Abstract
Epigenetic dysregulation is a prominent feature in cancer, as exemplified by frequent mutations in chromatin regulators, including the MLL/KMT2 family of histone methyltransferases. Although MLL1/KMT2A activity on H3K4 methylation is well documented, their non-canonical activities remain mostly unexplored. Here we show that MLL1/KMT2A methylates Borealin K143 in the intrinsically disordered region essential for liquid–liquid phase separation of the chromosome passenger complex (CPC). The co-crystal structure highlights the distinct binding mode of the MLL1 SET domain with Borealin K143. Inhibiting MLL1 activity or mutating Borealin K143 to arginine perturbs CPC phase separation, reduces Aurora kinase B activity, and impairs the resolution of erroneous kinetochore–microtubule attachments and sister-chromatid cohesion. They significantly increase chromosome instability and aneuploidy in a subset of hepatocellular carcinoma, resulting in growth inhibition. These results demonstrate a non-redundant function of MLL1 in regulating inner centromere liquid condensates and genome stability via a non-canonical enzymatic activity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Previously published data that were re-analysed here are available under accession codes GSE57957, GSE22058, GSE25097, GSE76297 and GSE112790 (refs. 34,35,36,37,38). The crystal structure model has been submitted to the PDB under accession code 7U5V (https://doi.org/10.2210/pdb7u5v/pdb). The human data were derived from the TCGA Research Network (http://cancergenome.nih.gov/). HCC patient aneuploidy and mRNA expression profiles are available from the GDC Data Portal (https://portal.gdc.cancer.gov) with accession no. phs000178. CRISPR Public 20Q1 pre-computed associations were generated in DepMap portal (http://depmap.org/portal). Full lists of MS data for the SILAC experiments are provided in Supplementary Tables 1–4. All original gel and microscopic images are deposited to Mendeley and are publicly available at https://doi.org/10.17632/jx3b22f6ft.1. Unprocessed gel images and all data presented in the graphs are available as source data files. All other data supporting the findings of this study are available from the corresponding author on reasonable request. Source data are provided with this paper.
Code availability
This paper does not report original code.
References
Rao, R. C. & Dou, Y. Hijacked in cancer: the KMT2 (MLL) family of methyltransferases. Nat. Rev. Cancer 15, 334–346 (2015).
Lauberth, S. M. et al. H3K4me3 interactions with TAF3 regulate preinitiation complex assembly and selective gene activation. Cell 152, 1021–1036 (2013).
Calo, E. & Wysocka, J. Modification of enhancer chromatin: what, how and why? Mol. Cell 49, 825–837 (2013).
Tanny, J. C. Chromatin modification by the RNA polymerase II elongation complex. Transcription 5, e988093 (2014).
Wang, H. et al. H3K4me3 regulates RNA polymerase II promoter-proximal pause-release. Nature 615, 339–348 (2023).
Sims, R. J. III et al. Recognition of trimethylated histone H3 lysine 4 facilitates the recruitment of transcription postinitiation factors and pre-mRNA splicing. Mol. Cell 28, 665–676 (2007).
Yan, J. et al. Histone H3 lysine 4 monomethylation modulates long-range chromatin interactions at enhancers. Cell Res. 28, 204–220 (2018).
Sun, Y. et al. HOXA9 reprograms the enhancer landscape to promote leukemogenesis. Cancer Cell 34, 643–658 (2018).
Dorighi, K. M. et al. Mll3 and Mll4 facilitate enhancer RNA synthesis and transcription from promoters independently of H3K4 monomethylation. Mol. Cell 66, 568–576.e4 (2017).
Milne, T. A. et al. MLL targets SET domain methyltransferase activity to Hox gene promoters. Mol. Cell 10, 1107–1117 (2002).
Wang, P. et al. Global analysis of H3K4 methylation defines MLL family member targets and points to a role for MLL1-mediated H3K4 methylation in the regulation of transcriptional initiation by RNA polymerase II. Mol. Cell. Biol. 29, 6074–6085 (2009).
Douillet, D. et al. Uncoupling histone H3K4 trimethylation from developmental gene expression via an equilibrium of COMPASS, Polycomb and DNA methylation. Nat. Genet. 52, 615–625 (2020).
Yu, B. D., Hess, J. L., Horning, S. E., Brown, G. A. & Korsmeyer, S. J. Altered Hox expression and segmental identity in Mll-mutant mice. Nature 378, 505–508 (1995).
Glaser, S. et al. The histone 3 lysine 4 methyltransferase, Mll2, is only required briefly in development and spermatogenesis. Epigenetics Chromatin 2, 5 (2009).
Lee, J. et al. Targeted inactivation of MLL3 histone H3-Lys-4 methyltransferase activity in the mouse reveals vital roles for MLL3 in adipogenesis. Proc. Natl Acad. Sci. USA 105, 19229–19234 (2008).
Xu, J. et al. Subtype-specific 3D genome alteration in acute myeloid leukaemia. Nature 611, 387–398 (2022).
Carmena, M., Wheelock, M., Funabiki, H. & Earnshaw, W. C. The chromosomal passenger complex (CPC): from easy rider to the godfather of mitosis. Nat. Rev. Mol. Cell Biol. 13, 789–803 (2012).
Vader, G., Medema, R. H. & Lens, S. M. The chromosomal passenger complex: guiding Aurora-B through mitosis. J. Cell Biol. 173, 833–837 (2006).
Trivedi, P. & Stukenberg, P. T. A centromere-signaling network underlies the coordination among mitotic events. Trends Biochem. Sci. 41, 160–174 (2016).
Hindriksen, S., Meppelink, A. & Lens, S. M. A. Functionality of the chromosomal passenger complex in cancer. Biochem. Soc. Trans. 43, 23–32 (2015).
Trivedi, P. et al. The inner centromere is a biomolecular condensate scaffolded by the chromosomal passenger complex. Nat. Cell Biol. 21, 1127–1137 (2019).
Poppe, B. et al. Expression analyses identify MLL as a prominent target of 11q23 amplification and support an etiologic role for MLL gain of function in myeloid malignancies. Blood 103, 229–235 (2004).
The Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature 474, 609–615 (2011).
The Cancer Genome Atlas Research Network. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N. Engl. J. Med. 372, 2481–2498 (2015).
Moore, K. E. et al. A general molecular affinity strategy for global detection and proteomic analysis of lysine methylation. Mol. Cell 50, 444–456 (2013).
Klein, U. R., Nigg, E. A. & Gruneberg, U. Centromere targeting of the chromosomal passenger complex requires a ternary subcomplex of Borealin, Survivin, and the N-terminal domain of INCENP. Mol. Biol. Cell 17, 2547–2558 (2006).
Southall, S. M., Wong, P.-S., Odho, Z., Roe, S. M. & Wilson, J. R. Structural basis for the requirement of additional factors for MLL1 SET domain activity and recognition of epigenetic marks. Mol. Cell 33, 181–191 (2009).
Kroschwald, S., Maharana, S. & Simon, A. Hexanediol: a chemical probe to investigate the material properties of membrane-less compartments. Matters https://doi.org/10.19185/matters.201702000010 (2017).
Jain, A. & Vale, R. D. RNA phase transitions in repeat expansion disorders. Nature 546, 243–247 (2017).
Nishiyama, T., Sykora, M. M., Huis in ‘t Veld, P. J., Mechtler, K. & Peters, J.-M. Aurora B and Cdk1 mediate Wapl activation and release of acetylated cohesin from chromosomes by phosphorylating Sororin. Proc. Natl Acad. Sci. USA 110, 13404–13409 (2013).
Ali, A., Veeranki, S. N., Chinchole, A. & Tyagi, S. MLL/WDR5 complex regulates Kif2A localization to ensure chromosome congression and proper spindle assembly during mitosis. Dev. Cell 41, 605–622.e7 (2017).
Gassmann, R. et al. Borealin: a novel chromosomal passenger required for stability of the bipolar mitotic spindle. J. Cell Biol. 166, 179–191 (2004).
Tao, Z., Cui, Y., Xu, X. & Han, T. FGFR redundancy limits the efficacy of FGFR4-selective inhibitors in hepatocellular carcinoma. Proc. Natl Acad. Sci. USA 119, e2208844119 (2022).
Burchard, J. et al. microRNA-122 as a regulator of mitochondrial metabolic gene network in hepatocellular carcinoma. Mol. Syst. Biol. 6, 402 (2010).
Sung, W.-K. et al. Genome-wide survey of recurrent HBV integration in hepatocellular carcinoma. Nat. Genet. 44, 765–769 (2012).
Chaisaingmongkol, J. et al. Common molecular subtypes among Asian hepatocellular carcinoma and cholangiocarcinoma. Cancer Cell 32, 57–70 (2017).
Shimada, S. et al. Comprehensive molecular and immunological characterization of hepatocellular carcinoma. EBioMedicine 40, 457–470 (2019).
Mah, W.-C. et al. Methylation profiles reveal distinct subgroup of hepatocellular carcinoma patients with poor prognosis. PLoS ONE 9, e104158 (2014).
Sheltzer, J. M. A transcriptional and metabolic signature of primary aneuploidy is present in chromosomally unstable cancer cells and informs clinical prognosis. Cancer Res. 73, 6401–6412 (2013).
Santaguida, S. & Amon, A. Short- and long-term effects of chromosome mis-segregation and aneuploidy. Nat. Rev. Mol. Cell Biol. 16, 473–485 (2015).
Milne, T. A. et al. Menin and MLL cooperatively regulate expression of cyclin-dependent kinase inhibitors. Proc. Natl Acad. Sci. USA 102, 749–754 (2005).
Tyagi, S., Chabes, A. L., Wysocka, J. & Herr, W. E2F activation of S phase promoters via association with HCF-1 and the MLL family of histone H3K4 methyltransferases. Mol. Cell 27, 107–119 (2007).
Blobel, G. A. et al. A reconfigured pattern of MLL occupancy within mitotic chromatin promotes rapid transcriptional reactivation following mitotic exit. Mol. Cell 36, 970–983 (2009).
Kelly, A. E. et al. Survivin reads phosphorylated histone H3 threonine 3 to activate the mitotic kinase Aurora B. Science 330, 235–239 (2010).
Dai, J., Sultan, S., Taylor, S. S. & Higgins, J. M. G. The kinase haspin is required for mitotic histone H3 Thr 3 phosphorylation and normal metaphase chromosome alignment. Genes Dev. 19, 472–488 (2005).
Wang, F. et al. Histone H3 Thr-3 phosphorylation by Haspin positions Aurora B at centromeres in mitosis. Science 330, 231–235 (2010).
Trivedi, P. & Stukenberg, P. T. A condensed view of the chromosome passenger complex. Trends Cell Biol. 30, 676–687 (2020).
Saito, M. et al. Acetylation of intrinsically disordered regions regulates phase separation. Nat. Chem. Biol. 15, 51–61 (2019).
Hofweber, M. et al. Phase separation of FUS is suppressed by its nuclear import receptor and arginine methylation. Cell 173, 706–719.e13 (2018).
Zeitlin, S. G., Barber, C. M., Allis, C. D. & Sullivan, K. F. Differential regulation of CENP-A and histone H3 phosphorylation in G2/M. J. Cell Sci. 114, 653–661 (2001).
Welburn, J. P. I. et al. Aurora B phosphorylates spatially distinct targets to differentially regulate the kinetochore-microtubule interface. Mol. Cell 38, 383–392 (2010).
Hadders, M. A. et al. Untangling the contribution of Haspin and Bub1 to Aurora B function during mitosis. J. Cell Biol. 219 e201907087 (2020).
Park, S. H. et al. Cryo-EM structure of the human MLL1 core complex bound to the nucleosome. Nat. Commun. 10, 5540 (2019).
Dou, Y. et al. Regulation of MLL1 H3K4 methyltransferase activity by its core components. Nat. Struct. Mol. Biol. 13, 713–719 (2006).
Kabsch, W. XDS. Acta Crystallogr. D 66, 125–132 (2010).
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010).
Slaughter, B. D. et al. Non-uniform membrane diffusion enables steady-state cell polarization via vesicular trafficking. Nat. Commun. 4, 1380 (2013).
Bekier, M. E., Mazur, T., Rashid, M. S. & Taylor, W. R. Borealin dimerization mediates optimal CPC checkpoint function by enhancing localization to centromeres and kinetochores. Nat. Commun. 6, 6775 (2015).
Smyth, G. K. in Bioinformatics and Computational Biology Solutions Using R and Bioconductor 397–420 (Springer, 2005).
Schwarzer, G. & Schwarzer, M. G. Package ‘meta’, version 9 (The R Foundation for Statistical Computing, 2012).
Page, A. et al. Alcohol directly stimulates epigenetic modifications in hepatic stellate cells. J. Hepatol. 62, 388–397 (2015).
Broad, A. J., DeLuca, K. F. & DeLuca, J. G. Aurora B kinase is recruited to multiple discrete kinetochore and centromere regions in human cells. J. Cell Biol. 219, e201905144 (2020).
The Cancer Genome Atlas Research Network. Comprehensive and integrative genomic characterization of hepatocellular carcinoma. Cell 169, 1327–1341.e23 (2017).
Acknowledgements
This work is supported by NIGMS grant GM082856 to Y.D., NCI grant CA250329 to Y.D. and U.-S.C., as well as an NCI Cancer Center Shared Grant award P30CA014089 to Norris Comprehensive Cancer Center at the University of Southern California. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. We are thankful to P. Luu at USC CNI (Core Center of Excellence in Nano Imaging) for help with DeltaVision microscopy. We are grateful for the generous gifts of the phospho-antibodies against AurkB substrates from I. Cheeseman and J. DeLuca, the Huh-7 and PLC/PRF/5 HCC cell lines from K. Machida, the HeLa Accept #2 cell line as well as the pEM784 and pEM791 plasmids from R. Ohi, the pET28-ISB plasmid from P. Todd Stukenberg, and the pCXN2-MLL1 plasmid from A. Muntean.
Author information
Authors and Affiliations
Contributions
L.S. was responsible for the experimental design, execution, data analysis and writing of the paper. Z.Y. performed biochemical purification and in vitro enzymatic assays. S.A., H.O. and U.-S.C. solved the crystal structure. J.X. performed the SILAC experiment. S.K. helped with the animal experiment under the supervision of W.A. W.Y. performed patient data meta-analysis. J.Y. consulted on patient data analysis. H.-J.L. advised on the clinical data analysis. H.W. provided help with the development of the Borealin methylation reagents. Y.D. provided supervision for the overall research and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cell Biology thanks Jinrong Min and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
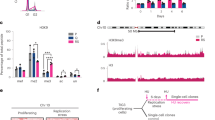
Extended Data Fig. 1 Borealin is a specific non-canonical substrate for MLL1.
(a) Schematics for the SILAC and quantitative mass spectrometry experiments. Two pair-wise Flag-tagged 3xMBT pulldown experiments were carried out. Venn diagram of proteins with > 2-fold enrichment in 3xMBT WT pulldown from Mll1 +/+ MEFs were shown (see text for details). (b) Silver stain of proteins eluted after GST-pulldown as indicated in (a). H3 or 3xMBT are indicated on right. (c) Quantitative RT-PCR for Borealin in WT or Mll1−/− MEF cells. Data from three independent biological replicates were presented after normalization against Gapdh. Expression of Borealin in WT MEF cells was arbitrarily set as 1. Data are presented as mean values +/− SEM. The p-value was calculated using two-tailed unpaired Student’s t-test. n.s, p > 0.05. (d) Coomassie stain of the purified recombinant ISB complex, as indicated on right. Imidazole concentration for elution was shown on top. This experiment is independently repeated five times with similar results. (e) Sequence alignment comparing human histone H3 flanking K4 and Borealin flanking K143 from indicated organisms. Human K143 and its corresponding counterparts are in red. Green rectangular indicates the highly conserved ARVK motif in mammals. (f) Dot-blot testing specificity of the anti-Borealin K143me1/2 antibody on increasing concentration of Borealin peptides (137-161aa) with 0, 1, or 2 methyl-groups as indicated on top. (g) Quantitative RT-PCR for relative expression level of MLL1 (KMT2A), KMT2D, EZH2, and G9a in HeLa cells treated with indicated shRNAs on bottom. Data from three independent biological replicates was presented after normalization against Gapdh. Expression of each gene in control shRNA transfected cells was arbitrarily set as 1. The p-value was calculated using two-tailed unpaired Student’s t-test. (h) Immunofluorescence for Borealin K143me1/2 in metaphase HeLa cells treated with indicated shRNAs on top. DNA was visualized by Hoechst. Scale bar, 5 μm. Representative images from three independent experiments were presented.
Extended Data Fig. 2 Establishing the WT and K143R Borealin HeLa cell lines.
(a) Immunoblot for HA-BorealinWT and Borealin143R after 3xMBTWT or 3xMBTD355N pulldown in mitotically synchronized 293T cells with or without transient expression of MLL1. Quantification of HA-Borealin in 3xMBTWT pulldown was shown on bottom. Data are presented as mean values +/− SEM. The p-value was calculated for n = 3 independent experiments using two-tailed unpaired Student’s t-test, n.s, p > 0.05. (b) Schematic representation of the Cre-recombinase-based knock-in system for generation of the inducible HA-Borealin-EGFP stable cell line. The recombination occurred via Lox2272 and Loxp site in both host cell line and donor plasmid. (c) Immunoblot for Borealin in HeLa cells expressing increasing amount of ectopic HA-BorealinWT or HA-BorealinK143R mutant. The cells were simultaneously treated with control or Borealin siRNA to remove endogenous Borealin as indicated on bottom. This experiment is independently repeated three times with similar results. (d) Immunoblots for proteins (indicated on right) from HA-BorealinWT or HA-BorealinK143R IP in mitotically synchronized 293T cells. This experiment is independently repeated two times with similar results. (e) Immunofluorescence for ACA or HA-Borealin in WT or K143R HeLa cells at different mitotic stages as indicated on top. DNA was visualized by Hoechst. Scale bar, 10 μm. This experiment is independently repeated three times with similar results.
Extended Data Fig. 3 MLL1 regulates CPC phase separation.
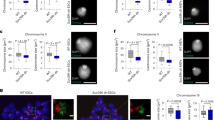
(a) Immunofluorescence for ACA and AurkB in HeLa cells treated with control or MLL1 siRNAs as indicated on left. Images were taken at various time points after NH4OAc washout. AurkB foci were extracted from the IF images as described in Methods. Enlarged insets show relative positions of AurkB foci and ACA. Representative images from three independent experiments were presented. Scale bar, 10 μm. (b) Quantification of AurkB foci in (a). Average results from n = 6 cells (30′ group) or n = 7 cells (120′ group) were presented. Standard deviation was presented as error bars. The p-values were calculated using two-tailed unpaired Student’s t-test. (c) Quantification of AurkB foci for HeLa cells expressing WT or V142W Borealin and treated as in (a). Average foci numbers from n = 12 cells were presented. Standard deviation was shown as error bar. The p value was calculated using two-tailed unpaired Student’s t-test. (d) Immunoblots with anti-MLL1C (~180KDa) antibody for indicated MEF lysates. The molecular weight markers were indicated on left. This experiment is independently repeated two times with similar results. (e) Immunofluorescence for ACA and AurkB in Mll1 set+/+ or Mll1 set∆/∆ MEFs. Images were taken at various time points after NH4OAc washout as treated in (a). Representative images from three independent experiments were presented. Scale bar, 10 μm. (f) Quantification of AurkB foci in (e). Average results from n = 10 cells were presented. Standard deviation was presented as error bar. The p-values were calculated using two-tailed unpaired Student’s t-test.
Extended Data Fig. 4 Borealin K143 methylation by MLL1 plays specific roles in faithful mitosis.
(a) Immunofluorescence for CENP-C and HA-Borealin on X-shaped mitotic chromosomes. Representative images from two independent experiments were presented. Scale bar, 1 μm. Distance was measured for 304 CENP-C pairs from 10 randomly selected Borealin WT cells as well as 423 pairs from 14 randomly selected K143R cells. (b) and (c) Quantification of mitotic phase distribution in HeLa cells treated with control or MLL1 siRNA (b) or in Borealin knockdown/rescue experiment (c). (d) Quantification of lagging chromosomes in anaphase HeLa cells treated with control or MLL1 siRNAs (left) or in Borealin knockdown/rescue experiment (right). For (b-d), average and standard deviation (error bars) from n = 3 (b, c, and d, right) or n = 5 (d, left) independent experiments were presented. At least 300 cells were analyzed for each group. The p-value was calculated using two-tailed unpaired Student’s t-test. n.s, p > 0.05. (e) Spindle assembly checkpoint (SAC) activation was analyzed for nocodazole-arrested HeLa cells treated with control, MLL1, or Borealin siRNA with or without ectopic Borealin. Average and standard deviation from 5 independent experiments were shown. The p-value was calculated using two-tailed unpaired Student’s t-test.n.s, p > 0.05. (f) Left, representative mitotic spreads from HeLa cells expressing ectopic WT or K143R Borealin. Scale bar, 2 μm. Right, chromosome numbers were quantified as multiples of 23. The dark line in the violin plot indicates mean value. Top and bottom dashed light blue lines represent 75% and 25% quantiles, respectively. The p-value was calculated using single-tailed F-test. (g) Growth curves of HeLa cells treated with control or Borealin siRNAs or Borealin knockdown cells rescued by ectopic WT or K143R Borealin. Average results from three independent experiments were presented. Standard deviation was shown as error bars. The p-value was calculated for Day 4 using two-tailed unpaired Student’s t-test. (h) Quantification of chromosome misalignment or spindle multipolarity in HeLa cell treated with Borealin siRNA and ectopically expressing WT or V142W Borealin. Average values from n = 3 independent experiments were presented. Standard deviations were presented as error bars. At least 300 cells in total were analyzed for each group. The p-value was calculated using two-tailed unpaired Student’s t-test.
Extended Data Fig. 5 MLL1 and its methyltransferase activity are indispensable for faithful mitosis.
(a) Top, schematics for MLL1 inhibition experiments. Bottom, quantification for chromosome misalignment or spindle multipolarity in HeLa cells treated with DMSO, MM589 or inactive MM599. (b) and (c) Representative images for chromosome misalignment (b) or spindle multipolarity (c) in Mll1 set+/+ or Mll1 set∆/∆ MEFs. Immunofluorescence for ACA (b) or αTubulin (c) was included. DNA was visualized by Hoechst. Scale bar, 10 μm. Quantification was shown on right. (d) Quantification of anaphase lagging chromosomes in Mll1 set+/+ or Mll1 set∆/∆ MEFs. For (a-d), average values from n = 5 independent experiments were presented. Standard deviations were presented as error bars. At least 400 cells in total were analyzed for each group. The p-values were calculated using two-tailed unpaired Student’s t-test. n.s, p > 0.05. (e) Immunoblots for MLL1 or KMT2B after siRNA treatments as indicated on top. Anti-actin was included as loading control. This experiment is independently repeated two times with similar results. (f) and (g) Representative images for chromosome misalignment (f) or spindle multipolarity (g) in HeLa cells treated with MLL1/KMT2A, KMT2B or both siRNA. Immunofluorescence for ACA (f) or α-Tubulin (g) were shown. DNA was visualized by Hoechst. Scale bar, 10 μm. Quantification for (f) and (g) is shown in (h) and (i), respectively. Average values from 5 independent experiments were presented. Standard deviations were presented as error bars. At least 400 cells in total were analyzed for each group. The p-values were calculated using two-tailed unpaired Student’s t-test. n.s., p > 0.05.
Extended Data Fig. 6 Borealin K143 methylation regulates AurkB activity at kinetochores.
(a-b) Immunofluorescence for CENPApS7 or H3pS10 in HeLa cells treated with control or MLL1 siRNAs (a) or in Borealin WT or K143R cells (b). Representative images from two independent experiments were presented. Scale bar, 10 μm. (c-f) Immunofluorescence for Hec1 pS44 (c), Dsn1 pS109 (d), Knl1 pS60 (e), and AurkB pT232 (f) as well as HA-Borealin in synchronized prometaphase WT or K143R cells as indicated on left. DNA was stained with Hoechst. Representative images from three independent experiments were presented. Scale bar, 10 μm. (g-j) Quantification of signals of respective phosphorylated proteins in (c-f) after normalizing against HA-Borealin. The graphs show the mean and S.D. A total of 15 cells were quantified per condition. Data are representative of two independent experiments. The p value was calculated using two-tailed unpaired Student’s t-test.
Extended Data Fig. 7 MLL1 enzymatic activity is required for AurkB activity at kinetochores.
(a-b) Immunofluorescence for AurkBpT232 (a) or Dsn1pS109 (b) in HeLa cells treated with siRNAs or inhibitors as indicated on top. DNA was visualized by Hoechst. The specific Dsn1pS109 signal was highlighted in the dashed rectangle. Scale bar, 10 μm. Quantification of fluorescence intensity for AurkBpT232 and Dsn1pS109 is shown on the right. Average intensity from n = 17 or 19 (siCon in a or b, repectively), n = 12 or 15 (siMLL1 in a or b, respectively), n = 20 or 14 (DMSO in a or b, respectively), n = 15 (ZM447439, MM-589, or MM-599 in both a and b) cells was presented for each condition. Standard deviation was presented as error bar. The p value was calculated by comparing to corresponding controls using two-tailed unpaired Student’s t-test. n.s, p > 0.05. (c) Immunofluorescence for AurkB pT232 (left) or Knl1 pS60 (right) in Mll1 set+/+ or Mll1 set∆/∆ MEFs. Centromere and DNA was visualized by ACA and Hoechst, respectively. Representative images from three independent experiments were presented. Scale bar, 10 μm. Quantification of signals of respective phosphorylated proteins is shown on the right. The line represents average from >120 kinetochore areas in 12 cells. The p value was calculated using two-tailed unpaired Student’s t-test. (d) Immunofluorescence for Dsn1 pS109 and Borealin in cells treated control, MLL1/KMT2A or KMT2B siRNAs. DNA was visualized by Hoechst. Representative images from two independent experiments were presented. Scale bar, 10 μm. Dashed rectangle was used to highlight specific Dsn1 pS109 signals.
Extended Data Fig. 8 MLL1 has potential functional interplay with Borealin in CPChigh HCC.
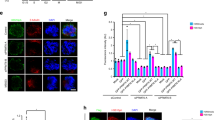
(a-c) Scatter plots of correlation coefficients and the corresponding P values for (a) MLL1 dependency vs Borealin expression level in 62 cancer types; (b) dependency of 139 epigenetic regulators vs Borealin expression level in HCC; (c) MLL1 dependency vs expression levels of 45 cell-cycle regulator genes from DepMap study. All correlation coefficients and their corresponding P values were derived from Spearman’s rank test and linear regression, respectively. (d) Meta-analyses for MLL1 expression in five independent datasets of human HCC (n = 743) and normal liver (n = 544) 34–38 as indicated on left. The expression level in normal samples is indicated by the line at 0. The vertical and horizontal lines represent point estimate and the 95% confidence interval of the study, respectively. The sample size of each study is represented by the box. Diamond shows the combined average point estimate and confidence interval of all studies. Detailed statistics was shown in Extended Data Table 3. (e) Immunohistochemistry for human HCC tissue microarray (TMA) using antibodies shown on the left. The samples were grouped based on MLL1 protein levels, with low (0–1) representing <25% MLL1+ cells, medium (2) representing 26–75% MLL1+ cells and high (3) representing >75% MLL1+ cells. (f) Box plot for Borealin positive cells in randomly selected areas. Six areas, 0.02 mm2 per area, were quantified from each tumor specimen. The central line represents median. The edges mark 75% and 25% quantiles and whiskers extend to minimum and maximum values. The p-values were determined for average Borealin positive cell numbers in n = 10 independent tumor specimens using one-way ANOVA with Tukey’s post hoc test. The F statistic is 140, and the p-value is 5.662 × 10−15. (g) Kaplan-Meier survival curve for HCC patients65 (n = 340) partitioned into two quantiles (top and bottom 50%) based on the expression of indicated genes. P-values were calculated by the log-rank test. (h) Scatter plot of MLL1 expression and aneuploidy scores of HCC tumors from TCGA HCC cohort with high CPC expression (n = 184/380). X axis represents TPM for Mll1 transcripts determined by the RSEM tool. Pearson correlation was performed to calculate P (two-tailed) and R values.
Extended Data Fig. 9 MLL1 is required for CPChigh HCC cell proliferation in vitro.
(a) Heatmap of Z-scores for expression of CPC components (top) in eight human HCC cell lines. Heatmap key was shown on right. Cell lines are divided into CPChigh (Huh-7, SNU-387, SNU-398, SNU-423) and CPClow (HepG2, PLC/PRF/5, SNU-475) subgroups according to the average Z-score of four components of CPC. An average Z-score above −0.05 is defined as CPChigh cell line. (b-d) Top, crystal violet stain of cell colonies of Huh-7 (b), SNU-475 (c), or PLC/PRF/5 (d) 72 hrs after control or MLL1 shRNA treatment. Representative images from three independent experiments were presented. Bottom, growth curves for Huh-7 (b), SNU-475 (c), or PLC/PRF/5 (d) cells treated with indicated shRNAs or compounds. Average results from three independent experiments were presented. Standard deviations were presented as error bars. The p-value was calculated two-tailed unpaired Student’s t-test for cells on Day 8 (Huh-7) or Day 9 (SNU-475 and PLC/PRF/5). n.s, p > 0.05. (e) Immunoblot for Borealin in SNU-398 cells expressing ectopic GFP-BorealinWT or GFP-BorealinK143R mutant. The cells were simultaneously treated with control or Borealin shRNA to remove endogenous Borealin as indicated on top. (f) Growth curves of SNU-398 cells expressing ectopic WT or K143R Borealin after depletion of endogenous Borealin. Average results from three independent experiments were presented. Standard deviations were shown as error bars. The p-value was calculated for Day 9 using two-tailed unpaired Student’s t-test.
Extended Data Fig. 10 MLL1 depletion does not affect tumor growth of CPClow HCC cell lines in vivo.
(a) Bioluminescence images of PLC/PRF/5 xenografts at Day1 and Day28 post subcutaneous injection. Control or MLL1 shRNA treatment was indicated on left. Heatmap key for bioluminescence intensity detected by IVIS Spectrum after GFP excitation is shown on the right. (b) Tumor xenografts isolated from mice at the experiment end point in Fig. 7c and Extended Data Fig. 10a, respectively. For shMLL1-treated SNU-398 cells, tumor formation was not observed and therefore shown as blank. Scale bar, 10mm. (c) Fold changes in PLC/PRF/5 xenograft tumor volume at various time points, respectively. Data were presented as average +/− SEM of six tumors in each group. P-values were calculated using two-tailed unpaired Student’s t-test for each data point. n.s., p > 0.05. (d) Kaplan-Meier survival curves of PLC/PRF/5 (n = 9 per group). Cells were treated with control or MLL1 shRNA before injection. Median survival were 31 days for control mice, and 31.5 days for shMLL1 mice. Experimental end point is declared when tumor volume reaches 1000 mm3. The p-value was determined by log-rank test. n.s., p > 0.05.
Supplementary information
Supplementary Information
Supplementary Figs. 1 and 2.
Supplementary Tables
Supplementary Tables 1 and 2. Full list of peptides and corresponding proteins identified by mass spectrometry from SILAC experiment with WT (light) 3xMBT pulldown as compared to D355N mutant (heavy) pulldown assay. Supplementary Tables 3 and 4. Full list of peptides and corresponding proteins identified by mass spectrometry from SILAC experiment of wild-type 3xMBT pulldown from WT (light) MEFs as compared to MLL1 KO (heavy) MEFs. Supplementary Table 5. List of 33 common proteins detected in two orthogonal SILAC experiments as in Fig. 1a. Supplementary Table 6. Statistics of data collection and refinement for structural model of co-crystallized MLL1SET and Borealin K143 peptide. Supplementary Table 7. List of correlation coefficients and corresponding P values used in Extended Data Fig. 8a–c. All correlation coefficients and P values were derived from Spearman’s rank test and linear regression, respectively. Supplementary Table 8. Statistics of differential expression of MLL1 in HCC tumours and control cohorts in five GEO datasets. Supplementary Table 9. Oligonucleotide sequences for siRNAs, shRNAs, and qRT-PCR primers used in this study.
Supplementary Data 1
A report produced by the wwPDB biocuration pipeline after annotation of the X-ray structure of co-crystallization of the MLL1 SET domain and Borealin peptide.
Source data
Source Data Fig. 1
Unprocessed autoradiographs/gels.
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Unprocessed WB/autoradiographs/gels.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Unprocessed gel.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 1
Unprocessed WB/gels.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Unprocessed WB.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Unprocessed WB.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Unprocessed WB.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Unprocessed WB.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sha, L., Yang, Z., An, S. et al. Non-canonical MLL1 activity regulates centromeric phase separation and genome stability. Nat Cell Biol 25, 1637–1649 (2023). https://doi.org/10.1038/s41556-023-01270-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41556-023-01270-1