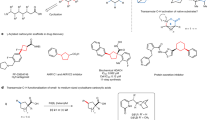
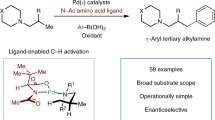
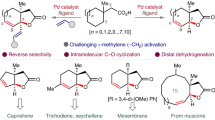
Abstract
Recent developments of bifunctional ligands have rapidly advanced palladium-catalysed C(sp3)–H activation reactions directed by native carboxylic acids. However, using this approach in inter- or intramolecular C(sp3)–H amination reactions is often hampered by N-coordination overriding the directing effect of the native carboxyl group. Here we report the design and development of chlorinated pyridine–pyridone ligands, which can overcome N-coordination and enable exclusive carboxylic-acid-directed lactamization and cycloamination of N-protected ω-amino acids. The compartmentalization of directed C(sp3)–H activation and C(sp3)–N bond formation in this reaction is distinct from existing C(sp3)–H amination approaches, in which both processes are directed by nitrogen. The protocols described in this report transform linear ω-amino acids into valuable cyclic β-amino acids possessing γ- and δ-lactam, pyrrolidine and tetrahydroquinoline scaffolds pertinent to drug discovery. The utility of this process was demonstrated by the formal synthesis of stemoamide.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All data are in the Supplementary Information. Computational data are available on Figshare: https://doi.org/10.6084/m9.figshare.25345330.v1 (ref. 53).
References
Kittakoop, P., Mahidol, C. & Ruchirawat, S. Alkaloids as important scaffolds in therapeutic drugs for the treatments of cancer, tuberculosis, and smoking cessation. Curr. Top. Med. Chem. 14, 239–252 (2014).
Cushnie, T. P. T., Cushnie, B. & Lamb, A. J. Alkaloids: an overview of their antibacterial, antibiotic-enhancing and antivirulence activities. Int. J. Antimicrob. Agents 44, 377–386 (2014).
Roughley, S. D. & Jordan, A. M. The medicinal chemist’s toolbox: an analysis of reactions used in the pursuit of drug candidates. J. Med. Chem. 54, 3451–3479 (2011).
Jeffrey, J. L. & Sarpong, R. Intramolecular C(sp3)–H amination. Chem. Sci. 4, 4092–4106 (2013).
Brown, D. G. & Boström, J. Analysis of past and present synthetic methodologies on medicinal chemistry: where have all the new reactions gone? J. Med. Chem. 59, 4443–4458 (2016).
Park, Y., Kim, Y. & Chang, S. Transition metal-catalyzed C–H amination: scope, mechanism, and applications. Chem. Rev. 117, 9247–9301 (2017).
He, C., Whitehurst, W. G. & Gaunt, M. J. Palladium-catalyzed C(sp3)–H bond functionalization of aliphatic amines. Chem 5, 1031–1058 (2019).
Trowbridge, A., Walton, S. M. & Gaunt, M. J. New strategies for the transition-metal catalyzed synthesis of aliphatic amines. Chem. Rev. 120, 2613–2692 (2020).
Che, C. M., Lo, V. K. Y., Zhou, C. Y. & Huang, J. S. Selective functionalisation of saturated C–H bonds with metalloporphyrin catalysts. Chem. Soc. Rev. 40, 1950–1975 (2011).
Roizen, J. L., Harvey, M. E. & Bois, J. D. Metal-catalyzed nitrogen-atom transfer methods for the oxidation of aliphatic C–H bonds. Acc. Chem. Res. 45, 911–922 (2012).
Paradine, S. M. & White, M. C. Iron-catalyzed intramolecular allylic C–H amination. J. Am. Chem. Soc. 134, 2036–2039 (2012).
Hennessy, E. T. & Betley, T. A. Complex N-heterocycle synthesis via iron-catalyzed, direct C–H bond amination. Science 340, 591–595 (2013).
Michaudel, Q., Thevenet, D. & Baran, P. S. Intermolecular Ritter-type C–H amination of unactivated sp3 carbons. J. Am. Chem. Soc. 134, 2547–2550 (2012).
Liu, T., Mei, T. S. & Yu, J. Q. γ,δ,ε-C(sp3)–H functionalization through directed radical H-abstraction. J. Am. Chem. Soc. 137, 5871–5874 (2015).
Sharma, A. & Hartwig, J. F. Metal-catalysed azidation of tertiary C–H bonds suitable for late-stage functionalization. Nature 517, 600–604 (2015).
Nakafuku, K. M. et al. Enantioselective radical C–H amination for the synthesis of β-amino alcohols. Nat. Chem. 12, 697–704 (2020).
Ali, S. Z. et al. Allylic C–H amination cross-coupling furnishes tertiary amines by electrophilic metal catalysis. Science 376, 276–283 (2022).
Cheung, K. P. S., Fang, J., Mukherjee, K., Mihranyan, A. & Gevorgyan, V. Asymmetric intermolecular allylic C–H amination of alkenes with aliphatic amines. Science 378, 1207–1213 (2022).
Thu, H. Y., Yu, W. Y. & Che, C. M. Intermolecular amidation of unactivated sp2 and sp3 C–H bonds via palladium-catalyzed cascade C–H activation/nitrene insertion. J. Am. Chem. Soc. 128, 9048–9049 (2006).
He, J., Shigenari, T. & Yu, J.-Q. Palladium(0)/PAr3-catalyzed intermolecular amination of C(sp3)–H bonds: synthesis of β-amino acids. Angew. Chem. Int. Ed. 127, 6645–6649 (2015).
Wasa, M. & Yu, J. Q. Synthesis of β-, γ-, and δ-lactams via Pd(II)-catalyzed C–H activation reactions. J. Am. Chem. Soc. 130, 14058–14059 (2008).
Nadres, E. T. & Daugulis, O. Heterocycle synthesis via direct C–H/N–H coupling. J. Am. Chem. Soc. 134, 7–10 (2012).
He, G., Zhao, Y., Zhang, S., Lu, C. & Chen, G. Highly efficient syntheses of azetidines, pyrrolidines, and indolines via palladium catalyzed intramolecular amination of C(sp3)–H and C(sp2)–H bonds at γ and δ positions. J. Am. Chem. Soc. 134, 3–6 (2012).
Zhao, J., Zhao, X. J., Cao, P., Liu, J. K. & Wu, B. Polycyclic azetidines and pyrrolidines via palladium-catalyzed intramolecular amination of unactivated C(sp3)–H bonds. Org. Lett. 19, 4880–4883 (2017).
Neumann, J. J., Rakshit, S., Droge, T. & Glorius, F. Palladium-catalyzed amidation of unactivated C(sp3)–H bonds: from anilines to indolines. Angew. Chem. Int. Ed. 48, 6892–6895 (2009).
McNally, A., Haffemayer, B., Collins, B. S. L. & Gaunt, M. J. Palladium-catalysed C–H activation of aliphatic amines to give strained nitrogen heterocycles. Nature 510, 129–133 (2014).
Smalley, A. P., Cuthbertson, J. D. & Gaunt, M. J. Palladium-catalyzed enantioselective C–H activation of aliphatic amines using chiral anionic BINOL-phosphoric acid ligands. J. Am. Chem. Soc. 139, 1412–1415 (2017).
Nappi, M., He, C., Whitehurst, W. G., Chappell, B. G. N. & Gaunt, M. J. Selective reductive elimination at alkyl palladium(IV) by dissociative ligand ionization: catalytic C(sp3)–H amination to azetidines. Angew. Chem. Int. Ed. 130, 3232–3236 (2018).
Liu, S. et al. Ligand enabled Pd(II)-catalyzed γ-C(sp3)–H lactamization of native amides. J. Am. Chem. Soc. 143, 21657–21666 (2021).
Zhuang, Z. et al. Ligand-enabled β-C(sp3)–H lactamization of tosyl-protected aliphatic amides using a practical oxidant. Angew. Chem. Int. Ed. 61, e202207354 (2022).
He, G., Zhang, S. Y., Nack, W. A., Li, Q. & Chen, G. Use of a readily removable auxiliary group for the synthesis of pyrrolidones by the palladium-catalyzed intramolecular amination of unactivated γ-C(sp3)–H bonds. Angew. Chem. Int. Ed. 52, 11124–11128 (2013).
Wang, Z. et al. Ligand-controlled divergent dehydrogenative reactions of carboxylic acids via C–H activation. Science 374, 1281–1285 (2021).
Sheng, T. et al. One-step synthesis of β-alkylidene-γ-lactones via ligand-enabled β,γ-dehydrogenation of aliphatic acids. J. Am. Chem. Soc. 144, 12924–12933 (2022).
Chan, H. S. S., Yang, J.-M. & Yu, J.-Q. Catalyst-controlled site-selective methylene C–H lactonization of dicarboxylic acids. Science 376, 1481–1487 (2022).
Uttry, A., Mal, S. & Van Gemmeren, M. Late-stage β-C(sp3)–H deuteration of carboxylic acids. J. Am. Chem. Soc. 143, 10895–10901 (2021).
Hu, L., Meng, G. & Yu, J.-Q. Ligand-enabled Pd(II)-catalyzed β-methylene C(sp3)–H arylation of free aliphatic acids. J. Am. Chem. Soc. 144, 20550–20553 (2022).
Yang, J. M. et al. Regio-controllable [2+2] benzannulation with two adjacent C(sp3)–H bonds. Science 380, 639–644 (2023).
Kang, G. et al. Transannular C–H functionalization of cycloalkane carboxylic acids. Nature 618, 519–525 (2023).
Tomberg, A. et al. Relative strength of common directing groups in palladium-catalyzed aromatic C−H activation. iScience 20, 373–391 (2019).
Zhu, C. & Falck, J. R. N-Acylsulfonamide assisted tandem C−H olefination/annulation: synthesis of isoindolinones. Org. Lett. 13, 1214–1217 (2011).
Péron, F., Fossey, C., Cailly, T. & Fabis, F. N-Tosylcarboxamide as a transformable directing group for Pd-catalyzed C–H ortho-arylation. Org. Lett. 14, 1827–1829 (2012).
Péron, F., Fossey, C., Sopkova-Deoliveirasantos, J., Cailly, T. & Fabis, F. Room-temperature ortho-alkoxylation and -halogenation of N-tosylbenzamides by using palladium(II)-catalyzed C–H activation. Chem. Eur. J. 20, 7507–7513 (2014).
Chen, G. et al. Ligand-accelerated enantioselective methylene C(sp3)–H bond activation. Science 353, 1023–1027 (2016).
Romero, E. A. et al. Understanding the activity and enantioselectivity of acetyl-protected aminoethyl quinoline ligands in palladium-catalyzed β-C(sp3)–H bond arylation reactions. J. Am. Chem. Soc. 141, 16726–16733 (2019).
Hiesinger, K., Dar’In, D., Proschak, E. & Krasavin, M. Spirocyclic scaffolds in medicinal chemistry. J. Med. Chem. 64, 150–183 (2021).
Marson, C. M. New and unusual scaffolds in medicinal chemistry. Chem. Soc. Rev. 40, 5514–5533 (2011).
Wang, J. R. et al. Palladium-catalyzed aerobic oxidation of amines. Tetrahedron Lett. 47, 8293–8297 (2006).
Pilli, R. A. & Ferreira de Oliveira, M. C. Recent progress in the chemistry of the Stemona alkaloids. Nat. Prod. Rep. 17, 117–127 (2000).
Pilli, R. A., Rosso, G. B. & Ferreira de Oliveira, M. D. C. The chemistry of Stemona alkaloids: an update. Nat. Prod. Rep. 27, 1908–1937 (2010).
Davies, S. G. et al. Diastereoselective Ireland–Claisen rearrangements of substituted allyl β-amino esters: applications in the asymmetric synthesis of C(5)-substituted transpentacins. Org. Biomol. Chem. 12, 2702–2728 (2014).
Faraggi, T. M., Li, W. & MacMillan, D. W. C. Decarboxylative oxygenation via photoredox catalysis. Isr. J. Chem. 60, 410–415 (2020).
Chavan, S. P., Harale, K. R., Puranik, V. G. & Gawade, R. L. Formal synthesis of (−)-stemoamide using a useful epimerization at C-8. Tetrahedron Lett. 53, 2647–2650 (2012).
Palladium-catalysed methylene C(sp3)–H lactamization and cycloamination enabled by chlorinated pyridine–pyridone ligands. Figshare https://doi.org/10.6084/m9.figshare.25345330.v1 (2024).
Acknowledgements
We thank M. Tomanik and K. Wu for proofreading and providing helpful suggestions in preparing the manuscript. We acknowledge The Scripps Research Institute, NIH (NIGMS, 2R01GM084019) and the Croucher Foundation (postdoctoral fellowship to H.S.S.C) for financial support.
Author information
Authors and Affiliations
Contributions
J.-Q.Y. and H.S.S.C. conceived the concept. H.S.S.C. discovered and developed the lactamization and cycloamination reactions, designed and synthesized ligands L2, L4, L7, L9, L10–L16 and L18, conducted preliminary mechanistic studies and finished the formal synthesis of stemoamide. Y.L. performed the computational studies. H.S.S.C. and J.-Q.Y. wrote the manuscript. J.-Q.Y. directed the project.
Corresponding author
Ethics declarations
Competing interests
J.-Q.Y. and H.S.S.C. are inventors on a patent application related to this work (US Patent Application 63/551,249) filed by The Scripps Research Institute. The authors declare no other competing interests.
Peer review
Peer review information
Nature Synthesis thanks Chuan He and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Thomas West, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–21, Tables 1 and 2, synthetic procedures, computational modelling details, control experiments and NMR spectra.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chan, H.S.S., Lu, Y. & Yu, JQ. Palladium-catalysed methylene C(sp3)–H lactamization and cycloamination enabled by chlorinated pyridine–pyridone ligands. Nat. Synth (2024). https://doi.org/10.1038/s44160-024-00517-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44160-024-00517-5