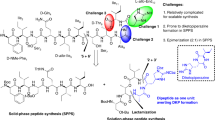
The emergence of drug-resistant bacterial pathogens has placed renewed emphasis on the total chemical synthesis of novel antibacterials. Tetracyclines, macrolides, streptogramins and lincosamides are now accessible through flexible and general synthetic routes. Pleuromutilins (antibiotics based on the fungal metabolite pleuromutilin) have remained resistant to this approach, in large part due to the difficulties encountered in the de novo construction of the decahydro-3a,9-propanocyclopenta[8]annulene skeleton. Here we present a platform for the total synthesis of pleuromutilins that provides access to diverse derivatives bearing alterations at previously inaccessible skeletal and peripheral positions. The synthesis is enabled by the serendipitous discovery of a vinylogous Wolff rearrangement, which serves to establish the C9 quaternary centre in the targets, and the development of a highly diastereoselective butynylation of an α-quaternary aldehyde, which forms the C14 secondary alcohol. The versatility of the route is demonstrated through the synthesis of seventeen structurally distinct derivatives, with many possessing potent antibacterial activity.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data are available in the main text or the Supplementary Information. Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre under deposition nos. CCDC 2114513 (S3), 2114514 (19), 2114515 (26), 2114516 (S20) and 2114517 (S37). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
Change history
25 October 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41557-022-01098-6
References
Fazakerley, N. J. & Procter, D. J. Synthesis and synthetic chemistry of pleuromutilin. Tetrahedron 70, 6911–6930 (2014).
Goethe, O., Heuer, A., Ma, X., Wang, Z. & Herzon, S. B. Antibacterial properties and clinical potential of pleuromutilins. Nat. Prod. Rep. 36, 220–247 (2019).
Davidovich, C. et al. Induced-fit tightens pleuromutilins binding to ribosomes and remote interactions enable their selectivity. Proc. Natl Acad. Sci. USA 104, 4291–4296 (2007).
Thirring, K. et al. Preparation of 12-epi-pleuromutilin derivatives as antimicrobial agents. AT Patent WO2015110481A1 (2015).
Berner, H. V., Schulz, G. H. & Schneider, H. Inversion of configuration of the vinylgroup at carbon 12 by reversible retro-en-cleavage. Monatsh. Fuer Chem. 117, 1073 (1986).
Wicha, W. et al. Efficacy of novel extended spectrum pleuromutilins against E. coli in vitro and in vivo. In 25th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) (Nabriva, 2015).
Paukner, S. et al. In vitro activity of the novel pleuromutilin BC-3781 tested against bacterial pathogens causing sexually transmitted diseases (STD). In 53rd Interscience Conference on Antimicrobial Agents and Chemotherapy (Nabriva, 2013).
Paukner, S. & Riedl, R. Pleuromutilins: potent drugs for resistant bugs—mode of action and resistance. Cold Spring Harb. Perspect. Med. 7, a027110 (2017).
Gentry, D. R., Rittenhouse, S. F., McCloskey, L. & Holmes, D. J. Stepwise exposure of Staphylococcus aureus to pleuromutilins is associated with stepwise acquisition of mutations in rplC and minimally affects susceptibility to retapamulin. Antimicrob. Agents Chemother. 51, 2048–2052 (2007).
Springer, D. M. et al. Synthesis and activity of a C-8 keto pleuromutilin derivative. Bioorg. Med. Chem. Lett. 13, 1751–1753 (2003).
Lykkeberg, A. K., Halling-Sorensen, B. & Jensen, L. B. Susceptibility of bacteria isolated from pigs to tiamulin and enrofloxacin metabolites. Vet. Microbiol. 121, 116–124 (2007).
Sun, F. et al. Unraveling the Metabolic Routes of Retapamulin: Insights into Drug Development of Pleuromutilins. Antimicrob. Agents Chemother. 62, e02388–17 (2018).
Daum, R. S., Kar, S. & Kirkpatrick, P. Retapamulin. Nat. Rev. Drug Discov. 6, 865–866 (2007).
Veve, M. P. & Wagner, J. L. Lefamulin: review of a promising novel pleuromutilin antibiotic. Pharmacotherapy 38, 935–946 (2018).
Liu, F. & Myers, A. G. Development of a platform for the discovery and practical synthesis of new tetracycline antibiotics. Curr. Opin. Chem. Biol. 32, 48–57 (2016).
Seiple, I. B. et al. A platform for the discovery of new macrolide antibiotics. Nature 533, 338–345 (2016).
Li, Q. et al. Synthetic group A streptogramin antibiotics that overcome Vat resistance. Nature 586, 145–150 (2020).
Mitcheltree, M. J., Stevenson, J. W., Pisipati, A. & Myers, A. G. A practical, component-based synthetic route to methylthiolincosamine permitting facile northern-half diversification of lincosamide antibiotics. J. Am. Chem. Soc. 143, 6829–6835 (2021).
Gibbons, E. G. Total synthesis of (±)-pleuromutilin. J. Am. Chem. Soc. 104, 1767–1769 (1982).
Boeckman, R. K., Springer, D. M. & Alessi, T. R. Synthetic studies directed toward naturally occurring cyclooctanoids. 2. A stereocontrolled assembly of (±)-pleuromutilin via a remarkable sterically demanding oxy-cope rearrangement. J. Am. Chem. Soc. 111, 8284–8286 (1989).
Fazakerley, N. J., Helm, M. D. & Procter, D. J. Total synthesis of (+)-pleuromutilin. Chem. Eur. J. 19, 6718–6723 (2013).
Murphy, S. K., Zeng, M. & Herzon, S. B. A modular and enantioselective synthesis of the pleuromutilin antibiotics. Science 356, 956 (2017).
Zeng, M., Murphy, S. K. & Herzon, S. B. Development of a modular synthetic route to (+)-pleuromutilin, (+)-12-epi-mutilins, and related structures. J. Am. Chem. Soc. 139, 16377–16388 (2017).
Farney, E. P., Feng, S. S., Schafers, F. & Reisman, S. E. Total synthesis of (+)-pleuromutilin. J. Am. Chem. Soc. 140, 1267–1270 (2018).
Nicholas, J. F. & Sergey, V. P. Synthesis of pleuromutilin. J. Am. Chem. Soc. 144, 10174–10179 (2022).
Lotesta, S. D. et al. Expanding the pleuromutilin class of antibiotics by de novo chemical synthesis. Chem. Sci. 2, 1258–1261 (2011).
Nagata, W., Yoshioka, M. & Murakami, M. Hydrocyanation. VI. Application of the new hydrocyanation methods to conjugate hydrocyanation of α,β-unsaturated ketones, conjugated dienones, and conjugated enamines and to preparation of α-cyanohydrins. J. Am. Chem. Soc. 94, 4654–4672 (1972).
Egger, H. & Reinshagen, H. New pleuromutilin derivatives with enhanced antimicrobial activity. II. Structure–activity correlations. J. Antibiot. 29, 923–927 (1976).
Smith, A. B. A vinylogous Wolff rearrangement; copper sulphate-catalysed decomposition of unsaturated diazomethyl ketones. Chem. Commun. 695–696 (1974).
Ireland, R. E. & Willard, A. K. The stereoselective generation of ester enolates. Tetrahedron Lett. 16, 3975–3978 (1975).
Justicia, J., Sancho-Sanz, I., Álvarez-Manzaneda, E., Oltra, J. E. & Cuerva, J. M. Efficient propargylation of aldehydes and ketones catalyzed by titanocene(III). Adv. Synth. Catal. 351, 2295–2300 (2009).
Bartolo, N. D., Read, J. A., Valentín, E. M. & Woerpel, K. A. Reactions of allylmagnesium reagents with carbonyl compounds and compounds with C═N double bonds: their diastereoselectivities generally cannot be analyzed using the Felkin–Anh and chelation-control models. Chem. Rev. 120, 1513–1619 (2020).
Muñoz-Bascón, J., Sancho-Sanz, I., Álvarez-Manzaneda, E., Rosales, A. & Oltra, J. E. Highly selective Barbier-type propargylations and allenylations catalyzed by titanocene(III). Chem. –Eur. J. 18, 14479–14486 (2012).
Frigerio, M. & Santagostino, M. A mild oxidizing reagent for alcohols and 1,2-diols: o-iodoxybenzoic acid (IBX) in DMSO. Tetrahedron Lett. 35, 8019–8022 (1994).
Montgomery, J. Nickel-catalyzed reductive cyclizations and couplings. Angew. Chem. Int. Ed. 43, 3890–3908 (2004).
Moslin, R. M., Miller-Moslin, K. & Jamison, T. F. Regioselectivity and enantioselectivity in nickel-catalysed reductive coupling reactions of alkynes. Chem. Commun. 4441–4449 (2007).
Crabtree, R. H. & Davis, M. W. Directing effects in homogeneous hydrogenation with [Ir(cod)(PCy3)(py)]PF6. J. Org. Chem. 51, 2655–2661 (1986).
Spartan 18 v.1.4.8 (Wavefunction, 2018).
Riedl, R. H., Heilmayer, W. & Spence, L. Process for the preparation for the preparation of pleuromutilins. AT Patent WO/2011/146954 (2011).
Lykkeberg, A. K., Cornett, C., Halling-Sørensen, B. & Hansen, S. H. Isolation and structural elucidation of tiamulin metabolites formed in liver microsomes of pigs. J. Pharm. Biomed. Anal. 42, 223–231 (2006).
Lykkeberg, A. K., Halling-Sørensen, B. & Jensen, L. B. Susceptibility of bacteria isolated from pigs to tiamulin and enrofloxacin metabolites. Vet. Microb. 121, 116–124 (2007).
Marx, J. N. & Norman, L. R. Synthesis of (–)-acorone and related spirocyclic sesquiterpenes. J. Org. Chem. 40, 1602–1606 (1975).
Ito, Y., Hirao, T. & Saegusa, T. Synthesis of α,β-unsaturated carbonyl compounds by palladium(II)-catalyzed dehydrosilylation of silyl enol ethers. J. Org. Chem. 43, 1011–1013 (1978).
Shvo, Y. & Arisha, A. H. I. Regioselective catalytic dehydrogenation of aldehydes and ketones. J. Org. Chem. 63, 5640–5642 (1998).
Bell, M. G. et al. Pyrazole derivatives useful as aldolsterone synthase inhibitors. US Patent WO 2012/173849 Al (2012).
Nakamura, E., Matsuzawa, S., Horiguchi, Y. & Kuwajima, I. Me3SiCl accelerated conjugate addition of stoichiometric organocopper reagents. Tetrahedron Lett. 27, 4029–4032 (1986).
Ito, Y., Fujii, S. & Saegusa, T. Reaction of 1-silyloxybicyclo[n.1.0]alkanes with iron(III) chlorides. A facile synthesis of 2-cycloalkenones via ring enlargement of cyclic ketones. J. Org. Chem. 41, 2073–2074 (1976).
Sammakia, T. & Jacobs, J. S. Picolinic acid as a partner in the Mitsunobu reaction: subsequent hydrolysis of picolinate esters under essentially neutral conditions with copper acetate in methanol. Tetrahedron Lett. 40, 2685–2688 (1999).
Richter, M. F. et al. Predictive compound accumulation rules yield a broad-spectrum antibiotic. Nature 545, 299–304 (2017).
Acknowledgements
We gratefully acknowledge financial support from the National Institutes of Health NIGMS (grant no. R35-GM131913), the Chemistry Biology Interface Training Program (grant no. T32GM067543 to M.D.) and Yale University (Wiberg Fellowship to O.G.). We acknowledge T. Smeltzer (Treehouse Biotech) and A. Heuer (Yale University) for preliminary experiments. We thank M. Espinosa (Yale University) for helpful suggestions and glovebox assistance. We acknowledge F. Menges for obtaining the high-resolution mass spectrometry data, B. Mercado for obtaining the X-ray crystallography data and E. Paulson for assistance with NMR processing.
Author information
Authors and Affiliations
Contributions
O.G. and S.B.H. initiated the project. O.G., S.B.H. and M.D. designed the synthetic routes. O.G. and M.D. performed the chemical syntheses and characterizations. All of the authors wrote and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Paul Hergenrother, William Wuest and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–7, and experimental procedures and detailed characterization data for all new compounds, and methods for antimicrobial susceptibility testing.
Supplementary Data 1
Crystallographic data for compound S3; CCDC reference 2114513.
Supplementary Data 2
Crystallographic data for compound 19; CCDC reference 2114514.
Supplementary Data 3
Crystallographic data for compound 26; CCDC reference 2114515.
Supplementary Data 4
Crystallographic data for compound S20; CCDC reference 2114516.
Supplementary Data 5
Crystallographic data for compound S35; CCDC reference 2114517.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Goethe, O., DiBello, M. & Herzon, S.B. Total synthesis of structurally diverse pleuromutilin antibiotics. Nat. Chem. 14, 1270–1277 (2022). https://doi.org/10.1038/s41557-022-01027-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-022-01027-7
This article is cited by
-
Structural basis of Cfr-mediated antimicrobial resistance and mechanisms to evade it
Nature Chemical Biology (2024)
-
Engineered and total biosynthesis of fungal specialized metabolites
Nature Reviews Chemistry (2024)
-
Porin-independent accumulation in Pseudomonas enables antibiotic discovery
Nature (2023)