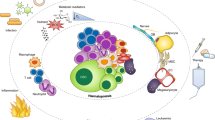
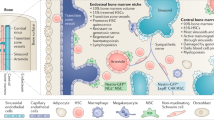
Abstract
Myeloid cell infiltration of solid tumours generally associates with poor patient prognosis and disease severity1,2,3,4,5,6,7,8,9,10,11,12,13. Therefore, understanding the regulation of myeloid cell differentiation during cancer is crucial to counteract their pro-tumourigenic role. Bone marrow (BM) haematopoiesis is a tightly regulated process for the production of all immune cells in accordance to tissue needs14. Myeloid cells differentiate during haematopoiesis from multipotent haematopoietic stem and progenitor cells (HSPCs)15,16,17. HSPCs can sense inflammatory signals from the periphery during infections18,19,20,21 or inflammatory disorders22,23,24,25,26,27. In these settings, HSPC expansion is associated with increased myeloid differentiation28,29. During carcinogenesis, the elevation of haematopoietic growth factors supports the expansion and differentiation of committed myeloid progenitors5,30. However, it is unclear whether cancer-related inflammation also triggers demand-adapted haematopoiesis at the level of multipotent HSPCs. In the BM, HSPCs reside within the haematopoietic niche which delivers HSC maintenance and differentiation cues31,32,33,34,35. Mesenchymal stem cells (MSCs) are a major cellular component of the BM niche and contribute to HSC homeostasis36,37,38,39,40,41. Modifications of MSCs in systemic disorders have been associated with HSC differentiation towards myeloid cells22,42. It is unknown if MSCs are regulated in the context of solid tumours and if their myeloid supportive activity is impacted by cancer-induced systemic changes. Here, using unbiased transcriptomic analysis and in situ imaging of HSCs and the BM niche during breast cancer, we show that both HSCs and MSCs are transcriptionally and spatially modified. We demonstrate that breast tumour can distantly remodel the cellular cross-talks in the BM niche leading to increased myelopoiesis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Source data for quantifications shown in figures are provided with the manuscript. Bulk and scRNA-seq data can be found on Gene Expression Omnibus under accession code GSE243964. Mus musculus genome GRCm38.p4 is available at https://www.ncbi.nlm.nih.gov/datasets/genome/GCF_000001635.24/. Any other data are available from the corresponding author upon reasonable request. Source data are provided with this paper.
Code availability
All codes and packages used in this study are either publicly available or can be found on GitHub (https://github.com/TeamPerie/Gerber_et_al_2023).
References
Cassetta, L. & Pollard, J. W. Targeting macrophages: therapeutic approaches in cancer. Nat. Rev. Drug Discov. 17, 887–904 (2018).
Shaul, M. E. & Fridlender, Z. G. Tumour-associated neutrophils in patients with cancer. Nat. Rev. Clin. Oncol. 16, 601–620 (2019).
Engblom, C. et al. Osteoblasts remotely supply lung tumors with cancer-promoting SiglecF high neutrophils. Science 358, eaal5081 (2017).
Alshetaiwi, H. et al. Defining the emergence of myeloid-derived suppressor cells in breast cancer using single-cell transcriptomics. Sci. Immunol. 5, eaay6017 (2020).
Casbon, A.-J. et al. Invasive breast cancer reprograms early myeloid differentiation in the bone marrow to generate immunosuppressive neutrophils. Proc. Natl Acad. Sci. USA 112, E566–E575 (2015).
Qian, B.-Z. et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 475, 222–225 (2011).
Franklin, R. A. et al. The cellular and molecular origin of tumor-associated macrophages. Science 344, 921–925 (2014).
Nixon, B. G. et al. Tumor-associated macrophages expressing the transcription factor IRF8 promote T cell exhaustion in cancer. Immunity 55, 2044–2058.e5 (2022).
Linde, N. et al. Macrophages orchestrate breast cancer early dissemination and metastasis. Nat. Commun. 9, 21 (2018).
Wu, W.-C. et al. Circulating hematopoietic stem and progenitor cells are myeloid-biased in cancer patients. Proc. Natl Acad. Sci. USA 111, 4221–4226 (2014).
Allen, B. M. et al. Systemic dysfunction and plasticity of the immune macroenvironment in cancer models. Nat. Med. https://doi.org/10.1038/s41591-020-0892-6 (2020).
Meyer, M. A. et al. Breast and pancreatic cancer interrupt IRF8-dependent dendritic cell development to overcome immune surveillance. Nat. Commun. https://doi.org/10.1038/s41467-018-03600-6 (2018).
Magidey-Klein, K. et al. IL-6 contributes to metastatic switch via the differentiation of monocytic-dendritic progenitors into prometastatic immune cells. J. Immunother. Cancer 9, e002856 (2021).
Cosgrove, J., Hustin, L. S. P., de Boer, R. J. & Perié, L. Hematopoiesis in numbers. Trends Immunol. 42, 1100–1112 (2021).
Spangrude, G. J., Heimfeld, S. & Weissman, I. L. Purification and characterization of mouse hematopoietic stem cells. Science 241, 58–62 (1988).
Oguro, H., Ding, L. & Morrison, S. J. SLAM family markers resolve functionally distinct subpopulations of hematopoietic stem cells and multipotent progenitors. Cell Stem Cell 13, 102–116 (2013).
Ikuta, K. & Weissman, I. L. Evidence that hematopoietic stem cells express mouse c-kit but do not depend on steel factor for their generation. Proc. Natl Acad. Sci. USA 89, 1502–1506 (1992).
Haltalli, M. L. R. et al. Manipulating niche composition limits damage to haematopoietic stem cells during Plasmodium infection. Nat. Cell Biol. 22, 1399–1410 (2020).
Isringhausen, S. et al. Chronic viral infections persistently alter marrow stroma and impair hematopoietic stem cell fitness. J. Exp. Med. 218, e20192070 (2021).
Essers, M. A. G. et al. IFNα activates dormant haematopoietic stem cells in vivo. Nature 458, 904–908 (2009).
Pietras, E. M. et al. Chronic interleukin-1 exposure drives haematopoietic stem cells towards precocious myeloid differentiation at the expense of self-renewal. Nat. Cell Biol. 18, 607–618 (2016).
Frodermann, V. et al. Exercise reduces inflammatory cell production and cardiovascular inflammation via instruction of hematopoietic progenitor cells. Nat. Med. 25, 1761–1771 (2019).
Ho, Y.-H. et al. Remodeling of bone marrow hematopoietic stem cell niches promotes myeloid cell expansion during premature or physiological aging. Cell Stem Cell https://doi.org/10.1016/j.stem.2019.06.007 (2019).
Bernitz, J. M., Kim, H. S., MacArthur, B., Sieburg, H. & Moore, K. Hematopoietic stem cells count and remember self-renewal divisions. Cell 167, 1296–1309.e10 (2016).
Geiger, H., de Haan, G. & Florian, M. C. The ageing haematopoietic stem cell compartment. Nat. Rev. Immunol. 13, 376–389 (2013).
Heidt, T. et al. Chronic variable stress activates hematopoietic stem cells. Nat. Med. 20, 754–758 (2014).
Severe, N. et al. Stress-induced changes in bone marrow stromal cell populations revealed through single-cell protein expression mapping. Cell Stem Cell https://doi.org/10.1016/j.stem.2019.06.003 (2019).
Caiado, F., Pietras, E. M. & Manz, M. G. Inflammation as a regulator of hematopoietic stem cell function in disease, aging, and clonal selection. J. Exp. Med. 218, e20201541 (2021).
Schultze, J. L., Mass, E. & Schlitzer, A. Emerging principles in myelopoiesis at homeostasis and during infection and inflammation. Immunity 50, 288–301 (2019).
Engblom, C., Pfirschke, C. & Pittet, M. J. The role of myeloid cells in cancer therapies. Nat. Rev. Cancer 16, 447–462 (2016).
Wilson, A. & Trumpp, A. Bone-marrow haematopoietic-stem-cell niches. Nat. Rev. Immunol. 6, 93–106 (2006).
Friedenstein, A. J., Chailakhyan, R. K., Latsinik, N. V., Panasyuk, A. F. & Keiliss-Borok, I. V. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 17, 331–340 (1974).
Sacchetti, B. et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 131, 324–336 (2007).
Uccelli, A., Moretta, L. & Pistoia, V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 8, 726–736 (2008).
Broudy, V. C. Stem cell factor and hematopoiesis. Blood 90, 1345–1364 (1997).
Greenbaum, A. et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature 495, 227–230 (2013).
Ding, L. & Morrison, S. J. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature 495, 231–235 (2013).
Asada, N. et al. Differential cytokine contributions of perivascular haematopoietic stem cell niches. Nat. Cell Biol. 19, 214–223 (2017).
Ding, L., Saunders, T. L., Enikolopov, G. & Morrison, S. J. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 481, 457–462 (2012).
Omatsu, Y. et al. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity 33, 387–399 (2010).
Balzano, M. et al. Nidogen-1 contributes to the interaction network involved in pro-B cell retention in the peri-sinusoidal hematopoietic stem cell niche. Cell Rep. 26, 3257–3271.e8 (2019).
Young, K. et al. Decline in IGF1 in the bone marrow microenvironment initiates hematopoietic stem cell aging. Cell Stem Cell 28, 1473–1482.e7 (2021).
Lin, E. Y. et al. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am. J. Pathol. 163, 2113–2126 (2003).
Muller, W. J., Sinn, E., Pattengale, P. K., Wallace, R. & Leder, P. Single-step induction of mammary adenocarcinoma in transgenic mice bearing the activated c-neu oncogene. Cell 54, 105–115 (1988).
Pietras, E. M. et al. Functionally distinct subsets of lineage-biased multipotent progenitors control blood production in normal and regenerative conditions. Cell Stem Cell 17, 35–46 (2015).
Wilson, A. et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell 135, 1118–1129 (2008).
Liang, R. et al. Restraining lysosomal activity preserves hematopoietic stem cell quiescence and potency. Cell Stem Cell 26, 359–376.e7 (2020).
Kang, M.-L., Kim, E.-A., Jeong, S.-Y. & Im, G.-I. Angiopoietin-2 enhances osteogenic differentiation of bone marrow stem cells: angiopoietin-2 enhances osteogenic differentiation. J. Cell. Biochem. 118, 2896–2908 (2017).
Maeda, A. et al. WNT1-induced secreted protein-1 (WISP1), a novel regulator of bone turnover and Wnt signaling. J. Biol. Chem. 290, 14004–14018 (2015).
Takahashi, N., Udagawa, N. & Suda, T. A new member of tumor necrosis factor ligand family, ODF/OPGL/TRANCE/RANKL, regulates osteoclast differentiation and function. Biochem. Biophys. Res. Commun. 256, 449–455 (1999).
Schepers, K. et al. Myeloproliferative neoplasia remodels the endosteal bone marrow niche into a self-reinforcing leukemic niche. Cell Stem Cell 13, 285–299 (2013).
Zhou, B. O., Yue, R., Murphy, M. M., Peyer, J. G. & Morrison, S. J. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell 15, 154–168 (2014).
Méndez-Ferrer, S. et al. Bone marrow niches in haematological malignancies. Nat. Rev. Cancer 20, 285–298 (2020).
Méndez-Ferrer, S. et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466, 829–834 (2010).
Pinho, S. et al. PDGFRα and CD51 mark human Nestin+ sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. J. Exp. Med. 210, 1351–1367 (2013).
Dykstra, B., Olthof, S., Schreuder, J., Ritsema, M. & de Haan, G. Clonal analysis reveals multiple functional defects of aged murine hematopoietic stem cells. J. Exp. Med. 208, 2691–2703 (2011).
Mitroulis, I. et al. Modulation of myelopoiesis progenitors is an integral component of trained immunity. Cell 172, 147–161.e12 (2018).
Mossadegh-Keller, N. et al. M-CSF instructs myeloid lineage fate in single haematopoietic stem cells. Nature 497, 239–243 (2013).
Zhang, J. et al. In situ mapping identifies distinct vascular niches for myelopoiesis. Nature https://doi.org/10.1038/s41586-021-03201-2 (2021).
Tikhonova, A. N. et al. The bone marrow microenvironment at single-cell resolution. Nature https://doi.org/10.1038/s41586-019-1104-8 (2019).
Gerber_et_al_2023. GitHub https://github.com/TeamPerie/Gerber_et_al_2023 (2023).
Butler, A., Hoffman, P., Smibert, P., Papalexi, E. & Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 36, 411–420 (2018).
Coutu, D. L., Kokkaliaris, K. D., Kunz, L. & Schroeder, T. Three-dimensional map of nonhematopoietic bone and bone-marrow cells and molecules. Nat. Biotechnol. 35, 1202–1210 (2017).
Acknowledgements
We thank A.-M. Lennon-Duménil and all INSERM U932. We thank E. Gomez-Perdiguero, A. Voissière, D. Gros and L. Jean-Pierre. J.H. team is supported by Fondation ARC (PJA2021060003760), Ligue contre le cancer (RS22/75-24) and the European Research Council (ERC). Y.G.-F. is supported by FRM (FDT202106013018). E.P. team is supported by FRM, ANR-10-IDEX-0001-02 PSL, LabEx DCBIOL, CIC IGR-Curie 1428 and INCa-DGOS-Inserm_12554. This work has received support under the programme France 2030 launched by the French Government. High-throughput sequencing was performed by the ICGex NGS platform of the Institut Curie supported by the grants ANR-10-EQPX-03 and ANR-10-INBS-09-08, by the ITMO-Cancer Aviesan (Plan Cancer III) and by the SiRIC-Curie program (SiRIC Grant INCa-DGOS-465 and INCa-DGOS- Inserm_12554). L.P. team received support from ANR-11-LABX-0038 and ANR-10-IDEX-0001-02 and from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme 758170-Microbar. D.P. laboratory is supported by grants from FRM (AJE20181239593), ATIP-AVENIR, Fondation ARC (R19084KS—RSE20008KSA) and ‘Ville de Paris Emergence’ (R20192KK—RPH20192KKA).
Author information
Authors and Affiliations
Contributions
Conceptualization: Y.G.-F. and J.H.; methodology: Y.G.-F., J.C., L.P., D.P., E.P. and J.H.; formal analysis: J.C., Y.M.-K., A.D.-S., M.D., K.S. and S.L.; investigation: Y.G.-F., J.C., Y.M.-K. and J.H.; resources: C.S., S.B., M.P.-A., N.B.-V., P.G., L.P., D.P., E.P. and J.H.; writing original draft: Y.G.-F. and J.H. Writing, review and editing: Y.G.-F., J.C., L.P., D.P., E.P. and J.H.; visualization: Y.G.-F., J.C., Y.M.-K., A.D.-S. and J.H.; supervision: D.P., L.P., E.P. and J.H.; funding acquisition: E.P. and J.H.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cell Biology thanks Iannis Aifantis and Su Liu for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Breast tumors remotely rewire HSPC differentiation towards myelopoiesis in various breast cancer models.
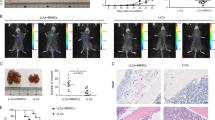
a, Number of EdU+ cycling LSK cells in the BM of WT (n = 6) or PyMT mice (n = 6, pool of 2 experiments). b, Lin−Sca1+Kit+ cell frequency and cell number in tumor-free (n = 9) or Py230 tumor-bearing mice (n = 9, pool of 2 experiments) and Neu mice (n = 6, one experiment). c, Representative pictures of femurs from tumor-free (n = 3) and Py230 (n = 3) or Neu mice (n = 3). d, e, Representative tissue histology of humerus sections from WT (d) and PyMT mice (e) stained with hematoxylin and eosin (performed twice on 2 WT and 2 PyMT mice). Epiphysis of humerus are a 4.6x zoom with 200μM scale bar. 40x zoom with a 20μM scale bar images from the endosteum and the BM of the same bone are also depicted. f, Total count of live cells in the BM of WT (n = 10) and PyMT mice (n = 11, pool of 4 experiments). g, Correlation between tumor weight and LSK cell number in the BM of PyMT (n = 9, pool of 3 experiments) and Py230 tumor-bearing mice (n = 7, pool of 2 experiments). h, i, Number of LSK cells at different tumor development stages in PyMT (h) or Py230 tumor-bearing mice (i). j, Cell number of CD45+Lin(CD19, NKp46, CD3, B220)- myeloid and Lin− lymphoid cells in the blood of tumor-free (n = 7), Py230 tumor-bearing (n = 7, pool of 2 experiments), WT (n = 5) and Neu (n = 4, one experiment) mice. k, l, Blood cell number of Lin-CD11b+Ly6G+ Neutrophils (k) and CD11b+Ly6G−Ly6CHi Monocytes (l) in WT (n = 7) and PyMT (n = 8, pool of 3 experiments), tumor-free (n = 7) and Py230 tumor-bearing mice (n = 7, pool of 2 experiments) or WT (n = 5) and Neu mice (n = 4, one experiment). m, n, Cell number of CD45+Lin(CD19, NKp46, CD3, B220)- myeloid and Lin− lymphoid cells in the blood of WT and PyMT mice (n = 6, pool of 2 experiments) (m) or tumor-free (n = 9, pool of 3 experiments) and Py230 tumor-bearing (n = 5 for 100mm3 and n = 3 for 500mm3 and 1500mm3) (n) at different tumor development stages. Data are the mean ± s.d. P value < 0.05 are depicted and were obtained using two-tailed t-test (a, b, f, k and l) corrected for multiple comparison using Holm-Šídák method (h, j and m), one-way Anova (i), two-way Anova (n) or simple linear regression (g).
Extended Data Fig. 2 Transcriptional rewiring of HSPCs in the BM during breast cancer.
a, Heatmap showing normalized expression of the top 10 most variable genes for each cluster (see Fig. 1H). b, Violin plots showing expression distributions of Meis1, Myl10, Gata1, Elane, Mpo, Dntt, Mki67, Top2a. c, UMAP showing the expression of HSC, MPP2, MPP3 and MPP4 gene signatures from Wilson et al., 2008 and Pietras et al., 2015. Gradient shows the background corrected mean expression of all genes in the signature, calculated using AddModuleScore function. d, Violin plots showing expression distribution of the PyMT response signature (upregulated DEG between PyMT and WT LSK) across clusters (see Fig. 1h). e, Frequency of WT and PyMT-derived cells in each cluster. f, Gating strategy to define LSK cell subsets by FACS. g, LSK cell subsets frequency in tumor-free (n = 9) or Py230 tumor-bearing mice (n = 9, pool of 2 experiments) and Neu mice (n = 6, one experiment). h, Frequencies and cell number of quiescent (TMRELow) and active (TMREHigh) HSCsLT in tumor-free (n = 3) or Py230 tumor-bearing mice (n = 3, one experiment), measured by FACS using TMRE staining. i, UMAP showing isolation of WT and PyMT G2M/S cycling cells from LSK scRNAseq dataset. Cell cycle was assigned based on gene expression profiles using the cyclone method in the scran package. j, UMAP showing the expression of MPP2, MPP3 and MPP4 gene signatures from Pietras et al., 2015 among cycling cells. k, Cell number-normalized density plot showing the cell distribution across the cycling cell dataset. l, Bone marrow cells were enriched for hematopoietic progenitor and stained with EdU to assess cell cycling activity. Frequency of EdU+LSK cell subsets by FACS (n = 6 WT and. 6 PyMT mice, pool of 2 experiments). Data are the mean ± s.d. P value < 0.05 are depicted and were obtained using two-tailed t-test (g, h, and l).
Extended Data Fig. 3 HSCsLT of tumor-bearing mice are myeloid-primed.
a, Frequency and cell number of total CD11b+ cells obtained after in vitro HSCLT myeloid differentiation from WT and PyMT mice (see Fig. 2a). Dots represent individual replicate wells (n = 9 mice, pool of 4 experiments). b, Total cell number obtained after in vitro HSCLT myeloid differentiation from tumor-free or Py230 tumor-bearing mice (n = 3, technical triplicates for each individual mouse, pool of 2 experiments). c, Frequency and cell numbers of Ly6G+CD11bint cells obtained after in vitro HSCLT myeloid differentiation from tumor-free or Py230 tumor-bearing mice (n = 3, triplicates for each individual mouse, pool of 2 experiments). d, e, Frequency of CD45.2+ donor-derived cells among TCRß+ T cells after the #1 transplant (d)(n = 17 WT and 12 PyMT mice at 2 and 4 weeks, n = 9 WT and 7 PyMT mice at 8 and 12 weeks, n = 16 WT and 12 PyMT mice at 16 weeks, pool of 2 experiments) and after the #2 transplant (e)(n = 14 WT and 11 PyMT mice at 2 weeks, n = 14 WT and 12 PyMT mice at 4, 8, 12 and 16 weeks, pool of 2 experiments) of HSCsLT from WT or PyMT mice in irradiated CD45.1+ recipients. Data are the mean ± s.d. P value < 0.05 are depicted and were obtained using two-tailed t-test (a, b, and c) corrected for multiple comparison using Holm-Šídák method (d and e).
Extended Data Fig. 4 Transcriptional rewiring of MSCs during breast cancer.
a, Concentration of OPG in the serum of WT or PyMT mice at different tumor development stages (n = 3 mice of 8, 12, 18 and 22w old and n = 4 mice of 24w old, pool of 2 experiments). b, Concentration of osteogenic factors in the serum of tumor-free (n = 5) or Py230 tumor-bearing mice (n = 4 for ANGPT2, n = 5 for OPG and WISP1, pool of 2 experiments). c, Concentration of OPG (n = 3 independent experiments) and WISP1 (n = 2 independent experiments) in PyMT tumor-conditioned media or Py230 cell line cultured media (n = 2 independent experiments). d, Concentration of osteogenic factors in the BM fluid of tumor-free (n = 5) and Py230 tumor-bearing mice (n = 5, pool of 2 experiments). e, Quantification of CD31−CD51+ stromal cells in tumor-free (n = 10) or Py230 tumor-bearing mice (n = 10, pool of 2 experiments). f, Heatmap of selected DEGs between clusters to identify cell subsets. g, Number of DEGs between PyMT and WT cells with adjusted p value < 0.05 for each cluster. h, Top5 pathways enriched in WT (grey) and PyMT (blue) MSCs according to REACTOME database. Data are the mean ± s.d. P value < 0.05 are depicted and were obtained using two-tailed t-test (a, b, d, and e).
Extended Data Fig. 5 Breast tumors remodel the BM stroma.
a, UMAP showing the expression of Vcam1, Cd200 and Pdpn by niche subsets analyzed by scRNAseq. b, FACS gating strategy of niche subsets to identify: CD31+VCAM1+CD200Low SECs, CD31+VCAM1−CD200High AECs, CD51+VCAM1+ MSCs, CD51+VCAM1−PDPN+ fibroblasts, CD51+VCAM1−PDPN−CD200+ osteoblasts. c, d, e, Frequency of BM CD31+ and CD31−CD51+ niche subsets analyzed by FACS in (c) WT and PyMT mice at different tumor development stages: 8-week-old (n = 5, pool of 2 experiments), 16-week-old (n = 4, pool of 2 experiments) and older than 20 weeks (n = 10 WT and 13 PyMT mice, pool of 4 experiments) (d) tumor-free (n = 3) and Py230 tumor-bearing mice (n = 3 at 500 mm3 and 1500 mm3 and n = 4 at 100 mm3, one experiment) and (e) WT (n = 5) or Neu mice older than 20 weeks (n = 4, one experiment). f, Principal component (PC) analysis using the top 1500 most variable genes of 10 000 sorted MSCs from tumor-free (grey) or Py230 tumor-bearing mice (orange) analyzed through bulk RNA sequencing (n = 4). Volcano plot showing the DEGs from MSCs of tumor-free and Py230 tumor-bearing mice. g, VCAM1 geometric mean of fluorescence intensity on MSC of control (n = 8) and PyMT (n = 11, pool of 4 experiments) or Py230 tumor-bearing mice (n = 10, pool of 2 experiments) or WT (n = 5) and Neu mice (n = 4, one experiment) assessed by FACS. h-i, The distance of each HSCLT from niche cells was calculated as described. The spot function was used to identify HSCLT based on the expression of CD150 and Lin. Volumetric iso-surfaces to define the bone and MSCs were generated using the intensity derived from the Col1 and LepR. The distance between each HSCLT and the closest surface pixel was then measured using ‘Distance Transformation’ tool using Imaris XT extension. h, Left plot: quantification of HSCLT distances to Col1+ bone in tumor-free (n = 1; HSCLT = 446) and Py230 tumor-bearing mice (n = 1; HSCLT = 236). Right plot: quantification of HSCLT distances to Col1+ bone in tumor-free (n = 2; HSCLT = 443) and PyMT tumor-bearing mice (n = 1; HSCLT = 613). i, Quantification of HSCLT distances to MSCs in tumor-free (n = 2; HSCLT = 443) and PyMT tumor-bearing mice (n = 1; HSCLT = 613). Data are the mean ± s.d. P value < 0.05 are depicted and were obtained using two-tailed t-test (d, e, and g) or two-tailed Mann-Whitney U test (c).
Supplementary information
Source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gerber-Ferder, Y., Cosgrove, J., Duperray-Susini, A. et al. Breast cancer remotely imposes a myeloid bias on haematopoietic stem cells by reprogramming the bone marrow niche. Nat Cell Biol 25, 1736–1745 (2023). https://doi.org/10.1038/s41556-023-01291-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41556-023-01291-w