Abstract
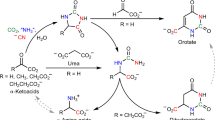
Efforts to decipher the prebiotic roots of metabolic pathways have focused on recapitulating modern biological transformations, with metals typically serving in place of cofactors and enzymes. Here we show that the reaction of glyoxylate with pyruvate under mild aqueous conditions produces a series of α-ketoacid analogues of the reductive citric acid cycle without the need for metals or enzyme catalysts. The transformations proceed in the same sequence as the reverse Krebs cycle, resembling a protometabolic pathway, with glyoxylate acting as both the carbon source and reducing agent. Furthermore, the α-ketoacid analogues provide a natural route for the synthesis of amino acids by transamination with glycine, paralleling the extant metabolic mechanisms and obviating the need for metal-catalysed abiotic reductive aminations. This emerging sequence of prebiotic reactions could have set the stage for the advent of increasingly sophisticated pathways operating under catalytic control.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The authors declare that all data supporting the findings of this study are available within the paper and its Supplementary Information.
References
Morowitz, H. J., Kostelnik, J. D., Yang, J. & Cody, G. D. The origin of intermediary metabolism. Proc. Natl Acad. Sci. USA 97, 7704–7708 (2000).
Huynen, M. A., Dandekar, T. & Bork, P. Variation and evolution of the citric-acid cycle: a genomic perspective. Trends Microbiol. 7, 281–291 (1999).
Meléndez-Hevia, E., Waddell, T. G. & Cascante, M. The puzzle of the krebs citric acid cycle: assembling the pieces of chemically feasible reactions, and opportunism in the design of metabolic pathways during evolution. J. Mol. Evol. 43, 293–303 (1996).
Nunoura, T. et al. A primordial and reversible TCA cycle in a facultatively chemolithoautotrophic thermophile. Science 563, 559–563 (2018).
Zubarev, D. Y., Rappoport, D. & Aspuru-Guzik, A. Uncertainty of prebiotic scenarios: the case of the non-enzymatic reverse tricarboxylic acid cycle. Sci. Rep. 5, 8009 (2015).
Wächtershäuser, G. Evolution of the first metabolic cycles. Proc. Natl Acad. Sci. USA 87, 200–204 (1990).
Cody, G. D. et al. Primordial carbonylated iron–sulfur compounds and the synthesis of pyruvate. Science 289, 1337–1340 (2000).
Cooper, G., Reed, C., Nguyen, D., Carter, M. & Wang, Y. Detection and formation scenario of citric acid, pyruvic acid, and other possible metabolism precursors in carbonaceous meteorites. Proc. Natl Acad. Sci. USA 108, 14015–14020 (2011).
Coggins, A. J. & Powner, M. W. Prebiotic synthesis of phosphoenol pyruvate by α-phosphorylation-controlled triose glycolysis. Nat. Chem. 9, 310 (2017).
Mall, A. et al. Reversibility of citrate synthase allows autotrophic growth of a thermophilic bacterium. Science 359, 563–567 (2018).
Lehninger, A. L., Nelson, D. L. & Cox, M. M. Lehninger Principles of Biochemistry (WH Freeman, 2013).
Zubay, G. The glyoxylate cycle, a possible evolutionary precursor of the TCA Cycle. Chemtracts 16, 783–788 (2003).
Peretó, J. Out of fuzzy chemistry: from prebiotic chemistry to metabolic networks. Chem. Soc. Rev. 41, 5394–5403 (2012).
Novikov, Y. & Copley, S. D. Reactivity landscape of pyruvate under simulated hydrothermal vent conditions. Proc. Natl Acad. Sci. USA 110, 13283–13288 (2013).
Zhang, X. V. & Martin, S. T. Driving parts of Krebs cycle in reverse through mineral photochemistry. J. Am. Chem. Soc. 128, 16032–16033 (2006).
Ralser, M. An appeal to magic? The discovery of a non-enzymatic metabolism and its role in the origins of life. Biochem. J 475, 2577–2592 (2018).
Sousa, F. L., Preiner, M. & Martin, W. F. Native metals, electron bifurcation, and CO2 reduction in early biochemical evolution. Curr. Opin. Microbiol. 43, 77–83 (2018).
Morowitz, H. J., Srinivasan, V. & Smith, E. Ligand field theory and the origin of life as an emergent feature of the periodic table of elements. Biol. Bull. 219, 1–6 (2010).
Keller, M. A., Piedrafita, G. & Ralser, M. The widespread role of non-enzymatic reactions in cellular metabolism. Curr. Opin. Biotechnol. 34, 153–161 (2015).
Hartman, H. Speculations on the origin and evolution of metabolism. J. Mol. Evol. 4, 359–370 (1975).
Muchowska, K. B. et al. Metals promote sequences of the reverse Krebs cycle. Nat. Ecol. Evol 1, 1716–1721 (2017).
Pascal, R. A possible prebiotic basis for metabolism. Nature 569, 47–48 (2019).
Orgel, L. E. The implausibility of metabolic cycles on the prebiotic earth. PLoS Biol. 6, 0005–0013 (2008).
Ross, D. S. The viability of a nonenzymatic reductive citric acid cycle—kinetics and thermochemistry. Orig. Life Evol. Biosph. 37, 61–65 (2007).
Kitadai, N., Kameya, M. & Fujishima, K. Origin of the reductive tricarboxylic acid (rTCA) cycle-type CO2 fixation: a perspective. Life 7, 39 (2017).
Maltais, T. R., VanderVelde, D., LaRowe, D. E., Goldman, A. D. & Barge, L. M. Reactivity of metabolic intermediates and cofactor stability under model early Earth conditions. Orig. Life Evol. Biosph. 50, 35–55 (2020).
Orgel, L. E. Self-organizing biochemical cycles. Proc. Natl Acad. Sci. USA 97, 12503–12507 (2000).
Springsteen, G., Yerabolu, J. R., Nelson, J., Rhea, C. J. & Krishnamurthy, R. Linked cycles of oxidative decarboxylation of glyoxylate as protometabolic analogs of the citric acid cycle. Nat. Commun. 9, 91 (2018).
Muchowska, K. B., Varma, S. J. & Moran, J. Synthesis and breakdown of universal metabolic precursors promoted by iron. Nature 569, 104–107 (2019).
Patel, B. H., Percivalle, C., Ritson, D. J., Duffy, C. D. & Sutherland, J. D. Common origins of RNA, protein and lipid precursors in a cyanosulfidic protometabolism. Nat. Chem. 7, 301–307 (2015).
Grabowski, J. J. Aqueous-phase pKa of the methyl group in acetic acid. Chem. Commun. 255–256 (1997).
Chiang, Y., Kresge, A. J. & Pruszynski, P. Keto–enol equilibria in the pyruvic acid system: determination of the keto–enol equilibrium constants of pyruvic acid and pyruvate anion and the acidity constant of pyruvate enol in aqueous solution. J. Am. Chem. Soc. 114, 3103–3107 (1992).
Cooper, A. J. L., Ginos, J. Z. & Meister, A. Synthesis and properties of the α-keto acids. Chem. Rev. 83, 321–358 (1983).
Buchanan, B. B. & Evans, M. C. W. The synthesis of alpha-ketoglutarate from succinate and carbon dioxide by a subcellular preparation of a photosynthetic bacterium. Biochemistry 54, 1212–1218 (1965).
Ruffo, A., Testa, E., Adinolfi, A. & Pelizza, G. Control of the citric acid cycle by glyoxylate. 1. A new inhibitor of aconitase formed by the condensation of glyoxylate with oxaloacetate. Biochem. J. 85, 588 (1962).
Payes, B. & Laties, G. G. The inhibition of several tricarboxylic acid cycle enzymes by γ-hydroxy-α-ketoglutarate. Biochem. Biophys. Res. Commun. 10, 460–466 (1963).
Wiley, R. H. & Kim, K. S. The bimolecular decarboxylative self-condensation of oxaloacetic acid. J. Org. Chem. 38, 3582–3585 (1973).
Herbst, R. M. & Engel, L. L. A reaction between alpha-ketonic acids and alpha-amino acids. J. Biol. Chem. 107, 505–512 (1934).
Potter-McIntyre, S. L. & McCollom, T. M. Jarosite and alunite in ancient terrestrial sedimentary rocks: reinterpreting martian depositional and diagenetic environmental conditions. Life 8, 1–22 (2018).
Wu, J. & Huang, S. X. Aluminum(iii) salts promoted transamination of glutamic acid for the synthesis of α-ketoglutaric acid. Chinese J. Org. Chem. 35, 1991–1993 (2015).
Metzler, D. E., Ikawa, M. & Snell, E. E. A general mechanism for vitamin B6-catalyzed reactions. J. Am. Chem. Soc. 76, 648–652 (1954).
Miller, S. L. Production of some organic compounds under possible primitive earth conditions. J. Am. Chem. Soc. 77, 2351–2361 (1955).
Kvenvolden, K. et al. Evidence for extraterrestrial amino-acids and hydrocarbons in the Murchison meteorite. Nature 228, 923–926 (1970).
Wu, L.-F. & Sutherland, J. D. Provisioning the origin and early evolution of life. Emerg. Top. Life Sci. 3, 459–468 (2019).
Hammond, G. S. & Wu, C.-H. S. in Oxidation of Organic Compounds Vol. 77, 186–207 (American Chemical Society, 1968); https://doi.org/10.1021/ba-1968-0077.ch075
Theil, E. C. & Goss, D. J. Living with iron (and oxygen): questions and answers about iron homeostasis. Chem. Rev. 109, 4568–4579 (2009).
Mohammed, F. S. et al. A plausible prebiotic origin of glyoxylate: nonenzymatic transamination reactions of glycine with formaldehyde. Synlett 28, 93–97 (2017).
Krishnamurthy, R. Life’s biological chemistry: a destiny or destination starting from prebiotic chemistry? Chem. Eur. J. 24, 16708–16715 (2018).
Yan, K. et al. Catalyst-free direct decarboxylative coupling of α-keto acids with thiols: a facile access to thioesters. Org. Biomol. Chem. 13, 7323–7330 (2015).
Bode, J. W., Fox, R. M. & Baucom, K. D. Chemoselective amide ligations by decarboxylative condensations of N-alkylhydroxylamines and alpha-ketoacids. Angew. Chem. Int. Ed 45, 1248–1252 (2006).
Acknowledgements
This work was jointly supported by NSF and the NASA Astrobiology Program under the Center for Chemical Evolution (grant no. CHE-1504217) and by a NASA Exobiology grant to R.K. (80NSSC18K1300). G.S. acknowledges a Henry Dreyfus Teacher-Scholar Award.
Author information
Authors and Affiliations
Contributions
R.T.S. and M.Y. contributed equally to this work. R.K., R.T.S and G.S. conceived the project. R.T.S, M.Y., R.K. and G.S. proposed and designed the experiments. R.T.S., G.S. and M.Y. carried out the experiments. All authors interpreted the data and discussed the experimental results. R.K. and G.S. supervised the research and wrote the paper with comments and feedback from R.T.S. and M.Y.
Corresponding authors
Ethics declarations
Competing interests
R.K. and M.Y. declare they have no competing interests. G.S. and R.T.S. declare that a US non-provisional (16/746,124) and PCT application (PCT/US20/14023) have been filed covering the synthesis of organic acids and α-ketoacids. G.S. and R.T.S. own Aconabolics LLC, a company with commercial interests in using α-ketoacids as diagnostic agents.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–17.
Rights and permissions
About this article
Cite this article
Stubbs, R.T., Yadav, M., Krishnamurthy, R. et al. A plausible metal-free ancestral analogue of the Krebs cycle composed entirely of α-ketoacids. Nat. Chem. 12, 1016–1022 (2020). https://doi.org/10.1038/s41557-020-00560-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-020-00560-7
This article is cited by
-
C2-addition patterns emerging from acetylene and nickel sulfide in simulated prebiotic hydrothermal conditions
Communications Chemistry (2023)
-
Aqueous microdroplets promote C–C bond formation and sequences in the reverse tricarboxylic acid cycle
Nature Ecology & Evolution (2023)
-
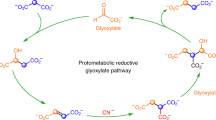
Cyanide as a primordial reductant enables a protometabolic reductive glyoxylate pathway
Nature Chemistry (2022)
-
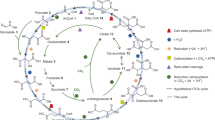
Proteome-wide 3D structure prediction provides insights into the ancestral metabolism of ancient archaea and bacteria
Nature Communications (2022)
-
Prebiotic synthesis of α-amino acids and orotate from α-ketoacids potentiates transition to extant metabolic pathways
Nature Chemistry (2022)