Abstract
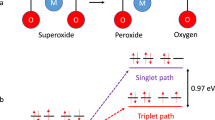
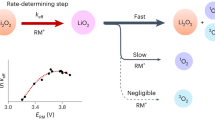
Aprotic alkali metal–O2 batteries face two major obstacles to their chemistry occurring efficiently, the insulating nature of the formed alkali superoxides/peroxides and parasitic reactions that are caused by the highly reactive singlet oxygen (1O2). Redox mediators are recognized to be key for improving rechargeability. However, it is unclear how they affect 1O2 formation, which hinders strategies for their improvement. Here we clarify the mechanism of mediated peroxide and superoxide oxidation and thus explain how redox mediators either enhance or suppress 1O2 formation. We show that charging commences with peroxide oxidation to a superoxide intermediate and that redox potentials above ~3.5 V versus Li/Li+ drive 1O2 evolution from superoxide oxidation, while disproportionation always generates some 1O2. We find that 1O2 suppression requires oxidation to be faster than the generation of 1O2 from disproportionation. Oxidation rates decrease with growing driving force following Marcus inverted-region behaviour, establishing a region of maximum rate.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the plots within this paper and other findings of this study are available from the corresponding author S.A.F. upon reasonable request. Source data are provided with this paper.
References
Luo, K. et al. Charge-compensation in 3d-transition-metal-oxide intercalation cathodes through the generation of localized electron holes on oxygen. Nat. Chem. 8, 684–691 (2016).
Lu, Y.-C. et al. Lithium–oxygen batteries: bridging mechanistic understanding and battery performance. Energy Environ. Sci. 6, 750–768 (2013).
Choi, J. W. & Aurbach, D. Promise and reality of post-lithium-ion batteries with high energy densities. Nat. Rev. Mater. 1, 16013 (2016).
Aurbach, D., McCloskey, B. D., Nazar, L. F. & Bruce, P. G. Advances in understanding mechanisms underpinning lithium–air batteries. Nat. Energy 1, 16128 (2016).
Wang, Y. et al. A solvent-controlled oxidation mechanism of Li2O2 in lithium–oxygen batteries. Joule 2, 2364–2380 (2018).
Ko, Y. et al. Redox mediators: a solution for advanced lithium–oxygen batteries. Trends Chem. 1, 349–360 (2019).
Gao, X., Chen, Y., Johnson, L. & Bruce, P. G. Promoting solution phase discharge in Li–O2 batteries containing weakly solvating electrolyte solutions. Nat. Mater. 15, 882–888 (2016).
Lim, H.-D. et al. Rational design of redox mediators for advanced Li–O2 batteries. Nat. Energy 1, 16066 (2016).
Aetukuri, N. B. et al. Solvating additives drive solution-mediated electrochemistry and enhance toroid growth in non-aqueous Li–O2 batteries. Nat. Chem. 7, 50–56 (2014).
Liu, T. et al. The effect of water on quinone redox mediators in nonaqueous Li–O2 batteries. J. Am. Chem. Soc. 140, 1428–1437 (2018).
Liang, Z. & Lu, Y.-C. Critical role of redox mediator in suppressing charging instabilities of lithium–oxygen batteries. J. Am. Chem. Soc. 138, 7574–7583 (2016).
McCloskey, B. D. et al. Twin problems of interfacial carbonate formation in nonaqueous Li–O2 batteries. J. Phys. Chem. Lett. 3, 997–1001 (2012).
McCloskey, B. D. et al. Limitations in rechargeability of Li–O2 batteries and possible origins. J. Phys. Chem. Lett. 3, 3043–3047 (2012).
Liu, T., Kim, G., Casford, M. T. L. & Grey, C. P. Mechanistic insights into the challenges of cycling a nonaqueous Na–O2 battery. J. Phys. Chem. Lett. 7, 4841–4846 (2016).
Burke, C. M. et al. Implications of 4 e– oxygen reduction via iodide redox mediation in Li–O2 batteries. ACS Energy Lett. 1, 747–756 (2016).
Chen, Y. et al. Charging a Li–O2 battery using a redox mediator. Nat. Chem. 5, 489–494 (2013).
Bergner, B. J. et al. TEMPO: A mobile catalyst for rechargeable Li–O2 batteries. J. Am. Chem. Soc. 136, 15054–15064 (2014).
Lu, Y.-C. & Shao-Horn, Y. Probing the reaction kinetics of the charge reactions of nonaqueous Li–O2 batteries. J. Phys. Chem. Lett. 4, 93–99 (2012).
Kang, S., Mo, Y., Ong, S. P. & Ceder, G. A facile mechanism for recharging Li2O2 in Li–O2 batteries. Chem. Mat. 25, 3328–3336 (2013).
Wang, J. et al. Identifying reactive sites and transport limitations of oxygen reactions in aprotic lithium–O2 batteries at the stage of sudden death. Angew. Chem. Int. Ed. 55, 5201–5205 (2016).
Ganapathy, S. et al. Nature of Li2O2 oxidation in a Li–O2 battery revealed by operando X-ray diffraction. J. Am. Chem. Soc. 136, 16335–16344 (2014).
Kwabi, D. G. et al. Controlling solution-mediated reaction mechanisms of oxygen reduction using potential and solvent for aprotic lithium–oxygen batteries. J. Phys. Chem. Lett. 7, 1204–1212 (2016).
Gao, X. et al. Phenol-catalyzed discharge in the aprotic lithium–oxygen battery. Angew. Chem. Int. Ed. 56, 6539–6543 (2017).
Ottakam Thotiyl, M. M., Freunberger, S. A., Peng, Z. & Bruce, P. G. The carbon electrode in nonaqueous Li–O2 cells. J. Am. Chem. Soc. 135, 494–500 (2013).
Wandt, J. et al. Singlet oxygen formation during the charging process of an aprotic lithium–oxygen battery. Angew. Chem. Int. Ed. 55, 6892–6895 (2016).
Mahne, N. et al. Singlet oxygen generation as a major cause for parasitic reactions during cycling of aprotic lithium–oxygen batteries. Nat. Energy 2, 17036 (2017).
Mourad, E. et al. Singlet oxygen from cation driven superoxide disproportionation and consequences for aprotic metal–O2 batteries. Energy Environ. Sci. 12, 2559–2568 (2019).
Schafzahl, L. et al. Singlet oxygen during cycling of the aprotic sodium–O2 battery. Angew. Chem. Int. Ed. 56, 15728–15732 (2017).
Liang, Z., Zou, Q., Xie, J. & Lu, Y.-C. Suppressing singlet oxygen generation in lithium-oxygen batteries with redox mediators. Energy Environ. Sci. 13, 1106–1126 (2020).
Park, J.-B. et al. Redox mediators for Li–O2 batteries: Status and perspectives. Adv. Mat. 30, 1704162 (2018).
Pande, V. & Viswanathan, V. Criteria and considerations for the selection of redox mediators in nonaqueous Li–O2 batteries. ACS Energy Lett. 2, 60–63 (2017).
Chen, Y., Gao, X., Johnson, L. R. & Bruce, P. G. Kinetics of lithium peroxide oxidation by redox mediators and consequences for the lithium–oxygen cell. Nat. Commun. 9, 767 (2018).
Gao, X. et al. A rechargeable lithium–oxygen battery with dual mediators stabilizing the carbon cathode. Nat. Energy 2, 17118 (2017).
Kundu, D., Black, R., Adams, B. & Nazar, L. F. A highly active low voltage redox mediator for enhanced rechargeability of lithium–oxygen batteries. ACS Cent. Sci. 1, 510–515 (2015).
Chase, G. V. et al. Soluble oxygen evolving catalysts for rechargeable metal-air batteries. US Patent App. 13/093,759 (2011).
Landa-Medrano, I. et al. Redox mediators: a shuttle to efficacy in metal–O2 batteries. J. Mat. Chem. A 7, 8746–8764 (2019).
Leverick, G. et al. Solvent-dependent oxidizing power of LiI redox couples for Li-O2 batteries. Joule. 3, 1106–1126 (2019).
Li, Q. et al. A spectroscopic study on singlet oxygen production from different reaction paths using solid inorganic peroxides as starting materials. Bull. Korean Chem. Soc. 28, 1656–1660 (2007).
Qingwei, L. et al. Singlet oxygen production in the reaction of potassium superoxide with chlorine. Chem. Lett. 36, 496–497 (2007).
Mayeda, E. A. & Bard, A. J. Production of singlet oxygen in electrogenerated radical ion electron transfer reactions. J. Am. Chem. Soc. 95, 6223–6226 (1973).
Senthil Kumar, S. & Bard, A. J. Background emission of electrogenerated chemiluminescence during oxidation of tri-n-propylamine from the dimeric 1Δg state of O2. Anal. Chem. 85, 292–295 (2013).
Ando, W. et al. Formation of sulfinyl oxide and singlet oxygen in the reaction of thianthrene cation radical and superoxide ion. J. Am. Chem. Soc. 102, 4526–4528 (1980).
Pierini, A., Brutti, S. & Bodo, E. Superoxide anions disproportionation induced by Li+ and H+: pathways to 1O2 release in Li–O2 batteries. ChemPhysChem 21, 2060–2067 (2020).
Marcus, R. A. Electron transfer reactions in chemistry. Theory and experiment. Rev. Mod. Phys. 65, 599–610 (1993).
Savéant, J.-M. Elements of Molecular and Biomolecular Electrochemistry (Wiley, 2006).
Henstridge, M. C., Laborda, E., Rees, N. V. & Compton, R. G. Marcus–Hush–Chidsey theory of electron transfer applied to voltammetry: A review. Electrochim. Acta 84, 12–20 (2012).
Feldberg, S. W. & Sutin, N. Distance dependence of heterogeneous electron transfer through the nonadiabatic and adiabatic regimes. Chem. Phys. 324, 216–225 (2006).
Fawcett, W. R. Liquids, Solutions, and Interfaces – From Classical Macroscopic Descriptions to Modern Microscopic Details (Oxford Univ. Press, 2004).
Hassoun, J., Croce, F., Armand, M. & Scrosati, B. Investigation of the O2 electrochemistry in a polymer electrolyte solid-state cell. Angew. Chem. Int. Ed. 50, 2999–3002 (2011).
Kwabi, D. G. et al. Experimental and computational analysis of the solvent-dependent O2/Li+-O2− redox couple: Standard potentials, coupling strength, and implications for lithium–oxygen batteries. Angew. Chem. Int. Ed. 55, 3129–3134 (2016).
Schafzahl, B. et al. Quantifying total superoxide, peroxide, and carbonaceous compounds in metal–O2 batteries and the solid electrolyte interphase. ACS Energy Lett. 3, 170–176 (2017).
Wilkinson, F., Helman, W. P. & Ross, A. B. Rate constants for the decay and reactions of the lowest electronically excited singlet-state of molecular-oxygen in solution. An expanded and revised compilation. J. Phys. Chem. Ref. Data 24, 663–1021 (1995).
Petit, Y. K. et al. DABCOnium: An efficient and high-voltage stable singlet oxygen quencher for metal–O2 cells. Angew. Chem. Int. Ed. 58, 6535–6539 (2019).
Acknowledgements
S.A.F. is indebted to the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement No. 636069) as well as IST Austria. O.F thanks the French National Research Agency (STORE-EX Labex Project ANR-10-LABX-76-01). We thank EL-Cell GmbH (Hamburg, Germany) for the pressure test cell. We thank R. Saf for help with the mass spectrometry, J. Schlegl for manufacturing instrumentation, M. Winkler of Acib GmbH, G. Strohmeier and R. Fürst for HPLC measurements and S. Mondal and S. Stadlbauer for kinetic measurements.
Author information
Authors and Affiliations
Contributions
S.A.F., Y.K.P., E.M., C.P., C.L., D.M. and S.M.B. performed the experiments and analysed the results. S.B., A.W. and E.Z. did the density functional theory calculations. C.S. helped with synthesis. O.F. helped with Marcus theory. S.A.F. conceived and directed the research, set up and performed experiments, analysed the results and wrote the manuscript. All authors contributed to the discussion and interpretation of the results.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Peer review information Nature Chemistry thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 3O2 loss upon superoxide disproportionation in presence of RMox.
KO2 powder was immersed in 0.1 M LiTFSI/TEGDME containing 0, 0.5, 1, or 2 equivalents of the indicated RMox. One equivalent is the amount to theoretically evolve all O2 (0.5 mol RMox/mol KO2) considering 0.5 mol O2/mol KO2 to evolve from disproportionation. Equal amounts of electrolytes were used and hence the RMox concentration adapted. a, The found amounts of 3O2 relative to the total amount expected from disproportionation and oxidation for the indicated RMox. The dashed lines are quadratic polynomial fits. To prove that RMox rather than RMred drives 3O2 loss, we also used the reduced form of DMPZ. b, The data in a plotted versus the redox potential of the RMs. The trendlines are to guide the eye. See Supplementary Note 7 for in-depth discussion.
Extended Data Fig. 2 Oxidation kinetics and RMox concentration.
a, Comparision of the mediated superoxidation kinetics k2 and apparent peroxide oxidation kinetics kapp including fits with the Marcus expression in equation (5). b, 1/k which is proportional to the required RMox concentration (\(c_{{{\rm{RM}}^{{\rm{ox}}}}}\) = ν/k) to drive a certain areal oxidation rate ν = k × \(c_{{{\rm{RM}}^{{\rm{ox}}}}}\).
Supplementary information
Supplementary Information
Supplementary Methods, Figs. 1–11, Notes 1–8 and References.
Supplementary Table 4
xyz-Coordinates of the structures of the three molecules (TDPA, TDPA+ and TDPA2+) optimized in methanol, 76 atoms, coordinates in Å.
Source data
Source Data Fig. 2
Source data
Source Data Fig. 3
Source data
Source Data Fig. 4
Source data
Source Data Extended Data Fig. 1
Source data
Source Data Extended Data Fig. 2
Source data
Rights and permissions
About this article
Cite this article
Petit, Y.K., Mourad, E., Prehal, C. et al. Mechanism of mediated alkali peroxide oxidation and triplet versus singlet oxygen formation. Nat. Chem. 13, 465–471 (2021). https://doi.org/10.1038/s41557-021-00643-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-021-00643-z
This article is cited by
-
Solving the Singlet Oxygen Puzzle in Metal-O2 Batteries: Current Progress and Future Directions
Electrochemical Energy Reviews (2024)
-
Why charging Li–air batteries with current low-voltage mediators is slow and singlet oxygen does not explain degradation
Nature Chemistry (2023)
-
Oxidative decomposition mechanisms of lithium carbonate on carbon substrates in lithium battery chemistries
Nature Communications (2022)
-
Threshold potentials for fast kinetics during mediated redox catalysis of insulators in Li–O2 and Li–S batteries
Nature Catalysis (2022)
-
Reactive pathways toward parasitic release of singlet oxygen in metal-air batteries
npj Computational Materials (2021)