Abstract
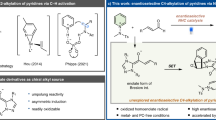
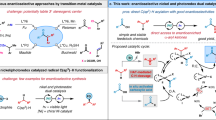
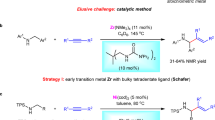
Achieving the transition metal-catalysed pyridine C3−H alkenylation, with pyridine as the limiting reagent, has remained a long-standing challenge. Previously, we disclosed that the use of strong coordinating bidentate ligands can overcome catalyst deactivation and provide Pd-catalysed C3 alkenylation of pyridines. However, this strategy proved ineffective when using pyridine as the limiting reagent, as it required large excesses and high concentrations to achieve reasonable yields, which rendered it inapplicable to complex pyridines prevalent in bioactive molecules. Here we report that a bifunctional N-heterocyclic carbene-ligated Ni–Al catalyst can smoothly furnish C3–H alkenylation of pyridines. This method overrides the intrinsic C2 and/or C4 selectivity, and provides a series of C3-alkenylated pyridines in 43–99% yields and up to 98:2 C3 selectivity. This method not only allows a variety of pyridine and heteroarene substrates to be used as the limiting reagent, but is also effective for the late-stage C3 alkenylation of diverse complex pyridine motifs in bioactive molecules.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The authors declare that all data supporting the findings of this study are available within the article and its supplementary information files. Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition number CCDC 2018614 (L10). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
References
Ping, L., Chung, D. S., Bouffard, J. & Lee, S. Transition metal-catalyzed site- and regio-divergent C–H bond functionalization. Chem. Soc. Rev. 46, 4299–4328 (2017).
Funken, N., Zhang, Y.-Q. & Gansäuer, A. Regiodivergent catalysis: a powerful tool for selective catalysis. Chem. Eur. J. 23, 19–32 (2017).
Neufeldt, S. R. & Sanford, M. S. Controlling site selectivity in palladium-catalyzed C−H bond functionalization. Acc. Chem. Res. 45, 936–946 (2012).
Takagi, J., Sato, K., Hartwig, J. F., Ishiyamaa, T. & Miyaura, N. Iridium-catalyzed C–H coupling reaction of heteroaromatic compounds with bis(pinacolato)diboron: regioselective synthesis of heteroarylboronates. Tetrahedron Lett. 43, 5649–5651 (2002).
Cheng, C. & Hartwig, J. F. Rhodium-catalyzed intermolecular C–H silylation of arenes with high steric regiocontrol. Science 343, 853–857 (2014).
Duong, H. A., Gilligan, R. E., Cooke, M. L., Phipps, R. J. & Gaunt, M. J. Copper(II)-catalyzed meta-selective direct arylation of α-aryl carbonyl compounds. Angew. Chem. Int. Ed. 50, 463–466 (2011).
Ackermann, L., Hofmann, N. & Vicente, R. Carboxylate-assisted ruthenium-catalyzed direct alkylations of ketimines. Org. Lett. 13, 1875–1877 (2011).
Saidi, O. et al. Ruthenium-catalyzed meta sulfonation of 2-phenylpyridines. J. Am. Chem. Soc. 133, 19298–19301 (2011).
Meng, G. et al. Achieving site-selectivity for C−H activation processes based on distance and geometry: a carpenter’s approach. J. Am. Chem. Soc. 142, 10571–10591 (2020).
Sambiagio, C. et al. A comprehensive overview of directing groups applied in metal-catalysed C–H functionalisation chemistry. Chem. Soc. Rev. 47, 6603–6743 (2018).
Rej, S., Ano, Y. & Chatani, N. Bidentate directing groups: an efficient tool in C–H bond functionalization chemistry for the expedient construction of C–C bonds. Chem. Rev. 120, 1788–1887 (2020).
Chen, Z. et al. Transition metal-catalyzed C–H bond functionalizations by the use of diverse directing groups. Org. Chem. Front. 2, 1107–1295 (2015).
Leow, D., Li, G., Mei, T. S. & Yu, J. Q. Activation of remote meta-C–H bonds assisted by an end-on template. Nature 486, 518–522 (2012).
Dey, A., Sinha, S. K., Achar, T. K. & Maiti, D. Accessing remote meta- and para-C(sp2)–H bonds with covalently attached directing groups. Angew. Chem. Int. Ed. 58, 10820–10843 (2019).
Mihai, M. T., Genov, G. R. & Phipps, R. J. Access to the meta position of arenes through transition metal catalysed C–H bond functionalisation: a focus on metals other than palladium. Chem. Soc. Rev. 47, 149–171 (2018).
Zhang, Z., Tanaka, K. & Yu, J.-Q. Remote site-selective C−H activation directed by a catalytic bifunctional template. Nature 543, 538–542 (2017).
Shi, H. et al. Differentiation and functionalization of remote C–H bonds in adjacent positions. Nat. Chem. 12, 399–404 (2020).
Davis, H. J. & Phipps, R. J. Harnessing non-covalent interactions to exert control over regioselectivity and site-selectivity in catalytic reactions. Chem. Sci. 8, 864–877 (2017).
Kim, D. S., Park, W.-J. & Jun, C.-H. Metal−organic cooperative catalysis in C−H and C−C bond activation. Chem. Rev. 117, 8977–9015 (2017).
Kuninobu, Y., Ida, H., Nishi, M. & Kanai, M. A meta-selective C−H borylation directed by a secondary interaction between ligand and substrate. Nat. Chem. 7, 712–717 (2015).
Roosen, P. C. et al. Outer-sphere direction in iridium C−H borylation. J. Am. Chem. Soc. 134, 11350–11353 (2012).
Davis, H. J., Genov, G. R. & Phipps, R. J. meta-Selective C–H borylation of benzylamine, phenethylamine and phenylpropylamine-derived amides enabled by a single anionic ligand. Angew. Chem. Int. Ed. 56, 13351–13355 (2017).
Davis, H. J., Mihai, M. T. & Phipps, R. J. Ion pair-directed regiocontrol in transition-metal catalysis: a meta-selective C−H borylation of aromatic quaternary ammonium salts. J. Am. Chem. Soc. 138, 12759–12762 (2016).
Chattopadhyay, B. et al. Ir-catalyzed ortho-borylation of phenols directed by substrate–ligand electrostatic interactions: a combined experimental/in silico strategy for optimizing weak interactions. J. Am. Chem. Soc. 139, 7864–7871 (2017).
Bisht, R. & Chattopadhyay, B. Formal Ir-catalyzed ligand-enabled ortho and meta borylation of aromatic aldehydes via in situ-generated imines. J. Am. Chem. Soc. 138, 84–87 (2016).
Li, H. L., Kuninobu, Y. & Kanai, M. Lewis Acid–base interaction-controlled ortho-selective C−H borylation of aryl sulfides. Angew. Chem. Int. Ed. 56, 1495–1499 (2017).
Hoque, M. E., Bisht, R., Haldar, C. & Chattopadhyay, B. Noncovalent interactions in Ir-catalyzed C−H activation: L-shaped ligand for para-selective borylation of aromatic esters. J. Am. Chem. Soc. 139, 7745–7748 (2017).
Yang, L., Uemura, N. & Nakao, Y. meta-Selective C−H borylation of benzamides and pyridines by an iridium−Lewis acid bifunctional catalyst. J. Am. Chem. Soc. 141, 7972–7979 (2019).
Lang, D. K., Kaur, R., Arora, R., Saini, B. & Arora, S. Nitrogen-containing heterocycles as anticancer agents: an overview. Anti-Cancer Agents Med. Chem. 20, 2150–2168 (2020).
Vitaku, E., Smith, D. T. & Njardarson, J. T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among US FDA approved pharmaceuticals. J. Med. Chem. 57, 10257–10274 (2014).
Das, R. & Kapur, M. Transition-metal-catalyzed C–H functionalization reactions of π-deficient heterocycles. Asian J. Org. Chem. 7, 1217–1235 (2018).
Murakami, K., Yamada, S., Kaneda, T. & Itami, K. C−H functionalization of azines. Chem. Rev. 117, 9302–9332 (2017).
Stephens, D. & Larionov, O. Recent advances in the C−H functionalization of the distal positions in pyridines and quinolines. Tetrahedron 71, 8683–8716 (2015).
Nakao, Y. Transition-metal-catalyzed C−H functionalization for the synthesis of substituted pyridines. Synthesis 2011, 3209–3219 (2011).
Rubio-Pérez, L. et al. A well-defined NHC–Ir(III) catalyst for the silylation of aromatic C–H bonds: substrate survey and mechanistic insights. Chem. Sci. 8, 4811–4822 (2017).
Wubbolt, S. & Oestreich, M. Catalytic electrophilic C–H silylation of pyridines enabled by temporary dearomatization. Angew. Chem. Int. Ed. 54, 15876–15879 (2015).
Cheng, C. & Hartwig, J. F. Iridium-catalyzed silylation of aryl C−H bonds. J. Am. Chem. Soc. 137, 592–595 (2015).
Ye, M. et al. Ligand-promoted C3-selective arylation of pyridines with Pd catalysts: gram-scale synthesis of (±)-preclamol. J. Am. Chem. Soc. 133, 19090–19093 (2011).
Li, B.-J. & Shi, Z.-J. Ir-catalyzed highly selective addition of pyridyl C−H bonds to aldehydes promoted by triethylsilane. Chem. Sci. 2, 488–493 (2011).
Mkhalid, I. A. I. et al. Ir-catalyzed borylation of C−H bonds in N-containing heterocycles: regioselectivity in the synthesis of heteroaryl boronate esters. Angew. Chem. Int. Ed. 45, 489–491 (2006).
Takagi, J., Sato, K., Hartwig, J. F., Ishiyama, T. & Miyaura, N. Iridium-catalyzed C−H coupling reaction of heteroaromatic compounds with bis(pinacolato)diboron: regioselective synthesis of heteroarylboronates. Tetrahedron Lett. 43, 5649–5651 (2002).
Ye, M., Gao, G.-L. & Yu, J.-Q. Ligand-promoted C-3 selective C−H olefination of pyridines with Pd catalysts. J. Am. Chem. Soc. 133, 6964–6967 (2011).
Cong, X., Tang, H., Wu, C. & Zeng, X. Role of mono-N-protected amino acid ligands in palladium(II)-catalyzed dehydrogenative Heck reactions of electron-deficient (hetero)arenes: experimental and computational studies. Organometallics 32, 6565–6575 (2013).
Yamada, S., Kaneda, T., Steib, P., Murakami, K. & Itami, K. Dehydrogenative synthesis of 2,2′-bipyridyls through regioselective pyridine dimerization. Angew. Chem. Int. Ed. 58, 8341–8345 (2019).
Kundu, A., Inoue, M., Nagae, H., Tsurugi, H. & Mashima, K. Direct ortho-C–H aminoalkylation of 2-substituted pyridine derivatives catalyzed by yttrium complexes with N,N′-diarylethylenediamido ligands. J. Am. Chem. Soc. 140, 7332–7342 (2018).
Song, G., O, W. W. N. & Hou, Z. Enantioselective C−H bond addition of pyridines to alkenes catalyzed by chiral half-sandwich rare-earth complexes. J. Am. Chem. Soc. 136, 12209–12212 (2014).
Liu, B., Huang, Y., Lan, J., Song, F. & You, J. Pd-catalyzed oxidative C−H/C−H cross-coupling of pyridines with heteroarenes. Chem. Sci. 4, 2163–2167 (2013).
Tobisu, M., Hyodo, I. & Chatani, N. Nickel-catalyzed reaction of arylzinc reagents with N-aromatic heterocycles: a straightforward approach to C–H bond arylation of electron-deficient heteroaromatic compounds. J. Am. Chem. Soc. 131, 12070–12071 (2009).
Lewis, J. C., Bergman, R. G. & Ellman, J. A. Rh(I)-catalyzed alkylation of quinolines and pyridines via C−H bond activation. J. Am. Chem. Soc. 129, 5332–5333 (2007).
Nakao, Y., Kanyiva, K. S. & Hiyama, T. A strategy for C–H activation of pyridines: direct C-2 selective alkenylation of pyridines by nickel/Lewis acid catalysis. J. Am. Chem. Soc. 130, 2448–2449 (2008).
Kawashima, T., Takao, T. & Suzuki, H. Dehydrogenative coupling of 4-substituted pyridines catalyzed by diruthenium complexes. J. Am. Chem. Soc. 129, 11006–11007 (2007).
Yang, L., Semba, K. & Nakao, Y. para-Selective C–H borylation of (hetero)arenes by cooperative iridium/aluminum catalysis. Angew. Chem. Int. Ed. 56, 4853–4857 (2017).
Andou, T., Saga, Y., Komai, H., Matsunaga, S. & Kanai, M. Cobalt-catalyzed C4-selective direct arylation of pyridines. Angew. Chem. Int. Ed. 52, 3213–3216 (2013).
Nakao, Y., Kanyiva, K. S. & Hiyama, T. A strategy for C−H activation of pyridines: direct C-2 selective alkenylation of pyridines by nickel/Lewis acid catalysis. J. Am. Chem. Soc. 130, 2448–2449 (2008).
Tsai, C.-C. et al. Bimetallic nickel aluminum mediated para-selective alkenylation of pyridine: direct observation of η2,η1-pyridine Ni(0)−Al(III) intermediates prior to C−H bond activation. J. Am. Chem. Soc. 132, 11887–11889 (2010).
Nakao, Y., Yamada, Y., Kashihara, N. & Hiyama, T. Selective C-4 alkylation of pyridine by nickel/Lewis acid catalysis. J. Am. Chem. Soc. 132, 13666–13668 (2010).
Evans, K. J. & Mansell, S. M. Functionalised N-heterocyclic carbene ligands in bimetallic architectures. Chem. Eur. J. 26, 5927–5941 (2020).
Dardun, V., Escomel, L., Jeanneau, E. & Camp, C. On the alcoholysis of alkyl-aluminum(III) alkoxy-NHC derivatives: reactivity of the Al-carbene Lewis pair versus Al-alkyl. Dalton Trans. 47, 10429–10433 (2018).
Khake, S. M. & Chatani, N. Nickel-catalyzed C–H functionalization using a non-directed strategy. Chem 6, 1056–1081 (2020).
Acknowledgements
We thank the National Natural Science Foundation of China (91856104 and 21871145), the Tianjin Applied Basic Research Project and Cutting-Edge Technology Research Plan (19JCZDJC37900) and ‘Frontiers Science Center for New Organic Matter’, Nankai University (63181206), for financial support (M.Y.). We gratefully acknowledge The Scripps Research Institute, the Lindemann Trust (N.Y.S.L.) and the NIH (National Institute of General Medical Sciences grant R01GM102265) for financial support (J.-Q.Y.).
Author information
Authors and Affiliations
Contributions
J.-Q.Y., M.Y. and T.Z. conceived the concept. T.Z. developed the conditions and performed the alkenylation of pyridines. Y.-X.L., J.-F.L. and Y.L. prepared the substrates and ligands. All authors analysed the results. N.Y.S.L. provided insightful suggestions in analysing the data and editing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Chemistry thanks Manmohan Kapur, Oleg Larionov and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Experimental procedures, mechanistic studies, Supplementary Figs. 1–90, Tables 1–7, X-ray crystallographic data and references.
Rights and permissions
About this article
Cite this article
Zhang, T., Luan, YX., Lam, N.Y.S. et al. A directive Ni catalyst overrides conventional site selectivity in pyridine C–H alkenylation. Nat. Chem. 13, 1207–1213 (2021). https://doi.org/10.1038/s41557-021-00792-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-021-00792-1
This article is cited by
-
A tautomerized ligand enabled meta selective C–H borylation of phenol
Nature Communications (2023)
-
Ni-catalyzed hydroarylation of alkynes with unactivated β-C(sp2)−H bonds
Nature Communications (2022)
-
Molecular editing of aza-arene C–H bonds by distance, geometry and chirality
Nature (2022)