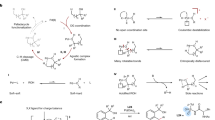
Abstract
The development of robust catalytic methods to assemble tertiary alkylamines provides a continual challenge to chemical synthesis. In this regard, transformation of a traditionally unreactive C–H bond, proximal to the nitrogen atom, into a versatile chemical entity would be a powerful strategy for introducing functional complexity to tertiary alkylamines. A practical and selective metal-catalysed C(sp3)–H activation facilitated by the tertiary alkylamine functionality, however, remains an unsolved problem. Here, we report a Pd(ii)-catalysed protocol that appends arene feedstocks to tertiary alkylamines via C(sp3)–H functionalization. A simple ligand for Pd(ii) orchestrates the C–H activation step in favour of deleterious pathways. The reaction can use both simple and complex starting materials to produce a range of multifaceted γ-aryl tertiary alkylamines and can be rendered enantioselective. The enabling features of this transformation should be attractive to practitioners of synthetic and medicinal chemistry as well as in other areas that use biologically active alkylamines.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data that support the findings of this study are available within the paper and its supplementary information files. Raw data are available from the corresponding author on reasonable request.
References
He, J., Wasa, M., Chan, K. S. L., Shao, Q. & Yu, J.-Q. Palladium-catalyzed transformations of alkyl C–H bonds. Chem. Rev. 117, 8754–8786 (2017).
Davies, H. M. L. & Morton, D. Guiding principles for site selective and stereoselective intermolecular C–H functionalization by donor/acceptor rhodium carbenes. Chem. Soc. Rev. 40, 1857–1869 (2011).
Capaldo, L. & Ravelli, D. Hydrogen atom transfer (HAT): a versatile strategy for substrate activation in photocatalyzed organic synthesis. Eur. J. Org. Chem. 15, 2056–2071 (2017).
Giri, R. et al. Palladium-catalyzed methylation and arylation of sp2 and sp3 C–H bonds in simple carboxylic acids. J. Am. Chem. Soc. 129, 3510–3511 (2007).
Chen, G. et al. Ligand-enabled β-C–H arylation of α-amino acids without installing exogenous directing groups. Angew. Chem. Int. Ed. 56, 1506–1509 (2017).
He, C., Whitehurst, W. G. & Gaunt, M. J. Palladium-catalyzed C(sp)3–H bond functionalization of aliphatic amines. Chem 5, 1031–1058 (2019).
Lyons, T. W. & Sanford, M. S. Palladium-catalyzed ligand-directed C–H functionalization reactions. Chem. Rev. 110, 1147–1169 (2010).
Rouquet, G. & Chatani, N. Catalytic functionalization of C(sp2)–H and C(sp3)–H bonds by using bidentate directing groups. Angew. Chem. Int. Ed. 52, 11726–11743 (2013).
Zaitsev, V. G., Shabashov, D. & Daugulis, O. Highly regioselective arylation of sp3 C–H bonds catalyzed by palladium acetate. J. Am. Chem. Soc. 127, 13154–13155 (2005).
He, G. & Chen, G. A practical strategy for the structural diversification of aliphatic scaffolds through the palladium-catalyzed picolinamide-directed remote functionalization of unactivated C(sp3)–H bonds. Angew. Chem. Int. Ed. 50, 5192–5196 (2011).
Chan, K. S. L. et al. Ligand-enabled cross-coupling of C(sp3)–H bonds with arylboron reagents via Pd(II)/Pd(0) catalysis. Nat. Chem. 6, 146–150 (2014).
Topczewski, J. J., Cabrera, P. J., Saper, N. I. & Sanford, M. S. Palladium-catalysed transannular C–H functionalization of alicyclic amines. Nature 531, 220–224 (2016).
Wu, Y., Chen, Y.-Q., Liu, T., Eastgate, M. D. & Yu, J.-Q. Pd-catalyzed γ-C(sp3)–H arylation of free amines using a transient directing group. J. Am. Chem. Soc. 138, 14554–14557 (2016).
Xu, Y., Young, M. C., Wang, C., Magness, D. M. & Dong, G. Catalytic C(sp3)–H arylation of free primary amines with an exo directing group generated in situ. Angew. Chem. Int. Ed. 55, 9084–9087 (2016).
Liu, Y. & Ge, H. Site-selective C–H arylation of primary aliphatic amines enabled by a catalytic transient directing group. Nat. Chem. 9, 26–32 (2017).
Kapoor, M., Liu, D. & Young, M. C. Carbon dioxide-mediated C(sp3)–H arylation of amine substrates. J. Am. Chem. Soc. 140, 6818–6822 (2018).
Roughley, S. D. & Jordan, A. M. The medicinal chemist’s toolbox: an analysis of reactions used in the pursuit of drug candidates. J. Med. Chem. 54, 3451–3479 (2011).
Cernak, T., Dykstra, K. D., Tyagarajan, S., Vachal, P. & Krska, S. W. The medicinal chemist’s toolbox for late stage functionalization of drug-like molecules. Chem. Soc. Rev. 45, 546–576 (2016).
Huang, L., Arndt, M., Gooβen, K., Heydt, H. & Gooβen, L. J. Late transition metal-catalyzed hydroamination and hydroamidation. Chem. Rev. 115, 2596–2697 (2015).
Musacchio, A. J. et al. Catalytic intermolecular hydroaminations of unactivated olefins with secondary alkyl amines. Science 355, 727–730 (2017).
Pirnot, M. T., Wang, Y.-M. & Buchwald, S. L. Copper hydride catalyzed hydroamination of alkenes and alkynes. Angew. Chem. Int. Ed. 55, 48–57 (2016).
Perez, F., Oda, S., Geary, L. M. & Krische, M. J. Ruthenium-catalyzed transfer hydrogenation for C–C bond formation: hydrohydroxyalkylation and hydroaminoalkylation via reactant redox pairs. Top. Curr. Chem. 374, 35 (2016).
Matier, C. D., Schwaben, J., Peters, J. C. & Fu, G. C. Copper-catalyzed alkylation of aliphatic amines induced by visible light. J. Am. Chem. Soc. 139, 17707–17710 (2017).
Hanna, S., Holder, J. C. & Hartwig, J. F. A multicatalytic approach to the hydroaminomethylation of α-olefins. Angew. Chem. Int. Ed. 58, 3368–3372 (2019).
Trowbridge, A., Reich, D. & Gaunt, M. J. Multicomponent synthesis of tertiary alkylamines by photocatalytic olefin-hydroaminoalkylation. Nature 561, 522–527 (2018).
Hsieh, S.-Y. & Bode, J. W. Lewis acid induced toggle from Ir(II) to Ir(IV) pathways in photocatalytic reactions: synthesis of thiomorpholines and thiazepanes from aldehydes and SLAP reagents. ACS Cent. Sci. 3, 66–72 (2017).
Xie, L.-G. & Dixon, D. J. Tertiary amine synthesis via reductive coupling of amides with Grignard reagents. Chem. Sci. 8, 7492–7497 (2017).
Grogan, G. Synthesis of chiral amines using redox biocatalysis. Curr. Opin. Chem. Biol. 43, 15–22 (2018).
Ouyang, K., Hao, W., Zhang, W.-X. & Xi, Z. Transition-metal-catalyzed cleavage of C–N single bonds. Chem. Rev. 115, 12045–12090 (2015).
Lawrence, J. D., Takahashi, M., Bae, C. & Hartwig, J. F. Regiospecific functionalization of methyl C–H bonds of alkyl groups in reagents with heteroatom functionality. J. Am. Chem. Soc. 126, 15334–15335 (2004).
Murphy, J. M., Lawrence, J. D., Kawamura, K., Incarvito, C. & Hartwig, J. F. Ruthenium-catalyzed regiospecific borylation of methyl C–H bonds. J. Am. Chem. Soc. 128, 13684–13685 (2006).
Li, Q., Liskey, C. W. & Hartwig, J. F. Regioselective borylation of the C–H bonds in alkylamines and alkyl ethers. Observation and origin of high reactivity of primary C–H bonds beta to nitrogen and oxygen. J. Am. Chem. Soc. 136, 8755–8765 (2014).
Lee, M. & Sanford, M. S. Platinum-catalyzed, terminal-selective C(sp3)–H oxidation of aliphatic amines. J. Am. Chem. Soc. 137, 12796–12799 (2015).
Mack, J. B. C., Gipson, J. D., Du Bois, J. & Sigman, M. S. Ruthenium-catalyzed C–H hydroxylation in aqueous acid enables selective functionalization of amine derivatives. J. Am. Chem. Soc. 139, 9503–9506 (2017).
Howell, J. M., Feng, K., Clark, J. R., Trzepkowski, L. J. & White, M. C. Remote oxidation of aliphatic C–H bonds in nitrogen-containing molecules. J. Am. Chem. Soc. 137, 14590–14593 (2015).
Schultz, D. M. et al. Oxyfunctionalization of the remote C–H bonds of aliphatic amines by decatungstate photocatalysis. Angew. Chem. Int. Ed. 56, 15274–15278 (2017).
Ghose, A. K., Herbertz, T., Hudkins, R. L., Dorsey, B. D. & Mallamo, J. P. Knowledge-based central nervous system (CNS) lead selection and lead optimization for CNS drug discovery. ACS Chem. Neurosci. 3, 50–68 (2012).
Ryabov, A. D., Sakodinskaya, I. & Yatsimirsky, A. Kinetics and mechanism of ortho-palladation of ring-substituted N,N-dimethylbenzylamines. J. Chem. Soc. Dalton Trans. 12, 2629–2638 (1985).
Nielsen, R. J. & Goddard, W. A. III Mechanism of the aerobic oxidation of alcohols by palladium complexes of N-heterocyclic carbenes. J. Am. Chem. Soc. 128, 9651–9660 (2006).
Yang, Y.-F., Hong, X., Yu, J.-Q. & Houk, K. N. Experimental-computational synergy for selective Pd(II)-catalyzed C–H activation of aryl and alkyl groups. Acc. Chem. Res. 50, 2853–2860 (2017).
Cheng, G.-J. et al. Role of N-acyl amino acid ligands in Pd(II)-catalyzed remote C–H activation of tethered arenes. J. Am. Chem. Soc. 136, 894–897 (2014).
Haines, B. E., Yu, J.-Q. & Musaev, D. G. Enantioselectivity model for Pd-catalyzed C–H functionalization mediated by the mono-N-protected amino acid (MPAA) family of ligands. ACS Catal. 7, 4344–4354 (2017).
Vasseur, A., Muzart, J. & Le Bras, J. Ubiquitous benzoquinones, multitalented compounds for palladium-catalyzed oxidative reactions. Eur. J. Org. Chem. 2015, 4053–4069 (2015).
Wu, Q. F. et al. Formation of α-chiral centers by asymmetric β-C(sp3)–H arylation, alkenylation, and alkynylation. Science 355, 499–503 (2017).
Saint-Denis, T. G., Zhu, R.-Y., Chen, G., Wu, Q.-F. & Yu, J.-Q. Enantioselective C(sp3)–H bond activation by chiral transition metal catalysts. Science 359, 747–759 (2018).
Mlynarski, S. N., Schuster, C. H. & Morken, J. P. Asymmetric synthesis from terminal alkenes by cascades of diboration and cross-coupling. Nature 505, 386–390 (2014).
Chen, G. et al. Ligand-accelerated enantioselective methylene C(sp3)–H bond activation. Science 353, 1023–1027 (2016).
Acknowledgements
We are grateful to the EPSRC UK National Mass Spectrometry Facility at Swansea University for HRMS analysis. We thank I. Michaelides (AstraZeneca) and M. Grayson (University of Bath) for useful discussion. We are grateful to La Caixa Foundation and the Cambridge European Trust (J.R.) and the Gates Cambridge Trust (N.J.F.) for scholarships, the EPSRC (EP/N031792/1), the Leverhulme Trust (RPG-2016-370 to M.N.), Mitsubishi (H.A.), H2020 Marie Curie Actions (702462 to M.N. and 656455 to M.E.B.) and the Royal Society for a Wolfson Merit Award (to M.J.G.)
Author information
Authors and Affiliations
Contributions
J.R., M.N. and M.J.G. conceived the project. J.R., M.N., H.A. and M.E.B. designed and performed the synthetic experiments. J.R. and N.J.F. designed and performed the computational studies. J.R., M.N., H.A., N.J.F. and M.J.G. prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information
Details of the materials and methods, experimental procedures, mechanistic studies, optimization studies, computational data and compound characterization data, including 1H NMR spectra, 13C NMR spectra and MS data.
Rights and permissions
About this article
Cite this article
Rodrigalvarez, J., Nappi, M., Azuma, H. et al. Catalytic C(sp3)–H bond activation in tertiary alkylamines. Nat. Chem. 12, 76–81 (2020). https://doi.org/10.1038/s41557-019-0393-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-019-0393-8
This article is cited by
-
Site-selective amination towards tertiary aliphatic allylamines
Nature Catalysis (2022)
-
C–H activation
Nature Reviews Methods Primers (2021)
-
Programmable late-stage C−H bond functionalization enabled by integration of enzymes with chemocatalysis
Nature Catalysis (2021)
-
Catalytic asymmetric reductive hydroalkylation of enamides and enecarbamates to chiral aliphatic amines
Nature Communications (2021)
-
Late-stage C–H functionalization offers new opportunities in drug discovery
Nature Reviews Chemistry (2021)