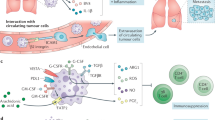
Abstract
Tumour-associated neutrophils can exert antitumour effects but can also assume a pro-tumoural phenotype in the immunosuppressive tumour microenvironment. Here we show that neutrophils can be polarized towards the antitumour phenotype by discoidal polymer micrometric ‘patches’ that adhere to the neutrophils’ surfaces without being internalized. Intravenously administered micropatch-loaded neutrophils accumulated in the spleen and in tumour-draining lymph nodes, and activated splenic natural killer cells and T cells, increasing the accumulation of dendritic cells and natural killer cells. In mice bearing subcutaneous B16F10 tumours or orthotopic 4T1 tumours, intravenous injection of the micropatch-loaded neutrophils led to robust systemic immune responses, a reduction in tumour burden and improvements in survival rates. Micropatch-activated neutrophils combined with the checkpoint inhibitor anti-cytotoxic T-lymphocyte-associated protein 4 resulted in strong inhibition of the growth of B16F10 tumours, and in complete tumour regression in one-third of the treated mice. Micropatch-loaded neutrophils could provide a potent, scalable and drug-free approach for neutrophil-based cancer immunotherapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. Raw bulk RNA-seq data for Fig. 3 and Extended Data Fig. 1 are available from NCBI Sequence Read Archive under accession number PRJNA1067075. Source data for tumour progression and survival data from Fig. 6 and Extended Data Figs. 4 and 5 are provided with this paper. All raw and analysed datasets generated during the study are available from the corresponding author on reasonable request.
References
Wang, L. L.-W. et al. Cell therapies in the clinic. Bioeng. Transl. Med. 6, e10214 (2021).
Ukidve, A. et al. Overcoming biological barriers to improve solid tumor immunotherapy. Drug Deliv. Transl. Res. 11, 2276–2301 (2021).
Rosenberg, S. A. et al. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat. Rev. Cancer 8, 299–308 (2008).
Larson, R. C. & Maus, M. V. Recent advances and discoveries in the mechanisms and functions of CAR T cells. Nat. Rev. Cancer 21, 145–161 (2021).
Laskowski, T. J., Biederstädt, A. & Rezvani, K. Natural killer cells in antitumour adoptive cell immunotherapy. Nat. Rev. Cancer 22, 557–575 (2022).
Masucci, M. T., Minopoli, M. & Carriero, M. V. Tumor associated neutrophils. their role in tumorigenesis, metastasis, prognosis and therapy. Front. Oncol. 9, 1146 (2019).
Coffelt, S. B., Wellenstein, M. D. & de Visser, K. E. Neutrophils in cancer: neutral no more. Nat. Rev. Cancer 16, 431–446 (2016).
Shaul, M. E. & Fridlender, Z. G. Tumour-associated neutrophils in patients with cancer. Nat. Rev. Clin. Oncol. 16, 601–620 (2019).
Fridlender, Z. G. et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: ‘N1’ versus ‘N2’ TAN. Cancer Cell 16, 183–194 (2009).
Andzinski, L. et al. Type I IFNs induce anti‐tumor polarization of tumor associated neutrophils in mice and human. Int. J. Cancer 138, 1982–1993 (2016).
Furumaya, C. et al. Plasticity in pro- and anti-tumor activity of neutrophils: shifting the balance. Front. Immunol. 11, 2100 (2020).
Giese, M. A., Hind, L. E. & Huttenlocher, A. Neutrophil plasticity in the tumor microenvironment. Blood 133, 2159–2167 (2019).
Clark, R. A. & Klebanoff, S. J. Neutrophil-mediated tumor cell cytotoxicity: role of the peroxidase system. J. Exp. Med. 141, 1442–1447 (1975).
Yee, P. P. et al. Neutrophil-induced ferroptosis promotes tumor necrosis in glioblastoma progression. Nat. Commun. 11, 5424 (2020).
Kotsafti, A. et al. Reactive oxygen species and antitumor immunity—from surveillance to evasion. Cancers 12, 1748 (2020).
Wang, X. et al. Understanding the multifaceted role of neutrophils in cancer and autoimmune diseases. Front. Immunol. 9, 2456 (2018).
Ohms, M., Möller, S. & Laskay, T. An attempt to polarize human neutrophils toward N1 and N2 phenotypes in vitro. Front. Immunol. 11, 532 (2020).
Matlung, H. L. et al. Neutrophils kill antibody-opsonized cancer cells by trogoptosis. Cell Rep. 23, 3946–3959.e6 (2018).
Ustyanovska Avtenyuk, N. et al. The neutrophil: the underdog that packs a punch in the fight against cancer. Int. J. Mol. Sci. 21, 7820 (2020).
Hirschhorn, D. et al. T cell immunotherapies engage neutrophils to eliminate tumor antigen escape variants. Cell 186, 1432–1447.e17 (2023).
Gungabeesoon, J. et al. A neutrophil response linked to tumor control in immunotherapy. Cell 186, 1448–1464.e20 (2023).
Linde, I. L. et al. Neutrophil-activating therapy for the treatment of cancer. Cancer Cell 41, 356–372.e10 (2023).
Quesada, J. R. et al. Clinical toxicity of interferons in cancer patients: a review. J. Clin. Oncol. 4, 234–243 (1986).
Xue, J. et al. Neutrophil-mediated anticancer drug delivery for suppression of postoperative malignant glioma recurrence. Nat. Nanotechnol. 12, 692–700 (2017).
Wu, M. et al. MR imaging tracking of inflammation-activatable engineered neutrophils for targeted therapy of surgically treated glioma. Nat. Commun. 9, 4777 (2018).
Abaricia, J. O. et al. Hydrophilic titanium surfaces reduce neutrophil inflammatory response and NETosis. Biomater. Sci. 8, 2289–2299 (2020).
Liu, L. et al. Surface-related triggering of the neutrophil respiratory burst. Characterization of the response induced by IgG adsorbed to hydrophilic and hydrophobic glass surfaces. Clin. Exp. Immunol. 109, 204–210 (1997).
Wang, Y. & Jönsson, F. Expression, role, and regulation of neutrophil fcγ receptors. Front. Immunol. 10, 1958 (2019).
Champion, J. A. & Mitragotri, S. Role of target geometry in phagocytosis. Proc. Natl Acad. Sci. USA 103, 4930–4934 (2006).
Pannuzzo, M. et al. Predicting the miscibility and rigidity of poly(lactic-co-glycolic acid)/polyethylene glycol blends via molecular dynamics simulations. Macromolecules 53, 3643–3654 (2020).
Kolaczkowska, E. & Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 13, 159–175 (2013).
Vogt, K. L., Summers, C., Chilvers, E. R. & Condliffe, A. M. Priming and de-priming of neutrophil responses in vitro and in vivo. Eur. J. Clin. Invest. 48, e12967 (2018).
Xie, Z. et al. Gene set knowledge discovery with Enrichr. Curr. Protoc. 1, e90 (2021).
Kuleshov, M. V. et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 44, W90–W97 (2016).
Chen, E. Y. et al. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinf. 14, 128 (2013).
Shaul, M. E. et al. Tumor-associated neutrophils display a distinct N1 profile following TGFβ modulation: a transcriptomics analysis of pro- vs. antitumor TANs. Oncoimmunology 5, e1232221 (2016).
Berraondo, P. et al. Cytokines in clinical cancer immunotherapy. Br. J. Cancer 120, 6–15 (2019).
Xiao, X. et al. Mechanisms of cytokine release syndrome and neurotoxicity of CAR T-cell therapy and associated prevention and management strategies. J. Exp. Clin. Cancer Res. 40, 367 (2021).
Lechner, M. G. et al. Immunogenicity of murine solid tumor models as a defining feature of in vivo behavior and response to immunotherapy. J. Immunother. 36, 477–489 (2013).
Martín-Fontecha, A. et al. Induced recruitment of NK cells to lymph nodes provides IFN-γ for TH1 priming. Nat. Immunol. 5, 1260–1265 (2004).
Buchbinder, E. I. & Desai, A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am. J. Clin. Oncol. 39, 98–106 (2016).
Bailey, S. R. et al. Four challenges to CAR T cells breaking the glass ceiling. Eur. J. Immunol. 53, 2250039 (2023).
Depil, S. et al. ‘Off-the-shelf’ allogeneic CAR T cells: development and challenges. Nat. Rev. Drug Discov. 19, 185–199 (2020).
Tong, L. et al. NK cells and solid tumors: therapeutic potential and persisting obstacles. Mol. Cancer 21, 206 (2022).
Habif, G. et al. Targeting natural killer cells in solid tumors. Cell. Mol. Immunol. 16, 415–422 (2019).
Heemskerk, N. et al. Augmented antibody-based anticancer therapeutics boost neutrophil cytotoxicity. J. Clin. Invest. 131, e134680 (2021).
Mantovani, A. et al. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 11, 519–531 (2011).
Gea-Banacloche, J. Granulocyte transfusions: a concise review for practitioners. Cytotherapy 19, 1256–1269 (2017).
Sharon, R. F. et al. Pre-emptive granulocyte transfusions enable allogeneic hematopoietic stem cell transplantation in pediatric patients with chronic infections. Bone Marrow Transplant. 37, 331–333 (2006).
Morishima, T. et al. Neutrophil differentiation from human-induced pluripotent stem cells. J. Cell. Physiol. 226, 1283–1291 (2011).
Saeki, K. et al. A feeder-free and efficient production of functional neutrophils from human embryonic stem cells. Stem Cells 27, 59–67 (2008).
Shields, C. W. et al. Cellular backpacks for macrophage immunotherapy. Sci. Adv. 6, eaaz6579 (2020).
Kapate, N. et al. A backpack-based myeloid cell therapy for multiple sclerosis. Proc. Natl Acad. Sci. USA 120, e2221535120 (2023).
Kapate, N. et al. Backpack-mediated anti-inflammatory macrophage cell therapy for the treatment of traumatic brain injury. PNAS Nexus 3, pgad434 (2023).
Wang, L. L.-W. et al. Preclinical characterization of macrophage-adhering gadolinium micropatches for MRI contrast after traumatic brain injury in pigs. Sci. Transl. Med. 16, eadk5413 (2024).
Prakash, S. et al. Polymer micropatches as natural killer cell engagers for tumor therapy. ACS Nano. 17, 15918–15930 (2023).
Mysore, V. et al. FcγR engagement reprograms neutrophils into antigen cross-presenting cells that elicit acquired anti-tumor immunity. Nat. Commun. 12, 4791 (2021).
Zheng, Y. et al. Enhancing adoptive cell therapy of cancer through targeted delivery of small-molecule immunomodulators to internalizing or noninternalizing receptors. ACS Nano. 11, 3089–3100 (2017).
Zhao, Z. et al. Systemic tumour suppression via the preferential accumulation of erythrocyte-anchored chemokine-encapsulating nanoparticles in lung metastases. Nat. Biomed. Eng. 5, 441–454 (2021).
Acknowledgements
We acknowledge R. Bronson of Rodent Histopathology Core at the Harvard Medical School for histological evaluation. We acknowledge the use of BioRender.com for schematic creation. We acknowledge the Harvard Center for Biological Imaging; the Allston Science and Engineering Complex’s Molecular and Cellular Biology Core; and the Harvard University Center for Nanoscale Systems (CNS), a member of the National Nanotechnology Coordinated Infrastructure Network (NNCI) supported by the National Science Foundation ECCS-2025158. Bulk RNA-seq was performed by Bauer Sequencing Core at Harvard University. We acknowledge funding from the John A. Paulson School of Engineering and Applied Sciences, and Wyss Institute for Biologically Inspired Engineering. N. Kapate was supported by the National Science Foundation Graduate Research Fellowship under grant number 1122374.
Author information
Authors and Affiliations
Contributions
N. Kumbhojkar and S.M. conceived the project and designed the experiments. N. Kumbhojkar, S.P., T.F., N. Kapate, R.A., S.D., V.C.S., K.S.P., A.P.G., M.M., M.G.B. and L.L.-W.W. performed the experiments. K.A.-B. and D.J.M designed and performed the RNA-seq data analysis. N. Kumbhojkar analysed data with co-authors. N. Kumbhojkar and S.M. wrote the paper with input from all the co-authors.
Corresponding author
Ethics declarations
Competing interests
N. Kumbhojkar, S.P., N. Kapate, L.L.-W.W. and S.M. are inventors of patent applications related to the technologies discussed in this work. These patents are owned and managed by Harvard University. S.M. declares the following interests: Hitch Bio, board member, equity; and Asalyxa, scientific advisory board (SAB), equity. D.J.M. declares the following competing interests: Novartis, sponsored research, licensed intellectual property; Immulus, equity; IVIVA, SAB; Attivare, SAB, equity; and Lyell, licensed intellectual property, equity. The other authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Xiaoyuan Chen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 The transcriptomic profile of the CAMP-treated neutrophils is altered.
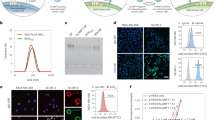
a, Volcano plot showing over 2000 differentially expressed genes between CAMP -ve NE and NE. Threshold P = 0.01. b, Significantly overexpressed pathways in CAMP -ve NE when compared to NE. Generated using EnrichR, focusing on MSigDB Hallmark 2020 database. c-d, Violin plots comparing aggregate expression distribution of genes related to IL-1 pathway (c) and IL-6-JAK-STAT3 pathway (d) according to MSigDB Hallmark 2020 database. Adhesion to CAMPs led to the increased aggregate expression of genes related to IL-1 and IL-6-JAK-STAT3 pathways. e-f, Violin Plots representing the expression of N2 related genes Arg-1 (e) and CD206 (f). Adhesion to CAMPs led to reduction in the expression of N2 related genes. Solid line within each violin represents median and dotted lines represent quartiles. Analysed using ordinary one-way ANOVA with Tukey’s multiple comparisons test.
Extended Data Fig. 2 Biodistribution of adoptively transferred neutrophils.
a, Experimental scheme for biodistribution in the B16F10 model. b, Representative IVIS images for organ accumulation of neutrophils in the B16F10 model. c, Experimental scheme for biodistribution in the 4T1-Luc model. d-f Biodistribution of untreated neutrophils (NE) and neutrophil-CAMPs (NE + CAMP) in the 4T1-Luc model at 24 (d), 48 (e) and 72 (f) hours after intravenous injections. n = 3 biological replicates. Data represented as mean ± SD. Adoptively transferred CAMP-treated neutrophils efficiently accumulated in the tumour, spleen, lungs, and liver of 4T1-Luc tumour bearing mice. g, IVIS images for tumour accumulation of neutrophils in the 4T1-Luc model at different timepoints. h, Kinetics of adoptively transferred neutrophils in the blood circulation as estimated by flow cytometry. Y axis represents %vivotrack680+ neutrophils of all neutrophils in circulation. n = 3 biological replicates. Data represented as mean ± SEM. Analysed by ordinary two-way ANOVA with Sidak’s multiple comparisons tests.
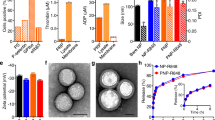
Extended Data Fig. 3 Treatment with CAMP-carrying neutrophils elicits a systemic immune response in the B16F10 model.
a, Experimental scheme for immunophenotyping. B16F10-tumour-bearing mice were treated with two doses of Saline, Neutrophil only (NE) or neutrophil-CAMPs (NE + CAMP). Blood, tumours, tumour-draining lymph nodes and spleens were harvested 48 hours after the second dose and analysed using flow cytometry. b, Neutrophil (%Ly6G+ Ly6C+ of CD45+) accumulation in the spleen was higher in the NE + CAMP group. c, Neutrophil accumulation in the tumour-draining lymph nodes was higher in the NE + CAMP group. d, CD8 + T cells in the spleen displayed higher expression of PD-1 in NE + CAMP group. e-f, NK cells (e) and CD8 + T cells (f) in the spleen displayed higher expression of LAMP-1 in the NE + CAMP group. g, The proportion of CD8+ effector memory T cells in the spleen was reduced in the NE + CAMP group. h, The proportion of CD8+ effector memory T cells in the tumour was marginally increased in the NE + CAMP group. i, CD8 + T cells in the blood in the NE + CAMP groups displayed activated phenotype as seen from the higher expression of PD-1. j-k, CD8 + T cells (j) and NK cells (k) in the tumours in the NE + CAMP group displayed modest activation with a marginally higher expression of CD69. l, Marginally higher accumulation of neutrophils in the tumours was observed in NE + CAMP group. n = 6 biological replicates for saline, n = 8 biological replicates for NE, n = 8 biological replicates for NE + CAMP. All data are represented as mean ± SEM. Sentences at the top of each panel represent the key observation that pertains to the NE + CAMP group. Data analysed using Ordinary one-way ANOVA with Tukey’s multiple comparisons test.
Extended Data Fig. 4 Systemic dose of CAMP-carrying neutrophils reduces tumor burden in 4T1-Luc model.
a, Experimental scheme for the treatment of 4T1-Luc tumours. Mice bearing orthotopic 4T1-Luc tumours were dosed twice with saline, neutrophils alone (NE) or neutrophil-CAMPs (NE + CAMP). First dose was performed when the tumours reached an average size of 50 mm3. b, Tumour growth kinetics of various treatments in the 4T1-Luc model. Neutrophil-CAMPs treatment significantly slowed the growth of 4T1-Luc tumours compared to the controls. n = 13 biological replicates for saline, n = 14 biological replicates for NE, n = 13 biological replicates for NE + CAMP. Data represented as mean ± SEM. Analysed by ordinary two-way ANOVA with Sidak’s multiple comparisons tests. c, Survival in the 4T1-Luc model. Neutrophil-CAMPs significantly improved the survival with 2/13 mice achieving complete regression by day 40. Analysed by log-rank (Mantle-Cox) test.
Extended Data Fig. 5 Systemic dose of CAMP-carrying neutrophils reduces tumor burden in the B16F10 model synergistically with aPD-1.
a, Experimental scheme for the treatment of B16F10 tumours. In addition to the two doses of saline/NE/NE+CAMPs, mice were treated with 100 μg intraperitoneal dose of aPD-1 antibody, 4 times, 3 days apart. b, Tumour growth kinetics of various aPD-1 combination treatments in the B16F10 model. Data represented as mean ± SEM. Analysed by ordinary two-way ANOVA with Sidak’s multiple comparisons tests. c, Survival in the B16F10 model for aPD-1 combination treatments. Analysed by log-rank (Mantle-Cox) test. d, Individual tumour growth curves for each group. n = 6 biological replicates for Saline + aPD-1, n = 6 biological replicates for NE + aPD-1, n = 11 biological replicates for NE + CAMP + aPD-1. CR represents complete regression.
Supplementary information
Supplementary Information
Supplementary methods, tables and figures.
Source data
Source Data Fig. 6 and Extended Data Figs. 4 and 5
Source data for tumour progression and for survival for Fig. 6 and Extended Data Figs. 4 and 5.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumbhojkar, N., Prakash, S., Fukuta, T. et al. Neutrophils bearing adhesive polymer micropatches as a drug-free cancer immunotherapy. Nat. Biomed. Eng (2024). https://doi.org/10.1038/s41551-024-01180-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41551-024-01180-z