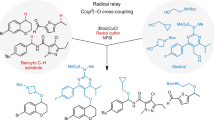
Abstract
Reactions that directly install nitrogen into C–H bonds of complex molecules are significant because of their potential to change the chemical and biological properties of a given compound. Although selective intramolecular C–H amination reactions are known, achieving high levels of reactivity while maintaining excellent site selectivity and functional-group tolerance remains a challenge for intermolecular C–H amination. Here, we report a manganese perchlorophthalocyanine catalyst [MnIII(ClPc)] for intermolecular benzylic C–H amination of bioactive molecules and natural products that proceeds with unprecedented levels of reactivity and site selectivity. In the presence of a Brønsted or Lewis acid, the [MnIII(ClPc)]-catalysed C–H amination demonstrates unique tolerance for tertiary amine, pyridine and benzimidazole functionalities. Mechanistic studies suggest that C–H amination likely proceeds through an electrophilic metallonitrene intermediate via a stepwise pathway where C–H cleavage is the rate-determining step of the reaction. Collectively, these mechanistic features contrast with previous base–metal-catalysed C–H aminations and provide new opportunities for tunable selectivities.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Richter, M. F. et al. Predictive compound accumulation rules yield a broad-spectrum antibiotic. Nature 545, 299–304 (2017).
Acred, P., Brown, D. M., Turner, D. H. & Wilson, M. J. Pharmacology and chemotherapy of ampicillin—a new broad-spectrum penicillin. Br. J. Pharmacol. 18, 356–369 (1962).
McGrath, N. A., Brichacek, M. & Njardarson, J. T. A graphical journey of innovative organic architectures that have improved our lives. J. Chem. Educ. 87, 1348–1349 (2010).
Hili, R. & Yudin, A. K. Making carbon–nitrogen bonds in biological and chemical synthesis. Nat. Chem. Biol. 2, 284–287 (2006).
Hubbard, B. K., Thomas, M. G. & Walsh, C. T. Biosynthesis of l-p-hydroxyphenylglycine, a non-proteinogenic amino acid constituent of peptide antibiotics. Chem. Biol. 7, 931–942 (2000).
Carey, J. S., Laffan, D., Thomson, C. & Williams, M. T. Analysis of the reactions used for the preparation of drug candidate molecules. Org. Biomol. Chem. 4, 2337–2347 (2006).
Dugger, R. W., Ragan, J. A. & Ripin, D. H. B. Survey of GMP bulk reactions run in a research facility between 1985 and 2002. Org. Process Res. Dev. 9, 253–258 (2005).
Roughley, S. D. & Jordan, A. M. The medicinal chemist’s toolbox: an analysis of reactions used in the pursuit of drug candidates. J. Med. Chem. 54, 3451–3479 (2011).
Li, J. W. H. & Vederas, J. C. Drug discovery and natural products: end of an era or an endless frontier? Science 325, 161–165 (2009).
DeCorte, B. L. Underexplored opportunities for natural products in drug discovery. J. Med. Chem. 59, 9295–9304 (2016).
Roizen, J. L., Harvey, M. E. & Du Bois, J. Metal-catalyzed nitrogen-atom transfer methods for the oxidation of aliphatic C–H bonds. Acc. Chem. Res. 45, 911–922 (2012).
Dequirez, G., Pons, V. & Dauban, P. Nitrene chemistry in organic synthesis: still in its infancy? Angew. Chem. Int. Ed. 51, 7384–7395 (2012).
Huard, K. & Lebel, H. N-tosyloxycarbamates as reagents in rhodium-catalyzed C–H amination reactions. Chem. Eur. J. 14, 6222–6230 (2008).
Fiori, K. W. & Du Bois, J. Catalytic intermolecular amination of C–H bonds: method development and mechanistic insights. J. Am. Chem. Soc. 129, 562–568 (2007).
Roizen, J. L., Zalatan, D. N. & Du Bois, J. Selective intermolecular amination of C–H bonds at tertiary carbon centers. Angew. Chem. Int. Ed. 52, 11343–11346 (2013).
Liang, C. et al. Efficient diastereoselective intermolecular rhodium-catalyzed C–H amination. Angew. Chem. Int. Ed. 45, 4641–4644 (2006).
Bess, E. N. et al. Analyzing site selectivity in Rh2(esp)2-catalyzed intermolecular C–H amination reactions. J. Am. Chem. Soc. 136, 5783–5789 (2014).
Li, J. et al. Simultaneous structure–activity studies and arming of natural products by C–H amination reveal cellular targets of eupalmerin acetate. Nat. Chem. 5, 510–517 (2013).
Paradine, S. M. & White, M. C. Iron-catalyzed intramolecular allylic C–H amination. J. Am. Chem. Soc. 134, 2036–2039 (2012).
Paradine, S. M. et al. A manganese catalyst for highly reactive yet chemoselective intramolecular C(sp 3) –H amination. Nat. Chem. 7, 987–994 (2015).
Hennessy, E. T. & Betley, T. A. Complex N-heterocycle synthesis via iron-catalyzed, direct C–H bond amination. Science 340, 591–595 (2013).
Hennessy, E. T., Liu, R. Y., Iovan, D. A., Duncan, R. A. & Betley, T. A. Iron-mediated intermolecular N-group transfer chemistry with olefinic substrates. Chem. Sci. 5, 1526–1532 (2014).
Liu, Y. et al. Nonheme iron-mediated amination of C(sp 3)–H bonds. Quinquepyridine-supported iron-imide/nitrene intermediates by experimental studies and DFT calculations. J. Am. Chem. Soc. 135, 7194–7204 (2013).
Lu, H., Subbarayan, V., Tao, J. & Zhang, X. P. Cobalt(II)-catalyzed intermolecular benzylic C–H amination with 2,2,2-trichloroethoxycarbonyl azide (TrocN3). Organometallics 29, 389–393 (2010).
Lu, H., Hu, Y., Jiang, H., Wojtas, L. & Zhang, X. P. Stereoselective radical amination of electron deficient C(sp 3)–H bonds by Co(II)-based metalloradical catalysis: direct synthesis of α-amino acid derivatives via α-C–H amination. Org. Lett. 14, 5158–5161 (2012).
Gephart, R. T. III & Warren, T. H. Copper-catalyzed sp 3 C–H amination. Organometallics 31, 7728–7752 (2012).
Fructos, M. R., Trofimenko, S., Díaz-Requejo, M. M. & Pérez, P. J. Facile amine formation by intermolecular catalytic amidation of carbon–hydrogen bonds. J. Am. Chem. Soc. 128, 11784–11791 (2006).
Huang, X., Bergsten, T. M. & Groves, J. T. Manganese-catalyzed late-stage aliphatic C–H azidation. J. Am. Chem. Soc. 137, 5300–5303 (2015).
Karimov, R. R., Sharma, A. & Hartwig, J. F. Late stage azidation of complex molecules. ACS Cent. Sci. 2, 715–724 (2016).
Ruppel, J. V., Kamble, R. M. & Zhang, X. P. Cobalt-catalyzed intramolecular C–H amination with arylsulfonyl azides. Org. Lett. 9, 4889–4892 (2007).
Safari, N. et al. Rapid and efficient synthesis of metallophthalocyanines in ionic liquid. J. Porphyr. Phthalocyanines 9, 256–261 (2005).
Du Bois, J. 2,2,2-Trichloroethoxysulfonamide. e-EROS Encyclopedia of Reagents for Organic Synthesis (2009). https://doi.org/10.1002/047084289X.rn01072
Asensio, G., González-Núñez, M. E., Bernardini, C. B., Mello, R. & Adam, W. Regioselective oxyfunctionalization of unactivated tertiary and secondary C–H bonds of alkylamines by methyl(trifluoromethyl)dioxirane in acid medium. J. Am. Chem. Soc. 115, 7250–7253 (1993).
Howell, J. M., Feng, K., Clark, J. R., Trzepkowski, L. J. & White, M. C. Remote oxidation of aliphatic C–H bonds in nitrogen-containing molecules. J. Am. Chem. Soc. 137, 14590–14593 (2015).
Lee, M. & Sanford, M. S. Platinum-catalyzed, terminal selective C(sp 3 )–H oxidation of aliphatic amines. J. Am. Chem. Soc. 137, 12796–12799 (2015).
Taylor, R. D., MacCoss, M. & Lawson, A. D. G. Rings in drugs. J. Med. Chem. 57, 5845–5859 (2014).
Stepan, A. F., Mascitti, V., Beaumont, K. & Kalgutkar, A. S. Metabolism-guided drug design. Med. Chem. Commun. 4, 631–652 (2013).
Cernak, T., Dykstra, K. D., Tyagarajan, S., Vachal, P. & Krska, S. W. The medicinal chemist’s toolbox for late stage functionalization of drug-like molecules. Chem. Soc. Rev. 45, 546–576 (2016).
Lund, B. W. et al. Discovery of a potent, orally available, and isoform-selective retinoic acid β2 receptor agonist. J. Med. Chem. 48, 7517–7519 (2005).
Alibert, S. et al. Effects of a series of dihydroanthracene derivatives on drug efflux in multidrug resistant cancer cells. Eur. J. Med. Chem. 38, 253–263 (2003).
Chambers, M. S. et al. Spiropiperidines as high-affinity, selective σ ligands. J. Med. Chem. 35, 2033–2039 (1992).
Wikström, H. et al. N-substituted 1,2,3,4,4a,5,6,10b-octahydrobenzo[f]quinolines and 3-phenylpiperidines: effects on central dopamine and σ receptors. J. Med. Chem. 30, 2169–2174 (1987).
Morgan, B. P. et al. Discovery of potent, nonsteroidal, and highly selective glucocorticoid receptor antagonists. J. Med. Chem. 45, 2417–2424 (2002).
Krishnegowda, G. et al. Synthesis and biological evaluation of a novel class of isatin analogs as dual inhibitors of tubulin polymerization and Akt pathway. Bioorg. Med. Chem. 19, 6006–6014 (2011).
Hille, U. E., Zimmer, C., Vock, C. A. & Hartmann, R. W. First selective CYP11B1 inhibitors for the treatment of cortisol-dependent diseases. ACS Med. Chem. Lett. 2, 2–6 (2011).
Jiang, X., Song, Z., Xu, C., Yao, Q. & Zhang, A. (d,l)-10-Camphorsulfonic-acid-catalysed synthesis of diaryl-fused 2,8-dioxabicyclo[3.3.1]nonanes from 2-hydroxychalcones and naphthol derivatives. Eur. J. Org. Chem. 418–425 (2014).
Lu, H., Jiang, H., Hu, Y., Wojtas, L. & Zhang, X. P. Chemoselective intramolecular allylic C–H amination versus C=C aziridination through Co(II)-based metalloradical catalysis. Chem. Sci. 2, 2361–2366 (2011).
Luo, Y.-R. Comprehensive Handbook of Chemical Bond Energies Ch. 3 (CRC, Boca Raton, FL, 2007).
Greenwood, N. N. & Earnshaw, A. Chemistry of the Elements. 2nd edn, Ch. 24. (Butterworth-Heinemann, Oxford, 1997).
Chen, M. S. & White, M. C. A predictably selective aliphatic C–H oxidation reaction for complex molecule synthesis. Science 318, 783–787 (2007).
Acknowledgements
Financial support for this work was provided by the NIGMS Maximizing Investigators’ Research Award MIRA (R35 GM122525). J.R.C. is an NIH Ruth Kirschstein Postdoctoral Fellow (1 F32GM112501-01A1). The authors thank D.L. Gray and T.J. Woods for crystallographic analysis of compounds 36, 37, 38 and 44. The authors thank L. Zhu for assistance with NMR spectroscopy and T. Nanjo, R. Ma, W. Liu and J. Griffin for checking the experimental procedures.
Author information
Authors and Affiliations
Contributions
M.C.W. and J.R.C. conceived and designed the project. J.R.C., K.F. and A.S. conducted the experiments and, with M.C.W., analysed the data. M.C.W. and J.R.C. prepared the manuscript with input from K.F. and A.S.
Corresponding author
Ethics declarations
Competing interests
The University of Illinois has filed a patent application on the [Mn(ClPc)] catalyst for intermolecular C–H functionalization. The [Mn(ClPc)] catalyst (product # 901425) will be offered by MilliporeSigma through a licence from the University of Illinois.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information
Supplementary experimental data, synthetic procedures and chemical compound characterization data
Crystallographic data
CIF for compound (±)-36 with embedded structure factors; CCDC reference: 1587014
Crystallographic data
CIF for compound (±)-37 with embedded structure factors; CCDC reference: 1587015
Crystallographic data
CIF for compound (-)-38; CCDC reference: 1587016
Crystallographic data
Structure factors for compound (-)-38; CCDC reference: 1587016
Crystallographic data
Description: CIF for compound (-)-44; CCDC reference: 1587017
Crystallographic data
Structure factors for compound (-)-44; CCDC reference: 1587017
Rights and permissions
About this article
Cite this article
Clark, J.R., Feng, K., Sookezian, A. et al. Manganese-catalysed benzylic C(sp3)–H amination for late-stage functionalization. Nature Chem 10, 583–591 (2018). https://doi.org/10.1038/s41557-018-0020-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-018-0020-0
This article is cited by
-
Prospects and challenges for nitrogen-atom transfer catalysis
Nature Reviews Chemistry (2023)
-
Unnatural α-amino acid synthesis
Nature Synthesis (2023)
-
Radical C(sp3)–H functionalization and cross-coupling reactions
Nature Reviews Chemistry (2022)
-
Late-stage C–H functionalization offers new opportunities in drug discovery
Nature Reviews Chemistry (2021)
-
Benzylic C−H acylation by cooperative NHC and photoredox catalysis
Nature Communications (2021)