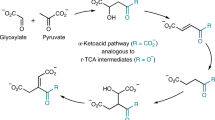
Abstract
Investigation of prebiotic metabolic pathways is predominantly based on abiotically replicating the reductive citric acid cycle. While attractive from a parsimony point of view, attempts using metal/mineral-mediated reductions have produced complex mixtures with inefficient and uncontrolled reactions. Here we show that cyanide acts as a mild and efficient reducing agent mediating abiotic transformations of tricarboxylic acid intermediates and derivatives. The hydrolysis of the cyanide adducts followed by their decarboxylation enables the reduction of oxaloacetate to malate and of fumarate to succinate, whereas pyruvate and α-ketoglutarate themselves are not reduced. In the presence of glyoxylate, malonate and malononitrile, alternative pathways emerge that bypass the challenging reductive carboxylation steps to produce metabolic intermediates and compounds found in meteorites. These results suggest a simpler prebiotic forerunner of today’s metabolism, involving a reductive glyoxylate pathway without oxaloacetate and α-ketoglutarate—implying that the extant metabolic reductive carboxylation chemistries are an evolutionary invention mediated by complex metalloproteins.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The authors declare that all data supporting the findings of this study are available within the paper and its Supplementary Information.
References
Wächtershäuser, G. Before enzymes and templates: theory of surface metabolism. Microbiol. Rev. 52, 452–484 (1988).
Morowitz, H. J., Kostelnik, J. D., Yang, J. & Cody, G. D. The origin of intermediary metabolism. Proc. Natl Acad. Sci. USA 97, 7704–7708 (2000).
Nunoura, T. et al. A primordial and reversible TCA cycle in a facultatively chemolithoautotrophic thermophile. Science 359, 559–563 (2018).
Mall, A. et al. Reversibility of citrate synthase allows autotrophic growth of a thermophilic bacterium. Science 359, 563–567 (2018).
Steffens, L. et al. High CO2 levels drive the TCA cycle backwards towards autotrophy. Nature 592, 784–788 (2021).
McMurry, J. & Begley, T. The Organic Chemistry of Biological Pathways (Roberts, 2005).
Dolan, S. K. & Welch, M. The glyoxylate shunt, 60 years on. Annu. Rev. Microbiol. 72, 309–330 (2018).
Zubay, G. The glyoxylate cycle, a possible evolutionay precursor of the TCA cycle. Chemtracts 16, 783–788 (2003).
Muchowska, K. B., Varma, S. J. & Moran, J. Synthesis and breakdown of universal metabolic precursors promoted by iron. Nature 569, 104–107 (2019).
Martin, W. F. Carbon–metal bonds: rare and primordial in metabolism. Trends Biochem. Sci. 44, 807–818 (2019).
Muchowska, K. B., Varma, S. J. & Moran, J. Nonenzymatic metabolic reactions and life’s origins. Chem. Rev. 120, 7708–7744 (2020).
Cody, G. D. et al. Primordial carbonylated iron-sulfur compounds and the synthesis of pyruvate. Science 289, 1337–1340 (2000).
Guzman, M. I. & Martin, S. T. Photo-production of lactate from glyoxylate: how minerals can facilitate energy storage in a prebiotic world. Chem. Commun. 46, 2265–2267 (2010).
Orgel, L. E. The implausibility of metabolic cycles on the prebiotic Earth. PLoS Biol. 6, e18 (2008).
Ferris, J. P. & Hagan, W. J. Jr. HCN and chemical evolution: the possible role of cyano compounds in prebiotic synthesis. Tetrahedron 40, 1093–1120 (1984).
Sutherland, J. D. The origin of life—out of the blue. Angew. Chem. Int. Ed. 55, 104–121 (2016).
Patel, B. H., Percivalle, C., Ritson, D. J., Duffy, C. D. & Sutherland, J. D. Common origins of RNA, protein and lipid precursors in a cyanosulfidic protometabolism. Nat. Chem. 7, 301–307 (2015).
Ritson, D. & Sutherland, J. D. Prebiotic synthesis of simple sugars by photoredox systems chemistry. Nat. Chem. 4, 895–899 (2012).
Coggins, A. J. & Powner, M. W. Prebiotic synthesis of phosphoenol pyruvate by α-phosphorylation-controlled triose glycolysis. Nat. Chem. 9, 310–317 (2017).
Canavelli, P., Islam, S. & Powner, M. W. Peptide ligation by chemoselective aminonitrile coupling in water. Nature 571, 546–549 (2019).
Foden, C. S. et al. Prebiotic synthesis of cysteine peptides that catalyze peptide ligation in neutral water. Science 370, 865–869 (2020).
Eschenmoser, A. The TNA-family of nucleic acid systems: properties and prospects. Orig. Life Evol. Biosphere 34, 277–306 (2004).
Butch, C. et al. Production of tartrates by cyanide-mediated dimerization of glyoxylate: a potential abiotic pathway to the citric acid cycle. J. Am. Chem. Soc. 135, 13440–13445 (2013).
Yerabolu, J. R., Liotta, C. L. & Krishnamurthy, R. Anchimeric-assisted spontaneous hydrolysis of cyanohydrins under ambient conditions: implications for cyanide-initiated selective transformations. Chem. Eur. J. 23, 8756–8765 (2017).
Cooper, G., Reed, C., Nguyen, D., Carter, M. & Wang, Y. Detection and formation scenario of citric acid, pyruvic acid, and other possible metabolism precursors in carbonaceous meteorites. Proc. Natl Acad. Sci. USA 108, 14015–14020 (2011).
Bender, M. L. & Connors, K. A. A non-enzymatic olefinic hydration under neutral conditions: the kinetics and mechanism of the hydration of fumaric acid monoanion. J. Am. Chem. Soc. 84, 1980–1986 (1962).
Colín-García, M., Negrón-Mendoza, A. & Ramos-Bernal, S. Organics produced by irradiation of frozen and liquid HCN solutions: implications for chemical evolution studies. Astrobiology 9, 279–288 (2009).
Keller, M. A., Kampjut, D., Harrison, S. A. & Ralser, M. Sulfate radicals enable a non-enzymatic Krebs cycle precursor. Nat. Ecol. Evol. 1, 0083 (2017).
Kunz, D. A., Chen, J.-L. & Pan, G. Accumulation of α-keto acids as essential components in cyanide assimilation by Pseudomonas fluorescens NCIMB 11764. Appl. Environ. Microbiol. 64, 4452–4459 (1998).
Stubbs, R. T., Yadav, M., Krishnamurthy, R. & Springsteen, G. A plausible metal-free ancestral analogue of the Krebs cycle composed entirely of α-ketoacids. Nat. Chem. 12, 1016–1022 (2020).
Ross, D. S. The viability of a nonenzymatic reductive citric acid cycle—kinetics and thermochemistry. Orig. Life Evol. Biosph. 37, 61–65 (2007).
Eschenmoser, A. & Loewenthal, E. Chemistry of potentially prebiological natural products. Chem. Soc. Rev. 21, 1–16 (1992).
Leopold, K. R. & Haim, A. Equilibrium, kinetics, and mechanism of the malonic acid–iodine reaction. Int. J. Chem. Kinet. 9, 83–95 (1977).
Ghorai, S., Laskin, A. & Tivanski, A. V. Spectroscopic evidence of keto-enol tautomerism in deliquesced malonic acid particles. J. Phys. Chem. A 115, 4373–4380 (2011).
Mainguet, S. E., Gronenberg, L. S., Wong, S. S. & Liao, J. C. A reverse glyoxylate shunt to build a non-native route from C4 to C2 in Escherichia coli. Metabol. Eng. 19, 116–127 (2013).
Toney, M. D. Carbon acidity in enzyme active sites. Front. Bioeng. Biotechnol. https://doi.org/10.3389/fbioe.2019.00025 (2019).
Wolfenden, R., Lewis, C. A. Jr & Yuan, Y. Kinetic challenges facing oxalate, malonate, acetoacetate, and oxaloacetate decarboxylases. J. Am. Chem. Soc. 133, 5683–5685 (2011).
Maltais, T. R., VanderVelde, D., LaRowe, D. E., Goldman, A. D. & Barge, L. M. Reactivity of metabolic intermediates and cofactor stability under model early earth conditions. Orig. Life Evol. Biosph. 50, 35–55 (2020).
Cody, G. D. et al. Geochemical roots of autotrophic carbon fixation: hydrothermal experiments in the system citric acid, H2O–(±FeS)–(±NiS). Geochim. Cosmochim. Acta 65, 3557–3576 (2001).
Varma, S. J., Muchowska, K. B., Chatelain, P. & Moran, J. Native iron reduces CO2 to intermediates and end-products of the acetyl-CoA pathway. Nat. Ecol. Evol. 2, 1019–1024 (2018).
Liu, Z. et al. Prebiotic photoredox synthesis from carbon dioxide and sulfite. Nat. Chem. 13, 1126–1132 (2021).
Ritson, D. J. A cyanosulfidic origin of the Krebs cycle. Sci. Adv. 7, eabh3981 (2021).
Springsteen, G., Yerabolu, J. R., Nelson, J., Rhea, C. J. & Krishnamurthy, R. Linked cycles of oxidative decarboxylation of glyoxylate as protometabolic analogs of the citric acid cycle. Nat. Commun. 9, 91 (2018).
Raulin, F., Bloch, S. & Toupance, G. Addition reactions of malonic nitriles with alkanethiol in aqueous solution. Orig. Life Evol. Biosph. 8, 247–257 (1977).
Freeman, F. Chemistry of malononitrile. Chem. Rev. 69, 591–624 (1969).
Grönberg, K. L. C., Gormal, C. A., Durrant, M. C., Smith, B. E. & Henderson, R. A. Why R-homocitrate is essential to the reactivity of FeMo-cofactor of nitrogenase: studies on NifV-extracted FeMo-cofactor. J. Am. Chem. Soc. 120, 10613–10621 (1998).
Fay, A. W. et al. Formation of a homocitrate-free iron-molybdenum cluster on NifEN: implications for the role of homocitrate in nitrogenase assembly. Dalton Trans. 39, 3124–3130 (2010).
Russell, J. B. & Forsberg, N. Production of tricarballylic acid by rumen microorganisms and its potential toxicity in ruminant tissue metabolism. Br. J. Nutr. 56, 153–162 (1986).
Sugimoto, N., Engelgau, P., Jones, A. D., Song, J. & Beaudry, R. Citramalate synthase yields a biosynthetic pathway for isoleucine and straight- and branched-chain ester formation in ripening apple fruit. Proc. Natl Acad. Sci. USA 118, e2009988118 (2021).
Pizzarello, S. & Garvie, L. A. J. Sutter’s Mill dicarboxylic acids as possible tracers of parent-body alteration processes. Meteor. Planet. Sci. 49, 2087–2094 (2014).
Pizzarello, S. & Shock, E. The organic composition of carbonaceous meteorites: the evolutionary story ahead of biochemistry. Cold Spring Harb. Perspect. Biol. 2, a002105–a002105 (2010).
Pizzarello, S. The chemistry of life’s origin: a carbonaceous meteorite perspective. Acc. Chem. Res. 39, 231–237 (2006).
Ball, R. & Brindley, J. The power without the glory: multiple roles of hydrogen peroxide in mediating the origin of life. Astrobiology 19, 675–684 (2019).
Springsteen, G., Rice, G., Yerabolu, J. & Krishnamurthy, R. The abiotic oxidation of organic acids to malonate. Synlett 28, 98–102 (2016).
Lazcano, A. & Miller, S. L. On the origin of metabolic pathways. J. Mol. Evol. 49, 424–431 (1999).
Harrison, S. A. & Lane, N. Life as a guide to prebiotic nucleotide synthesis. Nat. Commun. 9, 5176 (2018).
Krishnamurthy, R. Life’s biological chemistry: a destiny or destination starting from prebiotic chemistry? Chem. Eur. J. 24, 16708–16715 (2018).
Chen, X., Li, N. & Ellington, A. D. Ribozyme catalysis of metabolism in the RNA world. Chem. Biodiver. 4, 633–655 (2007).
Theil, E. C. & Goss, D. J. Living with iron (and oxygen): questions and answers about iron homeostasis. Chem. Rev. 109, 4568–4579 (2009).
Mohammed, F. S. et al. A plausible prebiotic origin of glyoxylate: nonenzymatic transamination reactions of glycine with formaldehyde. Synlett 28, 93–97 (2016).
Marin-Yaseli, M. R., Gonzalez-Toril, E., Mompean, C. & Ruiz-Bermejo, M. The role of aqueous aerosols in the “glyoxylate scenario”: an experimental approach. Chem. Eur. J. 22, 12785–12799 (2016).
Acknowledgements
This work was supported by a NASA (National Aeronautics and Space Administration) Exobiology grant 80NSSC18K1300 (R.K.) and jointly supported by National Science Foundation, the NASA Astrobiology Program under the Center for Chemical Evolution grant no. CHE-1504217 (R.K.) and a grant from the Simons Foundation 32712FY19 (R.K.). We thank A. Lazcano, G. Springsteen and L. Leman for feedback on the manuscript.
Author information
Authors and Affiliations
Contributions
R.K. conceived and directed the project. M.Y., S.P., J.R.Y. and R.K. proposed and designed the experiments; M.Y., S.P. and J.R.Y. carried out the experiments. M.Y. and S. P. contributed equally to this work. All authors interpreted the data and discussed the experimental results. R.K. wrote the paper with comments and feedback from M.Y., S.P. and J.R.Y.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Juli Pereto and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Cyanide mediated reduction of oxaloacetate to malate.
Time course 1H-NMR of the reaction of oxaloacetate with cyanide (a) after 30 min of addition of cyanide to oxaloacetic acid and (b) after 120 days. The formation of the intermediates, cyanohydrin adduct of oxaloacetate and 2-carboxymalate 4b en route to malate 5 was observed. Traces of pyruvate 21 was formed from the competing decarboxylation reaction of oxalocetate.
Extended Data Fig. 2 Reduction of the conjugated double bonds of fumarate (derivatives) by cyanide.
(a) 1H-NMR (D2O) documenting the cyanide-mediated reduction of fumarate to succinate. The reduction of fumarate proceeds cleanly over days to produce predominantly succinate. (b) The reduction of fumaramide 7 (potentially derived from fumaronitrile) proceeds via the partially hydrolyzed intermediate fumaramate 8. The presence of cyanide at pH 9 first hydrolyzes 7 to the half amide 8, which then undergoes the addition of cyanide followed by hydrolysis and decarboxylation to form succinate 10. Slight shift of succinate peak in NMR spectrum (C, after 11 days) is due to change in pH.
Extended Data Fig. 3 Cyanide interaction with β-ketoglutarate leading to the formation of citrate and citramalic acid.
(Top) The reaction scheme showing the pathway for the formation of citrate 14 and citramalic acid 19b. (Bottom) Stacked time course 1H-NMR (D2O) spectra of the cyanide mediated reduction of β-ketoglutarate to citrate and formation of citramalic acid as a side product.
Extended Data Fig. 4 A cyanide catalyzed novel non-oxidative decarboxylation of a conjugated α-ketoacid 21 with simultaneous reduction.
(a, b) 1H-NMR time-course stacked spectra of the reaction of fumaroyl formate 21 (in equilibrium with hydroxy-2-ketoglutarate 20) with cyanide forms a cyanohydrin adduct that undergoes decarboxylation and concomitant reduction of the double bond to yield succinate 10, bypassing fumarate (which is the product formed from oxidative decarboxylation30 of 21).
Extended Data Fig. 5 Reaction between carboxysuccinate and glyoxylate leading to isocitrate.
1H-NMR (D2O) showing (top) the reaction mixture resulting from the reaction of carboxysuccinate 9 to isocitrate 12, which exists as the open-chain and closed (lactone) diastereomers. The bottom spectrum is of the mixture of 1:1 authentic threo-D/L-isocitrate 12 and its lactone 12a shown for comparison.
Supplementary information
Supplementary Information
Supplementary Figs. 1–88 (1H and 13C NMR spectral data), materials and methods, procedures, experimental data, and references.
Rights and permissions
About this article
Cite this article
Yadav, M., Pulletikurti, S., Yerabolu, J.R. et al. Cyanide as a primordial reductant enables a protometabolic reductive glyoxylate pathway. Nat. Chem. 14, 170–178 (2022). https://doi.org/10.1038/s41557-021-00878-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-021-00878-w
This article is cited by
-
Primitive purine biosynthesis connects ancient geochemistry to modern metabolism
Nature Ecology & Evolution (2024)
-
Prebiotic synthesis of α-amino acids and orotate from α-ketoacids potentiates transition to extant metabolic pathways
Nature Chemistry (2022)
-
Cyanide at the origin of metabolism
Nature Chemistry (2022)
-
Frontiers in Prebiotic Chemistry and Early Earth Environments
Origins of Life and Evolution of Biospheres (2022)