Abstract
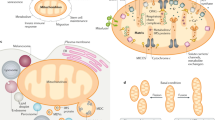
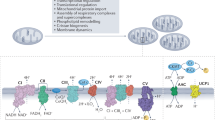
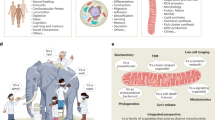
Although it is well described that mitochondria are at the epicentre of the energy demands of a cell, it is becoming important to consider how each cell tailors its mitochondrial composition and functions to suit its particular needs beyond ATP production. Here we provide insight into mitochondrial heterogeneity throughout development as well as in tissues with specific energy demands and discuss how mitochondrial malleability contributes to cell fate determination and tissue remodelling.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Spinelli, J. B. & Haigis, M. C. The multifaceted contributions of mitochondria to cellular metabolism. Nat. Cell Biol. 20, 745–754 (2018).
Bahat, A. & Gross, A. Mitochondrial plasticity in cell fate regulation. J. Biol. Chem. 294, 13852–13863 (2019).
Mehta, M. M., Weinberg, S. E. & Chandel, N. S. Mitochondrial control of immunity: beyond ATP. Nat. Rev. Immunol. 17, 608–620 (2017).
Fecher, C. et al. Cell-type-specific profiling of brain mitochondria reveals functional and molecular diversity. Nat. Neurosci. 22, 1731–1742 (2019).
Kajimura, S. & Saito, M. A new era in brown adipose tissue biology: molecular control of brown fat development and energy homeostasis. Annu. Rev. Physiol. 76, 225–249 (2014).
Yook, J.-S. et al. The SLC25A47 locus controls gluconeogenesis and energy expenditure. Proc. Natl Acad. Sci. USA 120, e2216810120 (2023).
Lesner, N. P. et al. Differential requirements for mitochondrial electron transport chain components in the adult murine liver. eLife 11, e80919 (2022).
Diaz-Cuadros, M. et al. Metabolic regulation of species-specific developmental rates. Nature 613, 550–557 (2023).
Burr, S. P. et al. Cell lineage-specific mitochondrial resilience during mammalian organogenesis. Cell 186, 1212–1229 (2023).
Huppertz, I. et al. Riboregulation of enolase 1 activity controls glycolysis and embryonic stem cell differentiation. Mol. Cell 82, 2666–2680 (2022).
Gu, W. et al. Glycolytic metabolism plays a functional role in regulating human pluripotent stem cell state. Cell Stem Cell 19, 476–490 (2016).
Xu, X. et al. Mitochondrial regulation in pluripotent stem cells. Cell Metab. 18, 325–332 (2013).
Intlekofer, A. M. & Finley, L. W. S. Metabolic signatures of cancer cells and stem cells. Nat. Metab. 1, 177–188 (2019).
Hicks, M. R. & Pyle, A. D. The emergence of the stem cell niche. Trends Cell Biol. 33, 112–123 (2023).
Mohyeldin, A., Garzón-Muvdi, T. & Quiñones-Hinojosa, A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell 7, 150–161 (2010).
De Almeida, M. J., Luchsinger, L. L., Corrigan, D. J., Williams, L. J. & Snoeck, H.-W. Dye-independent methods reveal elevated mitochondrial mass in hematopoietic stem cells. Cell Stem Cell 21, 725–729 (2017).
Ansó, E. et al. The mitochondrial respiratory chain is essential for haematopoietic stem cell function. Nat. Cell Biol. 19, 614–625 (2017).
Chakrabarty, R. P. & Chandel, N. S. Mitochondria as signaling organelles control mammalian stem cell fate. Cell Stem Cell 28, 394–408 (2021).
Zhang, H. et al. Distinct metabolic states can support self-renewal and lipogenesis in human pluripotent stem cells under different culture conditions. Cell Rep. 16, 1536–1547 (2016).
Zhu, Q., An, Y. A. & Scherer, P. E. Mitochondrial regulation and white adipose tissue homeostasis. Trends Cell Biol. 32, 351–364 (2022).
Kladnická, I. et al. Mitochondrial respiration of adipocytes differentiating from human mesenchymal stem cells derived from adipose tissue. Physiol. Res. 68, S287–S296 (2019).
Oguri, Y. et al. CD81 controls beige fat progenitor cell growth and energy balance via FAK signaling. Cell 182, 563–577 (2020).
Joffin, N. et al. Mitochondrial metabolism is a key regulator of the fibro-inflammatory and adipogenic stromal subpopulations in white adipose tissue. Cell Stem Cell 28, 702–717 (2021).
Kusminski, C. M. et al. MitoNEET-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity. Nat. Med. 18, 1539–1549 (2012).
Rodríguez-Colman, M. J. et al. Interplay between metabolic identities in the intestinal crypt supports stem cell function. Nature 543, 424–427 (2017).
Ludikhuize, M. C. et al. Mitochondria define intestinal stem cell differentiation downstream of a FOXO/Notch axis. Cell Metab. 32, 889–900 (2020).
Schell, J. C. et al. Control of intestinal stem cell function and proliferation by mitochondrial pyruvate metabolism. Nat. Cell Biol. 19, 1027–1036 (2017).
Nakamura-Ishizu, A., Ito, K. & Suda, T. Hematopoietic stem cell metabolism during development and aging. Dev. Cell 54, 239–255 (2020).
Vannini, N. et al. Specification of haematopoietic stem cell fate via modulation of mitochondrial activity. Nat. Commun. 7, 13125 (2016).
Lin, C. et al. Impaired mitochondrial oxidative metabolism in skeletal progenitor cells leads to musculoskeletal disintegration. Nat. Commun. 13, 6869 (2022).
Wanet, A., Arnould, T., Najimi, M. & Renard, P. Connecting mitochondria, metabolism, and stem cell fate. Stem. Cells Dev. 24, 1957–1971 (2015).
Picard, M. & Shirihai, O. S. Mitochondrial signal transduction. Cell Metab. 34, 1620–1653 (2022).
Arnold, P. K. et al. A non-canonical tricarboxylic acid cycle underlies cellular identity. Nature 603, 477–481 (2022).
Scandella, V., Petrelli, F., Moore, D. L., Braun, S. M. G. & Knobloch, M. Neural stem cell metabolism revisited: a critical role for mitochondria. Trends Endocrinol. Metab. 34, 446–461 (2023).
Knobloch, M. et al. A fatty acid oxidation-dependent metabolic shift regulates adult neural stem cell activity. Cell Rep. 20, 2144–2155 (2017).
Zhang, K. et al. Acquisition of cellular properties during alveolar formation requires differential activity and distribution of mitochondria. eLife 11, e68598 (2022).
Rangaraju, V. et al. Pleiotropic mitochondria: the influence of mitochondria on neuronal development and disease. J. Neurosci. 39, 8200–8208 (2019).
Zhao, J., Sun, Q., Zhou, L., Liu, K. & Jiao, K. Complex Regulation of Mitochondrial Function During Cardiac Development. J. Am. Heart Assoc. 8, e012731 (2019).
Cheong, A. et al. Nuclear encoded mitochondrial ribosomal proteins are required to initiate gastrulation. Development 147, dev188714 (2020).
Rodríguez-Nuevo, A. et al. Oocytes maintain ROS-free mitochondrial metabolism by suppressing complex I. Nature 607, 756–761 (2022).
Kirillova, A., Smitz, J. E. J., Sukhikh, G. T. & Mazunin, I. The role of mitochondria in oocyte maturation. Cells 10, 2484 (2021).
Wang, L. et al. Oxidative stress in oocyte aging and female reproduction. J. Cell. Physiol. 236, 7966–7983 (2021).
Folmes, C. D. L., Dzeja, P. P., Nelson, T. J. & Terzic, A. Metabolic plasticity in stem cell homeostasis and differentiation. Cell Stem Cell 11, 596–606 (2012).
Glancy, B., Kim, Y., Katti, P. & Willingham, T. B. The functional impact of mitochondrial structure across subcellular scales. Front. Physiol. 11, 541040 (2020).
Iwata, R. et al. Mitochondria metabolism sets the species-specific tempo of neuronal development. Science 379, eabn4705 (2023).
Steiner, P. Brain fuel utilization in the developing brain. Ann. Nutr. Metab. 75, 8–18 (2019).
Nagaraj, R. et al. Nuclear localization of mitochondrial TCA cycle enzymes as a critical step in mammalian zygotic genome activation. Cell 168, 210–223 (2017).
Zwick, R. K., Guerrero-Juarez, C. F., Horsley, V. & Plikus, M. V. Anatomical, physiological, and functional diversity of adipose tissue. Cell Metab. 27, 68–83 (2018).
Auger, C. & Kajimura, S. Adipose tissue remodeling in pathophysiology. Annu. Rev. Pathol. 18, 71–93 (2023).
Abe, I. et al. Lipolysis-derived linoleic acid drives beige fat progenitor cell proliferation. Dev. Cell 57, 2623–2637 (2022).
Giordano, A., Frontini, A. & Cinti, S. Convertible visceral fat as a therapeutic target to curb obesity. Nat. Rev. Drug Discov. 15, 405–424 (2016).
Bean, C. et al. The mitochondrial protein Opa1 promotes adipocyte browning that is dependent on urea cycle metabolites. Nat. Metab. 3, 1633–1647 (2021).
Lu, X. et al. Mitophagy controls beige adipocyte maintenance through a Parkin-dependent and UCP1-independent mechanism. Sci. Signal. 11, eaap8526 (2018).
Altshuler-Keylin, S. et al. Beige adipocyte maintenance is regulated by autophagy-induced mitochondrial clearance. Cell Metab. 24, 402–419 (2016).
Ghaben, A. L. & Scherer, P. E. Adipogenesis and metabolic health. Nat. Rev. Mol. Cell Biol. 20, 242–258 (2019).
Sun, K., Tordjman, J., Clément, K. & Scherer, P. E. Fibrosis and adipose tissue dysfunction. Cell Metab. 18, 470–477 (2013).
Chouchani, E. T. & Kajimura, S. Metabolic adaptation and maladaptation in adipose tissue. Nat. Metab. 1, 189–200 (2019).
Lee, Y. S. et al. Increased adipocyte O2 consumption triggers HIF-1α, causing inflammation and insulin resistance in obesity. Cell 157, 1339–1352 (2014).
Seo, J. B. et al. Knockdown of ANT2 reduces adipocyte hypoxia and improves insulin resistance in obesity. Nat. Metab. 1, 86–97 (2019).
Maqdasy, S. et al. Impaired phosphocreatine metabolism in white adipocytes promotes inflammation. Nat. Metab. 4, 190–202 (2022).
Rahbani, J. F. et al. Creatine kinase B controls futile creatine cycling in thermogenic fat. Nature 590, 480–485 (2021).
Serbulea, V. et al. Macrophage phenotype and bioenergetics are controlled by oxidized phospholipids identified in lean and obese adipose tissue. Proc. Natl Acad. Sci. USA 115, E6254–E6263 (2018).
Xu, L. et al. Macrophage polarization mediated by mitochondrial dysfunction induces adipose tissue inflammation in obesity. Int. J. Mol. Sci. 23, 9252 (2022).
Wang, Y. et al. Improvement of obesity-associated disorders by a small-molecule drug targeting mitochondria of adipose tissue macrophages. Nat. Commun. 12, 102 (2021).
Anvari, G. & Bellas, E. Hypoxia induces stress fiber formation in adipocytes in the early stage of obesity. Sci. Rep. 11, 21473 (2021).
Fung, T. S., Chakrabarti, R. & Higgs, H. N. The multiple links between actin and mitochondria. Nat. Rev. Mol. Cell Biol. 24, 651–667 (2023).
Li, S. et al. Transient assembly of F-actin on the outer mitochondrial membrane contributes to mitochondrial fission. J. Cell Biol. 208, 109–123 (2015).
Horn, A. et al. Mitochondrial redox signaling enables repair of injured skeletal muscle cells. Sci. Signal. 10, eaaj1978 (2017).
Sitar, T. et al. Molecular architecture of the spire–actin nucleus and its implication for actin filament assembly. Proc. Natl Acad. Sci. USA 108, 19575–19580 (2011).
Manor, U. et al. A mitochondria-anchored isoform of the actin-nucleating spire protein regulates mitochondrial division. eLife 4, e08828 (2015).
Pernas, L. & Scorrano, L. Mito-morphosis: mitochondrial fusion, fission, and cristae remodeling as key mediators of cellular function. Annu. Rev. Physiol. 78, 505–531 (2016).
Guidotti, S. et al. Glycogen synthase kinase-3β inhibition links mitochondrial dysfunction, extracellular matrix remodelling and terminal differentiation in chondrocytes. Sci. Rep. 7, 12059 (2017).
Bubb, K. et al. Mitochondrial respiratory chain function promotes extracellular matrix integrity in cartilage. J. Biol. Chem. 297, 101224 (2021).
Tharp, K. M. et al. Adhesion-mediated mechanosignaling forces mitohormesis. Cell Metab. 33, 1322–1341 (2021).
Park, J. S. et al. Mechanical regulation of glycolysis via cytoskeleton architecture. Nature 578, 621–626 (2020).
McLaughlin, K. L. et al. Novel approach to quantify mitochondrial content and intrinsic bioenergetic efficiency across organs. Sci. Rep. 10, 17599 (2020).
Magistretti, P. J. & Allaman, I. A cellular perspective on brain energy metabolism and functional imaging. Neuron 86, 883–901 (2015).
Ioannou, M. S. et al. Neuron-astrocyte metabolic coupling protects against activity-induced fatty acid toxicity. Cell 177, 1522–1535 (2019).
Bélanger, M., Allaman, I. & Magistretti, P. J. Brain energy metabolism: focus on astrocyte–neuron metabolic cooperation. Cell Metab. 14, 724–738 (2011).
Pekkurnaz, G. & Wang, X. Mitochondrial heterogeneity and homeostasis through the lens of a neuron. Nat. Metab. 4, 802–812 (2022).
Graham, L. C. et al. Proteomic profiling of neuronal mitochondria reveals modulators of synaptic architecture. Mol. Neurodegener. 12, 77 (2017).
Díaz-García, C. M. et al. Neuronal stimulation triggers neuronal glycolysis and not lactate uptake. Cell Metab. 26, 361–374 (2017).
Kuczynska, Z., Metin, E., Liput, M. & Buzanska, L. Covering the role of PGC-1α in the nervous system. Cells 11, 111 (2021).
Jensen, N. J., Wodschow, H. Z., Nilsson, M. & Rungby, J. Effects of ketone bodies on brain metabolism and function in neurodegenerative diseases. Int. J. Mol. Sci. 21, 8767 (2020).
Fritzen, A. M., Lundsgaard, A.-M. & Kiens, B. Tuning fatty acid oxidation in skeletal muscle with dietary fat and exercise. Nat. Rev. Endocrinol. 16, 683–696 (2020).
Hargreaves, M. & Spriet, L. L. Skeletal muscle energy metabolism during exercise. Nat. Metab. 2, 817–828 (2020).
Smith, J. A. B., Murach, K. A., Dyar, K. A. & Zierath, J. R. Exercise metabolism and adaptation in skeletal muscle. Nat. Rev. Mol. Cell Biol. 24, 607–632 (2023).
Deshmukh, A. S. et al. Deep muscle-proteomic analysis of freeze-dried human muscle biopsies reveals fiber type-specific adaptations to exercise training. Nat. Commun. 12, 304 (2021).
Reddy, A. et al. pH-gated succinate secretion regulates muscle remodeling in response to exercise. Cell 183, 62–75 (2020).
Ouyang, Q. et al. Rab8a as a mitochondrial receptor for lipid droplets in skeletal muscle. Dev. Cell 58, 289–305 (2023).
Benador, I. Y., Veliova, M., Liesa, M. & Shirihai, O. S. Mitochondria bound to lipid droplets: where mitochondrial dynamics regulate lipid storage and utilization. Cell Metab. 29, 827–835 (2019).
Benador, I. Y. et al. Mitochondria bound to lipid droplets have unique bioenergetics, composition, and dynamics that support lipid droplet expansion. Cell Metab. 27, 869–885 (2018).
Furrer, R. et al. Molecular control of endurance training adaptation in male mouse skeletal muscle. Nat. Metab. 5, 2020–2035 (2023).
Wang, X. et al. Scinderin promotes fusion of electron transport chain dysfunctional muscle stem cells with myofibers. Nat. Aging 2, 155–169 (2022).
Wicks, S. E. et al. Impaired mitochondrial fat oxidation induces adaptive remodeling of muscle metabolism. Proc. Natl Acad. Sci. USA 112, E3300–E3309 (2015).
Pereyra, A. S. et al. Skeletal muscle undergoes fiber type metabolic switch without myosin heavy chain switch in response to defective fatty acid oxidation. Mol. Metab. 59, 101456 (2022).
Koves, T. R. et al. Pyruvate-supported flux through medium-chain ketothiolase promotes mitochondrial lipid tolerance in cardiac and skeletal muscles. Cell Metab. 35, 1038–1056 (2023).
Yasuda, T., Ishihara, T., Ichimura, A. & Ishihara, N. Mitochondrial dynamics define muscle fiber type by modulating cellular metabolic pathways. Cell Rep. 42, 112434 (2023).
Song, H. et al. CREG1 improves the capacity of the skeletal muscle response to exercise endurance via modulation of mitophagy. Autophagy 17, 4102–4118 (2021).
Mishra, P., Varuzhanyan, G., Pham, A. H. & Chan, D. C. Mitochondrial dynamics is a distinguishing feature of skeletal muscle fiber types and regulates organellar compartmentalization. Cell Metab. 22, 1033–1044 (2015).
Hood, D. A., Memme, J. M., Oliveira, A. N. & Triolo, M. Maintenance of skeletal muscle mitochondria in health, exercise, and aging. Annu. Rev. Physiol. 81, 19–41 (2019).
Rasbach, K. A. et al. PGC-1α regulates a HIF2α-dependent switch in skeletal muscle fiber types. Proc. Natl Acad. Sci. USA 107, 21866–21871 (2010).
Auger, C., Alhasawi, A., Contavadoo, M. & Appanna, V. D. Dysfunctional mitochondrial bioenergetics and the pathogenesis of hepatic disorders. Front. Cell Dev. Biol. 3, 40 (2015).
Bórquez, J. C. et al. Mitofusin-2 induced by exercise modifies lipid droplet-mitochondria communication, promoting fatty acid oxidation in male mice with NAFLD. Metabolism 152, 155765 (2024).
Levine, D. C. et al. NADH inhibition of SIRT1 links energy state to transcription during time-restricted feeding. Nat. Metab. 3, 1621–1632 (2021).
Petersen, M. C., Vatner, D. F. & Shulman, G. I. Regulation of hepatic glucose metabolism in health and disease. Nat. Rev. Endocrinol. 13, 572–587 (2017).
Holeček, M. Roles of malate and aspartate in gluconeogenesis in various physiological and pathological states. Metabolism 145, 155614 (2023).
Bideyan, L., Nagari, R. & Tontonoz, P. Hepatic transcriptional responses to fasting and feeding. Genes Dev. 35, 635–657 (2021).
Méndez-Lucas, A. et al. PEPCK-M expression in mouse liver potentiates, not replaces, PEPCK-C mediated gluconeogenesis. J. Hepatol. 59, 105–113 (2013).
Bresciani, N. et al. The Slc25a47 locus is a novel determinant of hepatic mitochondrial function implicated in liver fibrosis. J. Hepatol. 77, 1071–1082 (2022).
Paris, J. & Henderson, N. C. Liver zonation, revisited. Hepatology 76, 1219–1230 (2022).
Ben-Moshe, S. & Itzkovitz, S. Spatial heterogeneity in the mammalian liver. Nat. Rev. Gastroenterol. Hepatol. 16, 395–410 (2019).
Brosch, M. et al. Epigenomic map of human liver reveals principles of zonated morphogenic and metabolic control. Nat. Commun. 9, 4150 (2018).
Goetzman, E. S. et al. Impaired mitochondrial medium-chain fatty acid oxidation drives periportal macrovesicular steatosis in sirtuin-5 knockout mice. Sci. Rep. 10, 18367 (2020).
Ben-Moshe, S. et al. Spatial sorting enables comprehensive characterization of liver zonation. Nat. Metab. 1, 899–911 (2019).
Golozoubova, V. et al. Only UCP1 can mediate adaptive nonshivering thermogenesis in the cold. FASEB J. 15, 2048–2050 (2001).
Tai, J. et al. Hem25p is required for mitochondrial IPP transport in fungi. Nat. Cell Biol. 25, 1616–1624 (2023).
Vandereyken, K., Sifrim, A., Thienpont, B. & Voet, T. Methods and applications for single-cell and spatial multi-omics. Nat. Rev. Genet. 24, 494–515 (2023).
Petrelli, F. et al. Mitochondrial pyruvate metabolism regulates the activation of quiescent adult neural stem cells. Sci. Adv. 9, eadd5220 (2023).
Tian, Z. & Liang, M. Renal metabolism and hypertension. Nat. Commun. 12, 963 (2021).
Kappler, L. et al. Linking bioenergetic function of mitochondria to tissue-specific molecular fingerprints. Am. J. Physiol. Endocrinol. Metab. 317, E374–E387 (2019).
Prola, A. et al. Cardiolipin content controls mitochondrial coupling and energetic efficiency in muscle. Sci. Adv. 7, eabd6322 (2021).
Sustarsic, E. G. et al. Cardiolipin synthesis in brown and beige fat mitochondria is essential for systemic energy homeostasis. Cell Metab. 28, 159–174 (2018).
Peng, M. et al. Aerobic glycolysis promotes T helper 1 cell differentiation through an epigenetic mechanism. Science 354, 481–484 (2016).
Cardoso, A. C. et al. Mitochondrial substrate utilization regulates cardiomyocyte cell cycle progression. Nat. Metab. 2, 167–178 (2020).
Schulze, P. C., Drosatos, K. & Goldberg, I. J. Lipid use and misuse by the heart. Circ. Res. 118, 1736–1751 (2016).
Hui, S. et al. Quantitative fluxomics of circulating metabolites. Cell Metab. 32, 676–688 (2020).
Paillard, M. et al. Tissue-specific mitochondrial decoding of cytoplasmic Ca2+ signals is controlled by the stoichiometry of MICU1/2 and MCU. Cell Rep. 18, 2291–2300 (2017).
Zhou, W. et al. SENP1–Sirt3 signaling promotes α-ketoglutarate production during M2 macrophage polarization. Cell Rep. 39, 110660 (2022).
Liu, P.-S. et al. α-Ketoglutarate orchestrates macrophage activation through metabolic and epigenetic reprogramming. Nat. Immunol. 18, 985–994 (2017).
Mills, E. L. et al. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell 167, 457–470 (2016).
Petrus, P. et al. Glutamine links obesity to inflammation in human white adipose tissue. Cell Metab. 31, 375–390 (2020).
Yoneshiro, T. et al. Metabolic flexibility via mitochondrial BCAA carrier SLC25A44 is required for optimal fever. eLife 10, e66865 (2021).
Yoneshiro, T. et al. BCAA catabolism in brown fat controls energy homeostasis through SLC25A44. Nature 572, 614–619 (2019).
Galy, B., Conrad, M. & Muckenthaler, M. Mechanisms controlling cellular and systemic iron homeostasis. Nat. Rev. Mol. Cell Biol. 25, 133–155 (2023).
Tran, D. H. et al. Mitochondrial NADP+ is essential for proline biosynthesis during cell growth. Nat. Metab. 3, 571–585 (2021).
Rönn, T. et al. Genes with epigenetic alterations in human pancreatic islets impact mitochondrial function, insulin secretion, and type 2 diabetes. Nat. Commun. 14, 8040 (2023).
Walejko, J. M. et al. Branched-chain α-ketoacids are preferentially reaminated and activate protein synthesis in the heart. Nat. Commun. 12, 1680 (2021).
Murashige, D. et al. Comprehensive quantification of fuel use by the failing and nonfailing human heart. Science 370, 364–368 (2020).
Flam, E. & Arany, Z. Metabolite signaling in the heart. Nat. Cardiovasc. Res. 2, 504–516 (2023).
Durante, W. The emerging role of l-glutamine in cardiovascular health and disease. Nutrients 11, 2092 (2019).
Muoio, D. M. Metabolic inflexibility: when mitochondrial indecision leads to metabolic gridlock. Cell 159, 1253–1262 (2014).
Maurer, J., Hoene, M. & Weigert, C. Signals from the circle: tricarboxylic acid cycle intermediates as myometabokines. Metabolites 11, 474 (2021).
Li, C. et al. Purkinje cell dopaminergic inputs to astrocytes regulate cerebellar-dependent behavior. Nat. Commun. 14, 1613 (2023).
Pickrell, A. M., Fukui, H., Wang, X., Pinto, M. & Moraes, C. T. The striatum is highly susceptible to mitochondrial oxidative phosphorylation dysfunctions. J. Neurosci. 31, 9895–9904 (2011).
Felix, J. B., Cox, A. R. & Hartig, S. M. Acetyl-CoA and metabolite fluxes regulate white adipose tissue expansion. Trends Endocrinol. Metab. 32, 320–332 (2021).
Kajimura, S., Spiegelman, B. M. & Seale, P. Brown and beige fat: physiological roles beyond heat generation. Cell Metab. 22, 546–559 (2015).
Wiley, S. E., Murphy, A. N., Ross, S. A., van der Geer, P. & Dixon, J. E. MitoNEET is an iron-containing outer mitochondrial membrane protein that regulates oxidative capacity. PNAS 104, 5318–5323 (2007).
Adusumilli, V. S. et al. ROS dynamics delineate functional states of hippocampal neural stem cells and link to their activity-dependent exit from quiescence. Cell Stem Cell 28, 300–314.e6 (2021).
Acknowledgements
We would like to acknowledge our funding sources: NIH (RO1 DK097441, RO1DK125283 and DK125281), Howard Hughes Medical Institute and the NIDDK (3DP1DK126160-04S1).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cell Biology thanks Navdeep Chandel and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Granath-Panelo, M., Kajimura, S. Mitochondrial heterogeneity and adaptations to cellular needs. Nat Cell Biol 26, 674–686 (2024). https://doi.org/10.1038/s41556-024-01410-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41556-024-01410-1