Abstract
The killing function of cytotoxic T cells can be enhanced biochemically. Here we show that blocking the mechanical sensor PIEZO1 in T cells strengthens their traction forces and augments their cytotoxicity against tumour cells. By leveraging cytotoxic T cells collected from tumour models in mice and from patients with cancers, we show that PIEZO1 upregulates the transcriptional factor GRHL3, which in turn induces the expression of the E3 ubiquitin ligase RNF114. RNF114 binds to filamentous actin, causing its downregulation and rearrangement, which depresses traction forces in the T cells. In mice with tumours, the injection of cytotoxic T cells collected from the animals and treated with a PIEZO1 antagonist promoted their infiltration into the tumour and attenuated tumour growth. As an immunomechanical regulator, PIEZO1 could be targeted to enhance the outcomes of cancer immunotherapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. The RNA-seq data are available from the NCBI Sequence Read Archive, under accession number PRJNA956426. The CUT&Tag-seq data are available from the GEO, under accession number GSE236492. The previously published ChIP-seq data that we reanalysed for this study are available from GEO under accession codes GSM2293976, GSM1502001, GSM1423150, GSM1273545, GSM2035772, GSM1666386, GSM2035818 and GSM2498560. Source data are provided with this paper. All other data supporting the findings of this study are available from the corresponding authors on reasonable request.
References
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723 (2010).
Topalian, S. L. et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J. Clin. Oncol. 41, 943–954 (2023).
Rittmeyer, A. et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 389, 255–265 (2017).
Garon, E. B. et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 372, 2018–2028 (2015).
Chowell, D. et al. Improved prediction of immune checkpoint blockade efficacy across multiple cancer types. Nat. Biotechnol. 40, 499–506 (2022).
Prieto, P. A. et al. CTLA-4 blockade with ipilimumab: long-term follow-up of 177 patients with metastatic melanoma. Clin. Cancer Res. 18, 2039–2047 (2012).
Eroglu, Z. et al. Long term survival with cytotoxic T lymphocyte-associated antigen 4 blockade using tremelimumab. Eur. J. Cancer 51, 2689–2697 (2015).
Zhang, T. et al. Targeting the tumor biophysical microenvironment to reduce resistance to immunotherapy. Adv. Drug Deliv. Rev. 186, 114319 (2022).
Lei, K., Kurum, A. & Tang, L. Mechanical immunoengineering of T cells for therapeutic applications. Acc. Chem. Res. 53, 2777–2790 (2020).
Zhu, C., Chen, W., Lou, J., Rittase, W. & Li, K. Mechanosensing through immunoreceptors. Nat. Immunol. 20, 1269–1278 (2019).
Hu, K. H. & Butte, M. J. T cell activation requires force generation. J. Cell Biol. 213, 535–542 (2016).
Meng, K. P., Majedi, F. S., Thauland, T. J. & Butte, M. J. Mechanosensing through YAP controls T cell activation and metabolism. J. Exp. Med. 217, e20200053 (2020).
Basu, R. et al. Cytotoxic T cells use mechanical force to potentiate target cell killing. Cell 165, 100–110 (2016).
Tamzalit, F. et al. Interfacial actin protrusions mechanically enhance killing by cytotoxic T cells. Sci. Immunol. 4, eaav5445 (2019).
Lei, K. et al. Cancer-cell stiffening via cholesterol depletion enhances adoptive T-cell immunotherapy. Nat. Biomed. Eng. 5, 1411–1425 (2021).
Tello-Lafoz, M. et al. Cytotoxic lymphocytes target characteristic biophysical vulnerabilities in cancer. Immunity 54, 1037–1054.e7 (2021).
Liu, Y. et al. Cell softness prevents cytolytic T-cell killing of tumor-repopulating cells. Cancer Res. 81, 476–488 (2021).
Ma, S. et al. A role of PIEZO1 in iron metabolism in mice and humans. Cell 184, 969–982.e13 (2021).
Coste, B. et al. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature 483, 176–181 (2012).
Coste, B. et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330, 55–60 (2010).
Ranade, S. S. et al. Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature 516, 121–125 (2014).
Woo, S. H. et al. Piezo2 is the principal mechanotransduction channel for proprioception. Nat. Neurosci. 18, 1756–1762 (2015).
Atcha, H. et al. Mechanically activated ion channel Piezo1 modulates macrophage polarization and stiffness sensing. Nat. Commun. 12, 3256 (2021).
Aykut, B. et al. Targeting Piezo1 unleashes innate immunity against cancer and infectious disease. Sci. Immunol. 5, eabb5168 (2020).
Solis, A. G. et al. Mechanosensation of cyclical force by PIEZO1 is essential for innate immunity. Nature 573, 69–74 (2019).
Liu, C. S. C. et al. Cutting edge: Piezo1 mechanosensors optimize human T cell activation. J. Immunol. 200, 1255–1260 (2018).
Jairaman, A. et al. Piezo1 channels restrain regulatory T cells but are dispensable for effector CD4(+) T cell responses. Sci. Adv. 7, eabg5859 (2021).
Kefalakes, H. et al. Liver-resident bystander CD8(+) T cells contribute to liver disease pathogenesis in chronic hepatitis D virus infection. Gastroenterology 161, 1567–1583.e9 (2021).
Huang, J. et al. The kinetics of two-dimensional TCR and pMHC interactions determine T-cell responsiveness. Nature 464, 932–936 (2010).
Huppa, J. B. et al. TCR-peptide-MHC interactions in situ show accelerated kinetics and increased affinity. Nature 463, 963–967 (2010).
Qin, Q. et al. Lisa: inferring transcriptional regulators through integrative modeling of public chromatin accessibility and ChIP-seq data. Genome Biol. 21, 32 (2020).
Seetharaman, S. & Etienne-Manneville, S. Cytoskeletal crosstalk in cell migration. Trends Cell Biol. 30, 720–735 (2020).
Van Allen, E. M. et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science 350, 207–211 (2015).
Lánczky, A. & Győrffy, B. Web-based survival analysis tool tailored for medical research (KMplot): development and implementation. J. Med. Internet Res. 23, e27633 (2021).
Li, T. et al. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 48, W509–W514 (2020).
Li, T. et al. TIMER: a web server for comprehensive analysis of tumor-infiltrating immune cells. Cancer Res. 77, e108–e110 (2017).
Hope, J. M. et al. Fluid shear stress enhances T cell activation through Piezo1. BMC Biol. 20, 61 (2022).
Abiff, M. et al. Piezo1 facilitates optimal T cell activation during tumor challenge. Oncoimmunology 12, 2281179 (2023).
De Jesus, M. et al. Topographical analysis of immune cell interactions reveals a biomechanical signature for immune cytolysis. Preprint at bioRxiv https://doi.org/10.1101/2023.04.16.537078 (2023).
Geng, J. et al. TLR4 signalling via Piezo1 engages and enhances the macrophage mediated host response during bacterial infection. Nat. Commun. 12, 3519 (2021).
Huse, M. Mechanical forces in the immune system. Nat. Rev. Immunol. 17, 679–690 (2017).
Alon, R. & Dustin, M. L. Force as a facilitator of integrin conformational changes during leukocyte arrest on blood vessels and antigen-presenting cells. Immunity 26, 17–27 (2007).
Han, S. J., Oak, Y., Groisman, A. & Danuser, G. Traction microscopy to identify force modulation in subresolution adhesions. Nat. Methods 12, 653–656 (2015).
Plotnikov, S. V., Pasapera, A. M., Sabass, B. & Waterman, C. M. Force fluctuations within focal adhesions mediate ECM-rigidity sensing to guide directed cell migration. Cell 151, 1513–1527 (2012).
Basu, R. & Huse, M. Mechanical communication at the immunological synapse. Trends Cell Biol. 27, 241–254 (2017).
Fleire, S. J. et al. B cell ligand discrimination through a spreading and contraction response. Science 312, 738–741 (2006).
Wang, J. et al. Profiling the origin, dynamics, and function of traction force in B cell activation. Sci. Signal. 11, eaai9192 (2018).
Choi, H. K. et al. Catch bond models may explain how force amplifies TCR signaling and antigen discrimination. Nat. Commun. 14, 2616 (2023).
Zhao, X. et al. Tuning T cell receptor sensitivity through catch bond engineering. Science 376, eabl5282 (2022).
Mitchell, M. J. & King, M. R. Fluid shear stress sensitizes cancer cells to receptor-mediated apoptosis via trimeric death receptors. New J. Phys. 15, 015008 (2013).
Park, J. S. et al. Mechanical regulation of glycolysis via cytoskeleton architecture. Nature 578, 621–626 (2020).
Acknowledgements
We acknowledge the use of High-performance Computing Platform at the Centre for Bioinformatics, Institute of Basic Medical Sciences Chinese Academy of Medical Sciences (CAMS), School of Basic Medicine, Peking Union Medical College. The National Natural Science Foundation of China discloses support for the research described in this study by Y.L. (82341009, 92169101), Y.Y. (82073184) and C.X. (11972002). The Beijing Natural Science Foundation discloses support for the research by C.X. (Z200017). CAMS Innovation Fund for Medical Sciences discloses support for the research by Y.L. (2021-I2M-1-064) and J.L. (2021-I2M-1-066). The National High Level Hospital Clinical Research Funding discloses support for the research by Y.Y. (2022-PUMCH-A-071; CAMS Innovation Fund for Medical Sciences 2022-I2M-C&T-B-012). The State Key Laboratory Special Fund discloses support for the research by Y.L. (2060204).
Author information
Authors and Affiliations
Contributions
Y.L. conceived the project. Y.L., R.P., W.S., Y.Y., D. Wen, F.L. and D. Wang performed the experiments. Y.L., K.L., J.L. and C.X. developed the methodology. Y.L., R.P., W.S., D. Wen, Y.Y., F.L., D. Wang, J.L. and C.X. performed data analysis. Y.L., N.Z., J.L. and R.P. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Li Tang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
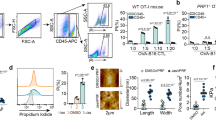
Extended Data Fig. 1 Inhibiting PIEZO1 activity strengthens T cell killing function.
a, b, The expression of Piezo1 from murine CD8+ T cells was analyzed by real-time PCR (a) or immunostained for PIEZO1 (b). Scale bar, 10 µm. c, d, The expression of PIEZO1 from murine CD8+ T cells upon activation by anti-CD3/CD28 beads for 48 hrs was detected by dSTORM (c) or flow cytometry (d). Scale bar, 5 µm. e, The expression of PIEZO1 from active and exhausted murine CD8+ T cells was determined by flow cytometry. f, Schematic of gating CD45− B16 tumor cells by flow cytometry. g, k, l, o, p, Anti-CD3/CD28 bead-activated OT-1 cytotoxic T cells (CTLs) pretreated with vehicle (Veh.) or GsMTx4 (1 µM) for 24 hrs were co-cultured with OVA-B16 cells at the effector/target (E/T) ratio of 2:1, 5:1 or 10:1 for different periods as indicated (24, 48 or 72 hrs). The tumor cell viability was measured by the Luminescent Cell Viability Assay (g). Flow cytometry analysis of the expression of TNF, IFN-γ (k), perforin, granzyme B (GzmB, l), CD107a (o) and FasL (p). h, Activated OT-1 CTLs pretreated with Vehicle (Veh.), Yoda1 (2 µM) or GsMTx4 (1 µM) for 24 hrs were co-cultured with OVA-MC38 cells at a ratio of 20:1 for 4 hrs. CD45− tumor cells apoptosis was determined by flow cytometry. i, j, Activated OT-1 CTLs were stimulated with fluid shear stress, squeezing force, hypertonic or hypotonic osmotic buffer. The intracellular Ca+ of CTLs was determined by flow cytometry (i). Some CTLs were co-cultured with OVA-B16 cells at the E/T ratio of 10:1 for 4 hrs. CD45− tumor cells apoptosis was determined by flow cytometry (j). m, Schematic of gating CD45+CD107a+ T cells by flow cytometry. n, Activated OT-1 CTLs were pretreated with Veh., Yoda1 (2 µM) or GsMTx4 (1 µM) for 24 hrs, and then co-cultured with OVA-MC38. Flow cytometry analysis of the expression of CD107a. q, Activated murine CD8+ T cells were treated with GsMTx4 (1 µM) for 24 hrs. Cell proliferation was determined by CCK8 kit at different time points as indicated. r, Activated murine CD8+ T cells were treated with Vehicle (Veh.) or GsMTx4 (1 µM) for 24 hrs. The expression of p-LCK and p-ZAP70 were determined by western blot. s, The expression of PIEZO1 in human CD8+ T cells from the BioGPS gene portal data. In (b-r), n = 3 independent experiments. Mann-Whitney test (c), two-tailed Student’s t-test (d and e), or one-way ANOVA followed by Bonferroni’s test (g, h, i, j, n and o). The data represent mean ± s.d.
Extended Data Fig. 2 The traction force exerted by T cells is enhanced by PIEZO1 blockade.
a, The traction force of CD8+ T cells on the polyacrylamide (PAA) gels with different stiffness, ranging from 0.3 kPa to 10.0 kPa. Scale bar, 5 µm. n = 60. b, The traction force of naive and activated CD8+ T cells from murine spleens (n = 111 for naive; n = 102 for active). Scale bar, 5 µm. c-g, Activated CD8+ T cells from murine spleens (c, d) or healthy donors (e, f, and g) were transfected with shRNAs targeting PIEZO1. The GFP+ T cells were sorted and the knockdown efficiency of PIEZO1 was confirmed by real-time PCR (c, e) or flow cytometry (d, f). In some experiments, sorted GFP+ T cells were performed the TFM (g, n = 100). Scale bar, 5 µm. h, Activated OT-1 CTLs were treated with fluid shear stress, squeezing force, hypertonic or hypotonic osmotic buffer. The traction force of CTLs was determined by TFM (n = 60). Scale bar, 5 µm. i, The traction force of activated OT-1 CTLs pretreated with Veh., cytochalasin D (1 µM) (n = 95). Scale bar, 5 µm. j, OT-1 CTLs were treated with cytochalasin D (1 µM), and then co-cultured with OVA-B16 cells. CD45− tumor cells apoptosis was determined by flow cytometry. Mann-Whitney test (b and i), two-tailed Student’s t-test (j) or one-way ANOVA followed by Bonferroni’s test (a, c-h). The data represent mean ± s.d.
Extended Data Fig. 3 Inhibiting PIEZO1 facilitates CTLs infiltration into tumor.
a, The expression of PIEZO1 in CD8+ T cells from tumor tissues or spleens was determined by flow cytometry. b-e, Representative gating procedures of CD45.1+TNF+ IFN-γ+ T cells (b), CD45.1+ PD-1+ T cells (c), CD45.1+CD107a+ T cells (d) and CD45.1+ T cells (e) from tumor tissues. f-i, Experimental design for CTLs transfer experiments (f). C57BJ/6 L mice bearing B16F10 tumor were administered with FTY720 (1 mg/kg) followed by adoptively transferred CD45.1+ Pmel-1 CTLs pretreated with vehicle or GsMTx4 (1 µM). Flow cytometry analysis of tumor-infiltrating CD45.1+ CTLs number (g, n = 6), the Ki67+ CTLs (h, n = 5) and the Annexin V−PI− CTLs (i, n = 6). j-n, CD45.1+ CTLs from Pmel-1 transgenic mice were stimulated as shown and transferred to B16F10-bearing mice (j). Flow cytometry analysis of CD45.1+ CTLs in tumors (k-n). The percentage of TNF+IFN-γ+ T cells (k), PD-1+, TIM3+ or LAG3+ T cells (l), CD107a+ T cells (m) and the number of CD45.1+ T cells in tumors (n). n = 5 mice. Two-tailed Student’s t-test (a, h, i, m and n), one-way ANOVA followed by Bonferroni’s test (g). The data represent mean ± s.d.
Extended Data Fig. 4 Inhibiting PIEZO1 suppresses tumor growth.
a-c,Pmel-1 CTLs were transferred into B16F10-luciferase bearing mice as indicated (a). Tumors were excised for the photograph (b), and quantified using in vivo fluorescence imaging system (c). N = 6; scale bar, 1 cm. d-f, Pmel-1 CTLs were transferred into B16F10-bearing mice as shown (d). The tumor growth was recorded (e, n = 8 for No transfer and CTLs/Veh.; n = 9 for CTLs/GsMTx4) and mouse survival was calculated (f, n = 10). g, h, Pmel-1 CTLs were activated as indicated and transferred to B16F10 bearing mice. Some mice were treated with GsMTx4 or anti-PD-1 antibodies (g). Tumor size was recorded (h, n = 10 for CTL/GsMTx4/aPD-1; n = 9 for the other groups). i, j, B16F10-bearing mice were treated with vehicle or GsMTx4 (0.8 mg/kg), and then the Gr-1+CD11b+ myeloid cells and CD4+CD25+FOXP3+ Treg cells in tumors were determined by flow cytometry (n = 6). k, l, B16F10-luciferase bearing mice were transferred with Pmel-1 CTLs pretreated with GsMTx4, and administered with anti-PD-1 antibody as indicated (k). The tumors were visualized by using an in vivo fluorescence imaging system (l), and then excised for the photograph (m). N = 6; scale bar, 1 cm. One-way ANOVA followed by Bonferroni’s test (e, h), log-rank test (f) or two-tailed student’s t-test (j). The data represent mean ± s.d. (i, j) or mean ± s.e.m. (e, h).
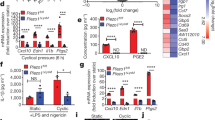
Extended Data Fig. 5 GRHL3 is identified as the downstream transcription factor in PIEZO1 pathway.
a, Pairwise spearman correlations of different treated groups transcriptional profiles. b, c, Activated CD8+ T cells from murine and healthy donors pretreated with Veh. or GsMTx4 (1 µM) for 24 hrs. The expression of GRHL3 was determined by real-time PCR (b) and western blot (c). d, CD8+ T cells from the peripheral blood (PB) of healthy donors activated with anti-CD3 and anti-CD28 antibodies were treated with Veh. or GsMTx4 (1 µM) for 24 hrs. T cells were immunostained for GRHL3. Scale bar, 5 µm. e-i, Activated CD8+ T cells from murine spleens (e, f) or healthy donors (g-i) were transfected with shRNAs targeting PIEZO1. The expression of GRHL3 was measured by real-time PCR (e, g), western blot (f, h) or immunofluorescent assay (i). j, Mice activated CD8+ T cells were transfected with shRNAs targeting Grhl3. The knockdown efficiency of Grhl3 was determined by western blot. k, Chromatin signatures at the Rnf114 locus in mouse skin cells. Promoters are shaded. l, Chromatin signatures at the RNF114 locus in human NHEK cells. Promoter are shaded. m, Chromatin signatures at the RNF114 locus in human CD8+ T cells. Promoter are shaded. In (b-k), n = 3 independent experiments. Two-tailed Student’s t-test (b), two-tailed Mann-Whitney test (d) or one-way ANOVA followed by Bonferroni’s test (e, g, and i). The data represent mean ± s.d.
Extended Data Fig. 6 T cells exert its traction force through PIEZO1-GRHL3-RNF114 pathway.
a, b, Activated CD8+ T cells from mice or healthy donors were treated with GsMTx4 (1 µM) for 24 hrs. The expression of RNF114 was determined by real-time PCR (a) and western blot (b). c, d, Activated CD8+ T cells from healthy donors were transfected with shRNAs targeting PIEZO1. The knockdown efficiency of RNF114 was determined by real-time PCR (c) and western blot (d). e, CD8+ T cells were treated with GsMTx4 for 24 hrs and then cultured in the absence of GsMTx4 for 24, 48, and 72 hrs. The expression of GRHL3 and RNF114 were determined by western blot. f, The localization of F-actin (green color) and RNF114 (magenta color) from CD8+ T cells was visualized by dSTORM microscope. White arrow indicated the sites of colocalization. g, Activated murine CD8+ T cells were transfected with shRNAs targeting Piezo1 and stained for F-actin. h, The immunostaining of F-actin from activated CD8+ T cells from mice treated with GsMTx4 (1 µM) for 24 hrs. i, Activated CD8+ T cells from healthy donors were transfected with shRNAs targeting RNF114. RNF114 expression was determined by western blot in sorted GFP+ T cells. j-l, GRHL3 or RNF114 was overexpressed upon PIEZO1 knockdown in Jurkat cells. The expression of PIEZO1, GRHL3 or RNF114 was determined by flow cytometry (j) or western blot (k, l). GRHL3 (S), short exposure time; GRHL3 (L), long exposure time. In (a-k), n = 3 independent experiments. In (f-h), scale bar, 5 µm. Two-tailed Student’s t-test (a) or one-way ANOVA followed by Bonferroni’s test (c, g, and j). The data represent mean ± s.d.
Extended Data Fig. 7 PIEZO1-GRHL3-RNF114 pathway regulates the traction force of T cells in cancer patients.
a, c, The mRNA expression of PIEZO1 (a) and GRHL3 (c) in CD8+ T cells from the PB of healthy donors (n = 6) and patients with stomach cancer (STAD, n = 6), hepatocellular carcinoma (HCC, n = 6), colorectal cancer (CRC, n = 10). b, The expression of PIEZO1 in CD8+ T cells from the PB of healthy donors (n = 5) and patients with colorectal cancer (CRC, n = 6) was determined by flow cytometry. d, Activated CD8+ T cells from the PB of healthy volunteers (n = 5) and patients with STAD (n = 6), CRC (n = 10) and HCC (n = 6) were immunostained for RNF114. Scale bar, 5 µm. e, The expression of GRHL3 and RNF114 in CD8+ T cells from the PB of healthy donors and patients with STAD, CRC and HCC were determined by western blot. f, The activated CD8+ T cells from CRC (n = 10) were treated with GsMTx4 (1 µM) for 24 hrs. GRHL3 mRNA level was determined by real-time PCR. g, l, m The activated CD8+ T cells from HCC (n = 6) patients were treated with GsMTx4 (1 µM) for 24 hrs. The expression of GRHL3 (g) or RNF114 (l) was determined by immunofluorescence assay. Some cells were performed the TFM (m, n = 100 cells from 6 patients). Scale bar, 5 µm. h, k, The activated CD8+ T cells from CRC (h, n = 3) or HCC (k, n = 3) were treated with GsMTx4 (1 µM) for 48 hrs. The expression of GRHL3 and RNF114 were determined by western blot. i, j, The mRNA expression of RNF114 in activated CD8+ T cells from the PB of CRC (n = 10) or HCC (n = 6) patients treated with GsMTx4 (1 µM) for 24 hrs. n, The same as (m), except that CD8+ T cells were from patients with STAD (n = 100 cells from 6 patients). One-way ANOVA followed by Bonferroni’s test (a, c, and d), Two-tailed Student’s t-test (b, f, g, i, j, and l) and two-tailed Mann-Whitney test (m and n). The data represent mean ± s.d.
Extended Data Fig. 8 GRHL3 or RNF114 expression is negatively correlated to both CD8+ T cell infiltration and overall survival in patients with multiple cancers.
a, Highly expressed GRHL3 shorten the overall survival (OS) in response to all immunotherapies in human cancers. b, c, Inverse correlation of the expression of GRHL3 with CD8+ T cells infiltration signature in patients with rectum adenocarcinoma (READ, n = 166), glioblastoma multiform (GBM, n = 153), lung squamous cell carcinoma (LUSC, n = 501) (b), skin cutaneous melanoma (SKCM-Primary, n = 471) and stomach adenocarcinoma (STAD, n = 415) (c). d, Correlation of a high RNF114 expression with comparatively poor prognosis for cancer patients in response to anti-CTLA4 therapy. e, Inverse correlation of the expression of RNF114 with CD8+ T cells infiltration signature in patients with stomach adenocarcinoma (STAD, n = 415), head and neck squamous cell carcinoma (HNSC, n = 522), prostate adenocarcinoma (PRAD, n = 498) and thymoma (THYM, n = 120). Log-rank test (a and d) or Pearson’s correlation test (b, c and e).
Supplementary information
Supplementary Information
Supplementary Tables 1–5.
Source data
Source Data for Extended Data Fig. 1
Unprocessed western blots.
Source Data for Extended Data Fig. 5
Unprocessed western blots.
Source Data for Extended Data Fig. 6
Unprocessed western blots.
Source Data for Extended Data Fig. 7
Unprocessed western blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pang, R., Sun, W., Yang, Y. et al. PIEZO1 mechanically regulates the antitumour cytotoxicity of T lymphocytes. Nat. Biomed. Eng (2024). https://doi.org/10.1038/s41551-024-01188-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41551-024-01188-5