Abstract
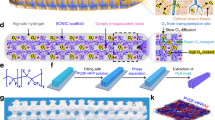
Cellular therapies for type-1 diabetes can leverage cell encapsulation to dispense with immunosuppression. However, encapsulated islet cells do not survive long, particularly when implanted in poorly vascularized subcutaneous sites. Here we show that the induction of neovascularization via temporary controlled inflammation through the implantation of a nylon catheter can be used to create a subcutaneous cavity that supports the transplantation and optimal function of a geometrically matching islet-encapsulation device consisting of a twisted nylon surgical thread coated with an islet-seeded alginate hydrogel. The neovascularized cavity led to the sustained reversal of diabetes, as we show in immunocompetent syngeneic, allogeneic and xenogeneic mouse models of diabetes, owing to increased oxygenation, physiological glucose responsiveness and islet survival, as indicated by a computational model of mass transport. The cavity also allowed for the in situ replacement of impaired devices, with prompt return to normoglycemia. Controlled inflammation-induced neovascularization is a scalable approach, as we show with a minipig model, and may facilitate the clinical translation of immunosuppression-free subcutaneous islet transplantation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. The raw and analysed datasets generated during the study are available for research purposes from the corresponding authors on reasonable request. Source data for the figures are provided with this paper.
Code availability
The SHARP source code is available on GitHub (https://github.com/alexanderuernst/SHARP).
References
DiMeglio, L. A., Evans-Molina, C. & Oram, R. A. Type 1 diabetes. Lancet 391, 2449–2462 (2018).
Cryer, P. E. Glycemic goals in diabetes: trade-off between glycemic control and iatrogenic hypoglycemia. Diabetes 63, 2188–2195 (2014).
Choudhary, P. et al. Evidence-informed clinical practice recommendations for treatment of type 1 diabetes complicated by problematic hypoglycemia. Diabetes Care 38, 1016–1029 (2015).
Marfil-Garza, B. A. et al. Pancreatic islet transplantation in type 1 diabetes: 20-year experience from a single-centre cohort in Canada. Lancet Diabetes Endocrinol. 10, 519–532 (2022).
Lemos, J. R. N. et al. Survival after islet transplantation in subjects with type 1 diabetes: twenty-year follow-up. Diabetes Care 44, e67–e68 (2021).
Lablanche, S. et al. Ten‐year outcomes of islet transplantation in patients with type 1 diabetes: data from the Swiss–French GRAGIL network. Am. J. Transplant. 21, 3725–3733 (2021).
Vantyghem, M.-C. et al. Ten-year outcome of islet alone or islet after kidney transplantation in type 1 diabetes: a prospective parallel-arm cohort study. Diabetes Care 42, 2042–2049 (2019).
Lemos, J. R. N. et al. Prolonged islet allograft function is associated with female sex in patients after islet transplantation. J. Clin. Endocrinol. Metab. 107, e973–e979 (2022).
Desai, T. & Shea, L. D. Advances in islet encapsulation technologies. Nat. Rev. Drug Discov. 16, 338–350 (2017).
Farina, M. et al. Cell encapsulation: overcoming barriers in cell transplantation in diabetes and beyond. Adv. Drug Deliv. Rev. 139, 92–115 (2019).
Fuchs, S. et al. Hydrogels in emerging technologies for type 1 diabetes. Chem. Rev. 121, 11458–11526 (2020).
Scharp, D. W. & Marchetti, P. Encapsulated islets for diabetes therapy: history, current progress, and critical issues requiring solution. Adv. Drug Deliv. Rev. 67, 35–73 (2014).
Orive, G. et al. Engineering a clinically translatable bioartificial pancreas to treat type I diabetes. Trends Biotechnol. 36, 445–456 (2018).
Marfil‐Garza, B. A., Polishevska, K., Pepper, A. R. & Korbutt, G. S. Current state and evidence of cellular encapsulation strategies in type 1 diabetes. Compr. Physiol. 10, 839–878 (2020).
Ramzy, A. et al. Implanted pluripotent stem-cell-derived pancreatic endoderm cells secrete glucose-responsive C-peptide in patients with type 1 diabetes. Cell Stem Cell 28, 2047–2061.e45 (2021).
Shapiro, A. M. J. et al. Insulin expression and C-peptide in type 1 diabetes subjects implanted with stem cell-derived pancreatic endoderm cells in an encapsulation device. Cell. Rep. Med. 2, 100466 (2021).
Goswami, D. et al. Design considerations for macroencapsulation devices for stem cell derived islets for the treatment of type 1 diabetes. Adv. Sci. 8, 2100820 (2021).
Pileggi, A. et al. Reversal of diabetes by pancreatic islet transplantation into a subcutaneous, neovascularized device. Transplantation 81, 1318–1324 (2006).
Sörenby, A. K. et al. Preimplantation of an immunoprotective device can lower the curative dose of islets to that of free islet transplantation—studies in a rodent model. Transplantation 86, 364–366 (2008).
Halberstadt, C. R. et al. Subcutaneous transplantation of islets into streptozocin-induced diabetic rats. Cell Transplant. 14, 595–605 (2005).
Mahou, R., Zhang, D. K. Y., Vlahos, A. E. & Sefton, M. V. Injectable and inherently vascularizing semi-interpenetrating polymer network for delivering cells to the subcutaneous space. Biomaterials 131, 27–35 (2017).
Coindre, V. F., Carleton, M. M. & Sefton, M. V. Methacrylic acid copolymer coating enhances constructive remodeling of polypropylene mesh by increasing the vascular response. Adv. Healthc. Mater. 8, e1900667 (2019).
Kawakami, Y. et al. Modified subcutaneous tissue with neovascularization is useful as the site for pancreatic islet transplantation. Cell Transplant. 9, 729–732 (2000).
Gu, Y. et al. Development of a new method to induce angiogenesis at subcutaneous site of streptozotocin-induced diabetic rats for islet transplantation. Cell Transplant. 10, 453–457 (2001).
Wang, W. et al. Reversal of diabetes in mice by xenotransplantation of a bioartificial pancreas in a prevascularized subcutaneous site. Transplantation 73, 122–129 (2002).
Weaver, J. D. et al. Vasculogenic hydrogel enhances islet survival, engraftment, and function in leading extrahepatic sites. Sci. Adv. 3, e1700184 (2017).
Song, W. et al. Engineering transferrable microvascular meshes for subcutaneous islet transplantation. Nat. Commun. 10, 4602 (2019).
Aghazadeh, Y. et al. Microvessels support engraftment and functionality of human islets and hESC-derived pancreatic progenitors in diabetes models. Cell Stem Cell 28, 1936–1949.e8 (2021).
Smink, A. M. et al. The efficacy of a prevascularized, retrievable poly (d, l,-lactide-co-ε-caprolactone) subcutaneous scaffold as transplantation site for pancreatic islets. Transplantation 101, e112–e119 (2017).
Stephens, C. H. et al. In situ type I oligomeric collagen macroencapsulation promotes islet longevity and function in vitro and in vivo. Am. J. Physiol. Endocrinol. Metab. 315, e650–e661 (2018).
Yu, M. et al. Islet transplantation in the subcutaneous space achieves long-term euglycaemia in preclinical models of type 1 diabetes. Nat. Metab. 2, 1013–1020 (2020).
Kuppan, P. et al. Co‐transplantation of human adipose‐derived mesenchymal stem cells with neonatal porcine islets within a prevascularized subcutaneous space augments the xenograft function. Xenotransplantation 27, e12581 (2020).
Barkai, U., Rotem, A. & de Vos, P. Survival of encapsulated islets: more than a membrane story. World J. Transplant. 6, 69–90 (2016).
Coronel, M. M., Liang, J.-P., Li, Y. & Stabler, C. Oxygen generating biomaterial improves the function and efficacy of beta cells within a macroencapsulation device. Biomaterials 210, 1–11 (2019).
Wang, L.-H. et al. An inverse-breathing encapsulation system for cell delivery. Sci. Adv. 7, eabd5835 (2021).
Carlsson, P.-O. et al. Transplantation of macroencapsulated human islets within the bioartificial pancreas βAir to patients with type 1 diabetes mellitus. Am. J. Transplant. 18, 1735–1744 (2018).
Ludwig, B. et al. Favorable outcome of experimental islet xenotransplantation without immunosuppression in a nonhuman primate model of diabetes. Proc. Natl Acad. Sci. USA 114, 11745–11750 (2017).
Ludwig, B. et al. Transplantation of human islets without immunosuppression. Proc. Natl Acad. Sci. USA 110, 19054–19058 (2013).
Neufeld, T. et al. The efficacy of an immunoisolating membrane system for islet xenotransplantation in minipigs. PLoS ONE 8, e70150 (2013).
Evron, Y. et al. Long-term viability and function of transplanted islets macroencapsulated at high density are achieved by enhanced oxygen supply. Sci. Rep. 8, 6508 (2018).
Burnett, D. R. et al. Glucose sensing in the peritoneal space offers faster kinetics than sensing in the subcutaneous space. Diabetes 63, 2498–2505 (2014).
Pepper, A. R. et al. A prevascularized subcutaneous device-less site for islet and cellular transplantation. Nat. Biotechnol. 33, 518–523 (2015).
An, D. et al. Designing a retrievable and scalable cell encapsulation device for potential treatment of type 1 diabetes. Proc. Natl Acad. Sci. USA 115, e263–e272 (2018).
Morris, R. M., Mortimer, T. O. & O’Neill, K. L. Cytokines: can cancer get the message? Cancers 14, 2178 (2022).
Lee, W. S., Yang, H., Chon, H. J. & Kim, C. Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp. Mol. Med. 52, 1475–1485 (2020).
Fahey, E. & Doyle, S. L. IL-1 family cytokine regulation of vascular permeability and angiogenesis. Front. Immunol. 10, 1426 (2019).
Fan, Y. et al. Interleukin-6 stimulates circulating blood-derived endothelial progenitor cell angiogenesis in vitro. J. Cereb. Blood Flow Metab. 28, 90–98 (2008).
Van Linthout, S., Miteva, K. & Tschöpe, C. Crosstalk between fibroblasts and inflammatory cells. Cardiovasc. Res. 102, 258–269 (2014).
Werner, S., Krieg, T. & Smola, H. Keratinocyte–fibroblast interactions in wound healing. J. Invest. Dermatol. 127, 998–1008 (2007).
Briššová, M. et al. Control and measurement of permeability for design of microcapsule cell delivery system. J. Biomed. Mater. Res. 39, 61–70 (1998).
Briššová, M. et al. Evaluation of microcapsule permeability via inverse size exclusion chromatography. Anal. Biochem. 242, 104–111 (1996).
Hoesli, C. A. et al. Reversal of diabetes by βTC3 cells encapsulated in alginate beads generated by emulsion and internal gelation. J. Biomed. Mater. Res. B 100, 1017–1028 (2012).
Pepper, A. R. et al. Diabetic rats and mice are resistant to porcine and human insulin: flawed experimental models for testing islet xenografts. Xenotransplantation 16, 502–510 (2009).
Komatsu, H. et al. Oxygen environment and islet size are the primary limiting factors of isolated pancreatic islet survival. PLoS ONE 12, e0183780 (2017).
Komatsu, H., Kandeel, F. & Mullen, Y. Impact of oxygen on pancreatic islet survival. Pancreas 47, 533–543 (2018).
Dolgin, E. Diabetes cell therapies take evasive action. Nat. Biotechnol. 40, 291–295 (2022).
Gala-Lopez, B. L. et al. Subcutaneous clinical islet transplantation in a prevascularized subcutaneous pouch-preliminary experience. CellR4 4, e2132 (2016).
Bachul, P. et al. 307.5: modified approach allowed for improved islet allotransplantation into pre-vascularized Sernova Cell Pouch device-preliminary results of the phase I/II clinical trial at University of Chicago. Transplantation 105, S25 (2021).
Colton, C. K. & Weir, G. Commentary-a hard lesson about transplanting islets into prevascularized devices. CellR4 5, e2251 (2017).
Yang, L. et al. Regenerating hair in prevascularized tissue space formed by a controllable foreign body reaction. Adv. Funct. Mater. 31, 2007483 (2021).
Lanza, R. P. et al. Treatment of severely diabetic pancreatectomized dogs using a diffusion-based hybrid pancreas. Diabetes 41, 886–889 (1992).
Lanza, R. P. et al. Successful xenotransplantation of a diffusion-based biohybrid artificial pancreas: a study using canine, bovine, and porcine islets. Transplant. Proc. 24, 669–671 (1992).
Lanza, R. P., Sullivan, S. J. & Chick, W. L. Islet transplantation with immunoisolation. Diabetes 41, 1503–1510 (1992).
Lanza, R. P. et al. Pancreatic islet transplantation using membrane diffusion chambers. Tranplant. Proc. 24, 2935–2936 (1992).
Lanza, R. P. et al. Xenotransplantation of canine, bovine, and porcine islets in diabetic rats without immunosuppression. Proc. Natl Acad. Sci. USA 88, 11100–11104 (1991).
Bochenek, M. A. et al. Alginate encapsulation as long-term immune protection of allogeneic pancreatic islet cells transplanted into the omental bursa of macaques. Nat. Biomed. Eng. 2, 810–821 (2018).
Vegas, A. J. et al. Combinatorial hydrogel library enables identification of materials that mitigate the foreign body response in primates. Nat. Biotechnol. 34, 345–352 (2016).
Dufrane, D., Goebbels, R.-M. & Gianello, P. Alginate macroencapsulation of pig islets allows correction of streptozotocin-induced diabetes in primates up to 6 months without immunosuppression.Transplantation 90, 1054–1062 (2010).
Dufrane, D. et al. The influence of implantation site on the biocompatibility and survival of alginate encapsulated pig islets in rats. Biomaterials 27, 3201–3208 (2006).
Liu, Q. et al. Zwitterionically modified alginates mitigate cellular overgrowth for cell encapsulation. Nat. Commun. 10, 5262 (2019).
Kuo, C. K. & Ma, P. X. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: part 1. Structure, gelation rate and mechanical properties. Biomaterials 22, 511–521 (2001).
Pham, T. T. et al. Hydrogel coatings: surface‐triggered in situ gelation for tunable conformal hydrogel coating of therapeutic cells and biomedical devices. Adv. Funct. Mater. 31, 2010169 (2021).
Fousteri, G., Ippolito, E., Ahmed, R. & Hamad, A. R. A. Beta-cell specific autoantibodies: are they just an indicator of type 1 diabetes?. Curr. Diabetes Rev. 13, 322–329 (2017).
Wang, X. et al. A nanofibrous encapsulation device for safe delivery of insulin-producing cells to treat type 1 diabetes. Sci. Transl. Med. 13, eabb4601 (2021).
Singh, M. K. A. & Steenbergen, W. Photoacoustic-guided focused ultrasound (PAFUSion) for identifying reflection artifacts in photoacoustic imaging. Photoacoustics 3, 123–131 (2015).
Shapiro, A. M. J., Pokrywczynska, M. & Ricordi, C. Clinical pancreatic islet transplantation. Nat. Rev. Endocrinol. 13, 268–277 (2017).
Wu, H. et al. In situ electrochemical oxygen generation with an immunoisolation device. Ann. N. Y. Acad. Sci. 875, 105–125 (1999).
Buchwald, P. A local glucose-and oxygen concentration-based insulin secretion model for pancreatic islets. Theor. Biol. Med. Model. 8, 20 (2011).
Buchwald, P. FEM-based oxygen consumption and cell viability models for avascular pancreatic islets. Theor. Biol. Med. Model. 6, 5 (2009).
Buchwald, P. et al. Glucose‐stimulated insulin release: parallel perifusion studies of free and hydrogel encapsulated human pancreatic islets. Biotechnol. Bioeng. 115, 232–245 (2018).
Buchwald, P. et al. Quantitative assessment of islet cell products: estimating the accuracy of the existing protocol and accounting for islet size distribution. Cell Transplant. 18, 1223–1235 (2009).
Ernst, A. U. et al. A predictive computational platform for optimizing the design of bioartificial pancreas devices. Nat. Commun. 13, 6031 (2022).
Lyon, J. et al. Research-focused isolation of human islets from donors with and without diabetes at the Alberta Diabetes Institute IsletCore. Endocrinology 157, 560–569 (2016).
Bhujbal, S. V., Paredes-Juarez, G. A., Niclou, S. P. & de Vos, P. Factors influencing the mechanical stability of alginate beads applicable for immunoisolation of mammalian cells. J. Mech. Behav. Biomed. Mater. 37, 196–208 (2014).
Acknowledgements
We thank the Cornell University Animal Health Diagnostic Center for histological sectioning and staining, and the University of Alberta IsletCore (http://www.bcell.org/adi-isletcore.html) for providing human islets. Human islets were provided with the assistance of the Human Organ Procurement and Exchange (HOPE) programme, Trillium Gift of Life Network (TGLN) and other Canadian organ procurement organizations. Islet isolation was approved by the Human Research Ethics Board at the University of Alberta (Pro00013094). All donors’ families gave informed consent for the use of pancreatic tissue in research. Some schematics were created with BioRender.com. This work was partially supported by the National Institutes of Health (NIH, 1R01DK105967 to M.M.), the Novo Nordisk Company (to M.M.), the Juvenile Diabetes Research Foundation (JDRF, 2-SRA-2018-472-S-B to M.M.) and the Hartwell Foundation (to M.M.). A.U.E. was supported by the National Science Foundation Graduate Research Fellowship under grant number DGE-1650441. B.A.M.-G. was supported by the Patronato del Instituto Nacional de Ciencias Medicas y Nutricion Salvador Zubiran (INCMNSZ) and the Fundación para la Salud y la Educación Dr Salvador Zubirán (FunSaEd). A.M.J.S. was supported through a Canada Research Chair in Regenerative Medicine and Transplantation Surgery at the University of Alberta. A.R.P. was supported through a Canada Research Chair in Cell Therapies for Diabetes at the University of Alberta. Other study support includes the Diabetes Research Institute Foundation of Canada (DRIFCan). O2M Technologies acknowledges the support of JDRF grant 3-SRA-2020-883-M-B, NIH/NCI SBIR grants R43CA224840 and R44CA224840, and NSF SBIR grants 1819583 and 2028829.
Author information
Authors and Affiliations
Contributions
L.-H.W., B.A.M.-G., M.M. and A.M.J.S. conceptualized the project. L.-H.W., B.A.M.-G., M.M. and A.M.J.S. developed the methodology. L.-H.W., B.A.M.-G., A.U.E., R.L.P., A.R.P., K.O., B.E., N.V., M.K., J.A.F., A.K.D., H.-J.G. and Y.-Z.Y. conducted investigations. L.-H.W. and B.A.M.-G. performed visualization. M.M. and A.M.J.S. acquired funding. L.-H.-W. and B.A.M.-G. administered the project. L.-H.W., B.A.M.-G., M.M. and A.M.J.S. supervised the project. L.-H.W. and B.A.M.-G. wrote the original draft. L.-H.W., B.A.M.-G., A.U.E., R.L.P., A.R.P., K.O., B.E., N.V., M.K., J.A.F., A.K.D., M.M. and A.M.J.S. reviewed and edited the paper.
Corresponding authors
Ethics declarations
Competing interests
A.M.J.S. serves as a consultant to ViaCyte Inc., Vertex Inc., Hemostemix Inc. and Aspect Biosystems Ltd. B.E. discloses financial interests in O2M. A.M.J.S. holds patents (US10434122B2 and CA2865122A1) for the ‘device-less’ prevascularization technique described in this paper. J.A.F. and M.M. are inventors on a patent application (US10493107B2) that covers the cell-encapsulation device described in this paper and are co-founders of Persista Bio. The other authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Matthias von Herrath, Corinne A. Hoesli and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Assessment of inflammatory responses promoting neovascularization.
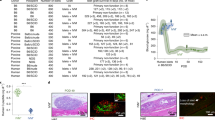
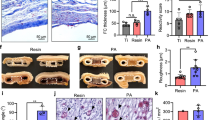
a, Schematic representation of the experimental design to evaluate the localized inflammatory response at the unmodified (control) and vascularized site. Three time points were evaluated: 1) 1-week post-catheter implantation in the vascularized site (n = 9), 2) At implantation of the device in the vascularized site (i.e., 4-6 weeks post-catheter implantation) (n = 5), and 3) 3 days post-device implantation at both the vascularized and unmodified/non-prevascularized site (n = 4). b−f, Inflammatory assays showing tissue cytokine levels at the unmodified and modified subcutaneous site; data points represent medians and interquartile ranges. Groups are compared using Mann-Whitney U tests for independent samples and Wilcoxon matched-pairs signed rank tests, as appropriate; Data are shown as median with interquartile range.
Extended Data Fig. 2 Evaluation of glucose-responsive insulin kinetics of encapsulated islets in perifusion simulation model.
a,b, Model settings for the perifusion simulation with non-encapsulated (a) and encapsulated (b) islets. The variable ci|j denotes the concentration species i in subdomain j. Top images show the 3-dimensional model; bottom images show the boundary conditions applied in the simulation on a representative 2-dimensional cross section. c–g, Settings and results of the in silico dynamic perifusion simulation to compare glucose-stimulated insulin secretion kinetics in non-encapsulated and encapsulated islets. (c) Inlet glucose concentration settings of the perifusion simulation, featuring three 60 min glucose regimens: an initial low-concentration (2.8 mM) phase, followed by a high-concentration (16.7 mM) phase, and finally a return to the low concentration (2.8 mM) regime; inset plots show the continuous transition of glucose concentration between regimens occurring over 2 min. Schematic showing the in silico representation of the perifusion test. Non-encapsulated (left) and encapsulated (right) islets were positioned in flowing media and exposed to the variable glucose regime, producing a simulated insulin outflux. (d) Glucose concentration (as a volume-average) in islets over time during the in silico perfusion test. (e) Surface plots of the glucose concentration in the perifusion system at 120 and 150 min with non-encapsulated (left) and encapsulated (right) islets. (f) Outlet flux (normalized by IEQ) of insulin over time during the in silico perifusion test. (g) Surface plots of insulin concentration in the perifusion system at 120 and 150 min with non-encapsulated (left) and encapsulated (right) islets.
Extended Data Fig. 3 Model physics and boundary conditions.
a, Schematic of a two-dimensional representation of the geometry dimensions applied in simulations of rat islet-containing devices. b, Mathematical representation of the external and internal boundary conditions. c, Values of the external boundary oxygen tension applied in simulations (based on the average value from EPR oxygen measurements) for devices at the unmodified and vascularized site, respectively.
Extended Data Fig. 4 Effect of vascularization on oxygenation outcomes.
a, Distribution of EPR oxygen measurements in the unmodified and vascularized sites (this figure is a copy of Fig. 2m); mean ± SD. b, Probability density functions of the best-fit normal distributions to the absolute pO2 measurements at the unmodified and vascularized sites shown in a. c–f, Results from Monte Carlo simulations of the rat islet device where the external boundary pO2, pext, was treated as a random variable described by the normal distributions shown in b. Mean pO2 (c and d) and net necrotic fraction within rat islets (e and f) in devices in the unmodified and vascularized sites. Data was compared in bar graphs (c and e) and in relation to the simulated value of pext (d and f). c and e: ****p < 0.0001 (two-sided Mann-Whitney U test); mean ± SD.
Extended Data Fig. 5 Glucose tolerance tests of mice with impaired encapsulation devices.
Blood glucose data from two animals (taken from Fig. 4g) showing hyperglycaemia and impaired glucose responsiveness.
Extended Data Fig. 6 In situ device replacement at the vascularized site.
a,b, Schematic showing the process of device replacement. Following device retrieval, the sharp end of the customized PE tube is inserted to maintain patency of the vascularized subcutaneous pocket. Once the PE tube is in place, a new device is inserted through the expanded funnel-shaped side of the customized PE tube. Following device implantation, the PE tube is completely removed. c, BG measurements of one mouse experiencing partial graft attrition of the initial transplantation, device replacement, and retrieval.
Supplementary information
Supplementary Information
Supplementary methods, figures, tables, references and video captions.
Supplementary Video 1
Glucose and insulin concentrations over time in the in silico perifusion test of non-encapsulated (free) and encapsulated islets.
Supplementary Video 2
Representative facile retrieval of a rat islet device in a mouse.
Supplementary Video 3
Transplantation and retrieval (4 months after transplantation) of a human islet device.
Supplementary Video 4
Surgical procedures for device implantation and retrieval (1 month after implantation) in minipigs.
Supplementary Video 5
Surgical procedure showing the implantation of a 12-cm long catheter into the subcutaneous space in a minipig.
Supplementary Video 6
Mechanical tests showing robustness of a potentially scalable encapsulation device under bending and compression.
Source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, LH., Marfil-Garza, B.A., Ernst, A.U. et al. Inflammation-induced subcutaneous neovascularization for the long-term survival of encapsulated islets without immunosuppression. Nat. Biomed. Eng (2023). https://doi.org/10.1038/s41551-023-01145-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41551-023-01145-8