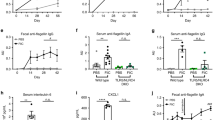
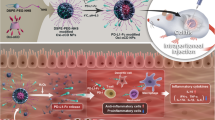
Abstract
Inflammatory bowel disease lacks a long-lasting and broadly effective therapy. Here, by taking advantage of the anti-infection and anti-inflammatory properties of natural antibodies against the small-molecule epitope phosphorylcholine (PC), we show in multiple mouse models of colitis that immunization of the animals with self-assembling supramolecular peptide nanofibres bearing PC epitopes induced sustained levels of anti-PC antibodies that were both protective and therapeutic. The strength and type of immune responses elicited by the nanofibres could be controlled through the relative valency of PC epitopes and exogenous T-cell epitopes on the nanofibres and via the addition of the adjuvant CpG. The nanomaterial-assisted induction of the production of therapeutic antibodies may represent a durable therapy for inflammatory bowel disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. The raw and analysed datasets generated during the study are available for research purposes from the corresponding author on reasonable request. Source data for the figures are provided with this paper.
References
GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 5, 17–30 (2020).
Guan, Q. A comprehensive review and update on the pathogenesis of inflammatory bowel disease. J. Immunol. Res. 2019, 7247238 (2019).
Chang, J. T. Pathophysiology of inflammatory bowel diseases. N. Engl. J. Med. 383, 2652–2664 (2020).
Huber, W. et al. [Life-threatening complications of Crohn’s disease and ulcerative colitis: a systematic analysis of admissions to an ICU during 18 years]. Dtsch Med. Wochenschr. 135, 668–674 (2010).
Papamichael, K. et al. Infliximab in inflammatory bowel disease. Ther. Adv. Chronic Dis. 10, 2040622319838443 (2019).
Feagan, B. G. et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N. Engl. J. Med. 375, 1946–1960 (2016).
Salvana, E. M. & Salata, R. A. Infectious complications associated with monoclonal antibodies and related small molecules. Clin. Microbiol. Rev. 22, 274–290 (2009).
Roda, G., Jharap, B., Neeraj, N. & Colombel, J. F. Loss of response to anti-TNFs: definition, epidemiology, and management. Clin. Transl. Gastroenterol. 7, e135 (2016).
Bachmann, M. F. & Whitehead, P. Active immunotherapy for chronic diseases. Vaccine 31, 1777–1784 (2013).
Zagury, D., Burny, A. & Gallo, R. C. Toward a new generation of vaccines: the anti-cytokine therapeutic vaccines. Proc. Natl Acad. Sci. USA 98, 8024–8029 (2001).
Durez, P. et al. Therapeutic vaccination with TNF-kinoid in TNF antagonist-resistant rheumatoid arthritis: a Phase II randomized, controlled clinical trial. PLoS ONE 9, e113465 (2014).
Houssiau, F. A. et al. IFN-alpha kinoid in systemic lupus erythematosus: results from a Phase IIb, randomised, placebo-controlled study. Ann. Rheum. Dis. 79, 347–355 (2020).
Cavelti-Weder, C. et al. Development of an interleukin-1beta vaccine in patients with type 2 diabetes. Mol. Ther. 24, 1003–1012 (2016).
Gronwall, C., Vas, J. & Silverman, G. J. Protective roles of natural IgM antibodies. Front. Immunol. 3, 66 (2012).
Bunker, J. J. et al. Natural polyreactive IgA antibodies coat the intestinal microbiota. Science 358, eaan6619 (2017).
Baumgarth, N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat. Rev. Immunol. 11, 34–46 (2011).
Shaw, P. X., Goodyear, C. S., Chang, M. K., Witztum, J. L. & Silverman, G. J. The autoreactivity of anti-phosphorylcholine antibodies for atherosclerosis-associated neo-antigens and apoptotic cells. J. Immunol. 170, 6151–6157 (2003).
Harnett, W. & Harnett, M. M. Phosphorylcholine: friend or foe of the immune system? Immunol. Today 20, 125–129 (1999).
Su, J. et al. Natural antibodies against phosphorylcholine as potential protective factors in SLE. Rheumatology 47, 1144–1150 (2008).
Fiskesund, R. et al. IgM phosphorylcholine antibodies inhibit cell death and constitute a strong protection marker for atherosclerosis development, particularly in combination with other auto-antibodies against modified LDL. Results Immunol. 2, 13–18 (2012).
Gil-Borras, R. et al. B1a lymphocytes (CD19+CD5+) deficiency in patients with Crohn’s disease and its relation with disease severity. Dig. Dis. 36, 194–201 (2018).
Polese, L. et al. B1a lymphocytes in ulcerative colitis. Int. J. Colorectal Dis. 22, 1005–1011 (2007).
Shimomura, Y. et al. Regulatory role of B-1 B cells in chronic colitis. Int. Immunol. 20, 729–737 (2008).
Allaoui-Attarki, K. et al. Protective immunity against Salmonella typhimurium elicited in mice by oral vaccination with phosphorylcholine encapsulated in poly(DL-lactide-co-glycolide) microspheres. Infect. Immun. 65, 853–857 (1997).
Tanaka, N. et al. Intranasal immunization with phosphorylcholine induces antigen specific mucosal and systemic immune responses in mice. Vaccine 25, 2680–2687 (2007).
Baatarjav, T. et al. Mucosal immune features to phosphorylcholine by nasal Flt3 ligand cDNA-based vaccination. Vaccine 29, 5747–5757 (2011).
Caligiuri, G. et al. Phosphorylcholine-targeting immunization reduces atherosclerosis. J. Am. Coll. Cardiol. 50, 540–546 (2007).
Yoshimatsu, H., Kataoka, K., Fujihashi, K., Miyake, T. & Ono, Y. A nasal double DNA adjuvant system induces atheroprotective IgM antibodies via dendritic cell-B-1a B cell interactions. Vaccine 40, 1116–1127 (2022).
Rudra, J. S., Tian, Y. F., Jung, J. P. & Collier, J. H. A self-assembling peptide acting as an immune adjuvant. Proc. Natl Acad. Sci. USA 107, 622–627 (2010).
Mora-Solano, C. et al. Active immunotherapy for TNF-mediated inflammation using self-assembled peptide nanofibers. Biomaterials 149, 1–11 (2017).
Rudra, J. S. et al. Self-assembled peptide nanofibers raising durable antibody responses against a malaria epitope. Biomaterials 33, 6476–6484 (2012).
Rudra, J. S. et al. Suppression of cocaine-evoked hyperactivity by self-adjuvanting and multivalent peptide nanofiber vaccines. ACS Chem. Neurosci. 7, 546–552 (2016).
Shores, L. S. et al. Multifactorial design of a supramolecular peptide anti-IL-17 vaccine toward the treatment of psoriasis. Front. Immunol. 11, 1855 (2020).
Fries, C. N. et al. HIV envelope antigen valency on peptide nanofibers modulates antibody magnitude and binding breadth. Sci. Rep. 11, 14494 (2021).
Hainline, K. M. et al. Modular complement assemblies for mitigating inflammatory conditions. Proc. Natl Acad. Sci. USA 118, e2018627118 (2021).
Chen, J. et al. The use of self-adjuvanting nanofiber vaccines to elicit high-affinity B cell responses to peptide antigens without inflammation. Biomaterials 34, 8776–8785 (2013).
Pompano, R. R. et al. Titrating T-cell epitopes within self-assembled vaccines optimizes CD4+ helper T cell and antibody outputs. Adv. Healthc. Mater. 3, 1898–1908 (2014).
Rudra, J. S. et al. Modulating adaptive immune responses to peptide self-assemblies. ACS Nano 6, 1557–1564 (2012).
Link, A. & Bachmann, M. F. Immunodrugs: breaking B- but not T-cell tolerance with therapeutic anticytokine vaccines. Immunotherapy 2, 561–574 (2010).
Chu, R. S., McCool, T., Greenspan, N. S., Schreiber, J. R. & Harding, C. V. CpG oligodeoxynucleotides act as adjuvants for pneumococcal polysaccharide–protein conjugate vaccines and enhance antipolysaccharide immunoglobulin G2a (IgG2a) and IgG3 antibodies. Infect. Immun. 68, 1450–1456 (2000).
Kim, T. H. et al. CpG-DNA exerts antibacterial effects by protecting immune cells and producing bacteria-reactive antibodies. Sci. Rep. 8, 16236 (2018).
Dintzis, H. M., Dintzis, R. Z. & Vogelstein, B. Molecular determinants of immunogenicity: the immunon model of immune response. Proc. Natl Acad. Sci. USA 73, 3671–3675 (1976).
Batista, F. D. & Neuberger, M. S. B cells extract and present immobilized antigen: implications for affinity discrimination. EMBO J. 19, 513–520 (2000).
Veneziano, R. et al. Role of nanoscale antigen organization on B-cell activation probed using DNA origami. Nat. Nanotechnol. 15, 716–723 (2020).
Groenning, M. et al. Study on the binding of Thioflavin T to beta-sheet-rich and non-beta-sheet cavities. J. Struct. Biol. 158, 358–369 (2007).
Yang, Y., Tung, J. W., Ghosn, E. E., Herzenberg, L. A. & Herzenberg, L. A. Division and differentiation of natural antibody-producing cells in mouse spleen. Proc. Natl Acad. Sci. USA 104, 4542–4546 (2007).
Bennett, N. R., Zwick, D. B., Courtney, A. H. & Kiessling, L. L. Multivalent antigens for promoting B and T cell activation. ACS Chem. Biol. 10, 1817–1824 (2015).
Yuseff, M. I., Pierobon, P., Reversat, A. & Lennon-Dumenil, A. M. How B cells capture, process and present antigens: a crucial role for cell polarity. Nat. Rev. Immunol. 13, 475–486 (2013).
Alexander, J. et al. Development of high potency universal DR-restricted helper epitopes by modification of high affinity DR-blocking peptides. Immunity 1, 751–761 (1994).
Scott, M. G., Briles, D. E., Shackelford, P. G., Smith, D. S. & Nahm, M. H. Human antibodies to phosphocholine. IgG anti-PC antibodies express restricted numbers of V and C regions. J. Immunol. 138, 3325–3331 (1987).
Hjelholt, A., Christiansen, G., Sorensen, U. S. & Birkelund, S. IgG subclass profiles in normal human sera of antibodies specific to five kinds of microbial antigens. Pathog. Dis. 67, 206–213 (2013).
Michaelsen, T. E., Kolberg, J., Aase, A., Herstad, T. K. & Hoiby, E. A. The four mouse IgG isotypes differ extensively in bactericidal and opsonophagocytic activity when reacting with the P1.16 epitope on the outer membrane PorA protein of Neisseria meningitidis. Scand. J. Immunol. 59, 34–39 (2004).
Nimmerjahn, F. & Ravetch, J. V. Divergent immunoglobulin G subclass activity through selective Fc receptor binding. Science 310, 1510–1512 (2005).
Eichele, D. D. & Kharbanda, K. K. Dextran sodium sulfate colitis murine model: an indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis. World J. Gastroenterol. 23, 6016–6029 (2017).
Melgar, S. et al. Validation of murine dextran sulfate sodium-induced colitis using four therapeutic agents for human inflammatory bowel disease. Int. Immunopharmacol. 8, 836–844 (2008).
Dieleman, L. A. et al. Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology 107, 1643–1652 (1994).
Dieleman, L. A. et al. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin. Exp. Immunol. 114, 385–391 (1998).
O’Garra, A. & Howard, M. IL-10 production by CD5 B cells. Ann. N. Y. Acad. Sci. 651, 182–199 (1992).
Bleich, A. et al. CpG motifs of bacterial DNA exert protective effects in mouse models of IBD by antigen-independent tolerance induction. Gastroenterology 136, 278–287 (2009).
Obermeier, F. et al. Contrasting activity of cytosin-guanosin dinucleotide oligonucleotides in mice with experimental colitis. Clin. Exp. Immunol. 134, 217–224 (2003).
Obermeier, F. et al. CpG motifs of bacterial DNA exacerbate colitis of dextran sulfate sodium-treated mice. Eur. J. Immunol. 32, 2084–2092 (2002).
Babickova, J. et al. Sex differences in experimentally induced colitis in mice: a role for estrogens. Inflammation 38, 1996–2006 (2015).
Chassaing, B., Aitken, J. D., Malleshappa, M. & Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. https://doi.org/10.1002/0471142735.im1525s104 (2014).
Alex, P. et al. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm. Bowel Dis. 15, 341–352 (2009).
Jofra, T. et al. Experimental colitis in IL-10-deficient mice ameliorates in the absence of PTPN22. Clin. Exp. Immunol. 197, 263–275 (2019).
Hale, L. P., Fitzhugh, D. J. & Staats, H. F. Oral immunogenicity of the plant proteinase bromelain. Int. Immunopharmacol. 6, 2038–2046 (2006).
Hale, L. P., Gottfried, M. R. & Swidsinski, A. Piroxicam treatment of IL-10-deficient mice enhances colonic epithelial apoptosis and mucosal exposure to intestinal bacteria. Inflamm. Bowel Dis. 11, 1060–1069 (2005).
Stevceva, L., Pavli, P., Husband, A. J. & Doe, W. F. The inflammatory infiltrate in the acute stage of the dextran sulphate sodium induced colitis: B cell response differs depending on the percentage of DSS used to induce it. BMC Clin. Pathol. 1, 3 (2001).
Glassner, K. L., Abraham, B. P. & Quigley, E. M. M. The microbiome and inflammatory bowel disease. J. Allergy Clin. Immunol. 145, 16–27 (2020).
Schwab, C. et al. Longitudinal study of murine microbiota activity and interactions with the host during acute inflammation and recovery. ISME J. 8, 1101–1114 (2014).
Stojanov, S., Berlec, A. & Strukelj, B. The influence of probiotics on the Firmicutes/Bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms 8, 1715 (2020).
Madsen, K. L., Doyle, J. S., Jewell, L. D., Tavernini, M. M. & Fedorak, R. N. Lactobacillus species prevents colitis in interleukin 10 gene-deficient mice. Gastroenterology 116, 1107–1114 (1999).
Nagalingam, N. A., Kao, J. Y. & Young, V. B. Microbial ecology of the murine gut associated with the development of dextran sodium sulfate-induced colitis. Inflamm. Bowel Dis. 17, 917–926 (2011).
Guan, Q. et al. Development of recombinant vaccines against IL-12/IL-23 p40 and in vivo evaluation of their effects in the downregulation of intestinal inflammation in murine colitis. Vaccine 27, 7096–7104 (2009).
Ma, Y. et al. Targeting TGF-beta1 by employing a vaccine ameliorates fibrosis in a mouse model of chronic colitis. Inflamm. Bowel Dis. 16, 1040–1050 (2010).
Simanovich, E., Brod, V. & Rahat, M. A. Active vaccination with EMMPRIN-derived multiple antigenic peptide (161-MAP) reduces angiogenesis in a dextran sodium sulfate (DSS)-induced colitis model. Front. Immunol. 9, 2919 (2018).
Votaw, N. L. et al. Randomized peptide assemblies for enhancing immune responses to nanomaterials. Biomaterials 273, 120825 (2021).
Duan, L., Rao, X. & Sigdel, K. R. Regulation of inflammation in autoimmune disease. J. Immunol. Res. 2019, 7403796 (2019).
Bashi, T. et al. Successful modulation of murine lupus nephritis with tuftsin-phosphorylcholine. J. Autoimmun. 59, 1–7 (2015).
Ben-Ami Shor, D. et al. Phosphorylcholine-tuftsin compound prevents development of dextransulfate-sodium-salt induced murine colitis: implications for the treatment of human inflammatory bowel disease. J. Autoimmun. 56, 111–117 (2015).
Ben-Ami Shor, D. et al. Immunomodulation of murine chronic DSS-induced colitis by tuftsin-phosphorylcholine. J. Clin. Med. 9, 65 (2019).
Elkins, K. L., Rhinehart-Jones, T. R., Stibitz, S., Conover, J. S. & Klinman, D. M. Bacterial DNA containing CpG motifs stimulates lymphocyte-dependent protection of mice against lethal infection with intracellular bacteria. J. Immunol. 162, 2291–2298 (1999).
Rose, W. A. 2nd, Sakamoto, K. & Leifer, C. A. TLR9 is important for protection against intestinal damage and for intestinal repair. Sci. Rep. 2, 574 (2012).
Atreya, R. et al. Clinical effects of a topically applied Toll-like receptor 9 agonist in active moderate-to-severe ulcerative colitis. J. Crohns Colitis 10, 1294–1302 (2016).
Bruhns, P. Properties of mouse and human IgG receptors and their contribution to disease models. Blood 119, 5640–5649 (2012).
Krieg, A. M. Therapeutic potential of Toll-like receptor 9 activation. Nat. Rev. Drug Discov. 5, 471–484 (2006).
Man, S. M., Kaakoush, N. O. & Mitchell, H. M. The role of bacteria and pattern-recognition receptors in Crohn’s disease. Nat. Rev. Gastroenterol. Hepatol. 8, 152–168 (2011).
Shen, Z. H. et al. Relationship between intestinal microbiota and ulcerative colitis: mechanisms and clinical application of probiotics and fecal microbiota transplantation. World J. Gastroenterol. 24, 5–14 (2018).
Berry, D. et al. Phylotype-level 16S rRNA analysis reveals new bacterial indicators of health state in acute murine colitis. ISME J. 6, 2091–2106 (2012).
Federici, S. et al. Targeted suppression of human IBD-associated gut microbiota commensals by phage consortia for treatment of intestinal inflammation. Cell 185, 2879–2898.e24 (2022).
Palma, J., Tokarz-Deptula, B., Deptula, J. & Deptula, W. Natural antibodies – facts known and unknown. Cent. Eur. J. Immunol. 43, 466–475 (2018).
Si, Y., Wen, Y., Kelly, S. H., Chong, A. S. & Collier, J. H. Intranasal delivery of adjuvant-free peptide nanofibers elicits resident CD8+ T cell responses. J. Control. Release 282, 120–130 (2018).
Nguyen, H. D., Aljamaei, H. M. & Stadnyk, A. W. The production and function of endogenous interleukin-10 in intestinal epithelial cells and gut homeostasis. Cell Mol. Gastroenterol. Hepatol. 12, 1343–1352 (2021).
Maseda, D. et al. Regulatory B10 cells differentiate into antibody-secreting cells after transient IL-10 production in vivo. J. Immunol. 188, 1036–1048 (2012).
Wang, L. et al. T regulatory cells and B cells cooperate to form a regulatory loop that maintains gut homeostasis and suppresses dextran sulfate sodium-induced colitis. Mucosal Immunol. 8, 1297–1312 (2015).
Eastaff-Leung, N., Mabarrack, N., Barbour, A., Cummins, A. & Barry, S. Foxp3+ regulatory T cells, Th17 effector cells, and cytokine environment in inflammatory bowel disease. J. Clin. Immunol. 30, 80–89 (2010).
Boehm, F. et al. Deletion of Foxp3(+) regulatory T cells in genetically targeted mice supports development of intestinal inflammation. BMC Gastroenterol. 12, 97 (2012).
Wen, J. et al. The role of Th17/Treg balance and Th22 cell in the pathogenesis of DSS-induced colitis in mice. Eur. J. Inflamm. 13, 101–108 (2015).
Melgar, S., Karlsson, A. & Michaelsson, E. Acute colitis induced by dextran sulfate sodium progresses to chronicity in C57BL/6 but not in BALB/c mice: correlation between symptoms and inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 288, G1328–G1338 (2005).
Colombel, J. F. et al. Discrepancies between patient-reported outcomes, and endoscopic and histological appearance in UC. Gut 66, 2063–2068 (2017).
Berg, D. J. et al. Rapid development of colitis in NSAID-treated IL-10-deficient mice. Gastroenterology 123, 1527–1542 (2002).
Burich, A. et al. Helicobacter-induced inflammatory bowel disease in IL-10- and T cell-deficient mice. Am. J. Physiol. Gastrointest. Liver Physiol. 281, G764–778, (2001).
Bolyen, E. et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37, 852–857 (2019).
Acknowledgements
We thank Y. Wu, B. Cossette and S. Shetty for helpful advice; the staff of the Duke Surgery Substrate Services Core and Research Support, especially Histology Core members P. Newell and C. Leonard. Timeline images in Figs. 4–6, 8 and Supplementary Figs. 8 and 19 were drawn using Biorender.com. This work was supported by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health (NIH/NIBIB, R01 EB009701) and National Institute of Allergy and Infectious Diseases of the National Institutes of Health (NIH/NIAID, R01 AI172151), both awarded to J.H.C. E.J.C was supported by the National Science Foundation Graduate Research Fellowship Program (DGE-1644868). MALDI–TOF was conducted on an instrument supported by the North Carolina Biotechnology Center (2017-IDG-1018). Transmission electron microscopy was performed on an instrument at the Duke University Shared Materials Instrumentation Facility (SMIF), a member of the North Carolina Research Triangle Nanotechnology Network (RTNN), which is supported by the National Science Foundation (ECCS-2025064) as part of the National Nanotechnology Coordinated Infrastructure (NNCI).
Author information
Authors and Affiliations
Contributions
E.J.C. designed and performed experiments, analysed and interpreted data, and wrote and edited the manuscript. E.F.R., H.F.H., M.E.W. and N.L.V. performed experiments, analysed data and edited the manuscript. A.R.A. and T.S. conceptualized confocal tissue section quantification and analysis. L.P.H. designed experiments, analysed and interpreted data, and wrote and edited the manuscript. J.H.C. designed experiments, analysed and interpreted data, wrote and edited the manuscript, and supervised the research.
Corresponding author
Ethics declarations
Competing interests
E.J.C. and J.H.C. are listed as inventors on a pending patent application (PCT/US2023/019876) for the described technology. The other authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Jinyao Liu, Gregg Silverman and Omid Veiseh for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Intraperitoneal immunization with PC-Q11 and CpG adjuvant increases antibody and T-cell responses.
Mice were immunized either i.p. or s.c. at weeks 0, 2 and 4 with PC-Q11 co-assembled with PADRE-Q11 and with or without CpG adjuvant. a, Serum total anti-PC IgG responses showed that i.p. immunization raised the greatest responses and that these responses were vastly improved with the addition of CpG. b, Serum anti-PC IgM responses showed significantly greater IgM for mice immunized i.p. with CpG. c, Week 5 ELISpot of splenocytes restimulated with PADRE peptide showed heightened T-cell responses after immunizations containing CpG with biasing towards IFNγ for i.p. immunized mice. SFC = spot forming cell. AUC = area under the curve. Mean + or ± s.e.m. are shown. Statistical significance determined by two-way RM ANOVA with Tukey’s multiple comparison test for a and b, two-way ANOVA with Šídák’s multiple comparisons test for c. n = 5 mice.
Extended Data Fig. 2 Additional cysteines in nanofibres do not improve anti-PC antibody responses.
Negative-stained TEMs of (a) PC-Q11 (1 mM PC-Q11 co-assembled with 0.2 mM Cys4-Q11, 0.05 mM PADRE-Q11, and 0.75 mM Q11) and (b) PCM-Q11 (0.2 mM PCM-Q11 co-assembled with 0.2 mM Cys4-Q11, 0.05 mM PADRE-Q11, and 1.55 mM Q11) both co-assembled with 10% Cys4-Q11 showed that the addition of excess free cysteines did not inhibit nanofiber formation. c, Mice were immunized i.p. at weeks 0, 2 and 4 with PC-Q11 or PCM-Q11 co-assembled with PADRE-Q11 and with or without Cys4-Q11. Serum total anti-PC IgG responses indicated that the addition of Cys4-Q11 did not significantly enhance anti-PC IgG antibody levels for PC-Q11 or PCM-Q11. Mean + s.e.m. are shown. AUC = area under the curve. ns = not significant via two-way RM ANOVA with Tukey’s multiple comparison test. n = 5 mice.
Extended Data Fig. 3 Increasing PCM-Q11 epitope content 2.5x does not increase anti-PC antibody responses.
Mice were immunized i.p. at weeks 0, 2, 4 and 9 with 10% PCM-Q11 as used in other experiments (0.2 mM PCM-Q11, 1.75 mM Q11, and 0.05 mM PADRE-Q11) or 25% PCM-Q11 with 2.5x the amount of PC epitope (0.5 mM PCM-Q11, 1.45 mM Q11, and 0.05 mM PADRE-Q11) with CpG adjuvant. Serum total anti-PC IgG responses showed that both 10% PCM-Q11 and 25% PCM-Q11 raised robust anti-PC IgG responses that were not statistically different from each other via two-way RM ANOVA. AUC = area under the curve. Mean ± s.e.m. are shown. n = 5 mice.
Extended Data Fig. 4 Administration of an anti-CD4 monoclonal antibody depletes the CD4+ T-cell population.
a, Timeline of CD4+ T-cell depletion experiment. Mice were injected i.p. with 200 μg of either anti-CD4 or isotype control antibody on days -3, 1, 3, 7, 11, 15 and 19 and immunized i.p. on day 0 and 14 with PC-Q11 or PCM-Q11 co-assembled with PADRE-Q11 and CpG adjuvant. b,c, Representative flow plots verifying CD4+ T-cell depletion in unimmunized mice at (b) day 0 and (c) day 22 in the spleen, draining lymph nodes (axial, brachial, and inguinal), and mesenteric lymph nodes. d,e, Representative histograms of the CD4+ population in naïve or depleted mice at (d) day 0 and (e) day 22. n = 2 mice per group per timepoint.
Extended Data Fig. 5 Bacteria cultured from the spleen and serum FITC-dextran levels were not significantly altered after chronic colitis compared to healthy controls.
a, Spleen CFUs, an indication of bacterial spread from colon damage, were not different among groups. b, Measures of FITC-dextran in serum after oral gavage were not different among groups. Data from chronic colitis experiment 2. Mean ± s.e.m. are shown. Lack of statistical significance determined by one-way ANOVA with Tukey’s multiple comparison test. n = 10 mice.
Extended Data Fig. 6 PC immunization reduces bleeding but does not prevent histological damage in an Il10–/– colitis model.
a, Timeline of Il10-/- colitis model where mice were immunized i.p. on days -35, -21 and -7 with PCM-Q11 co-assembled with PADRE-Q11 and CpG adjuvant or PBS (disease control) followed by piroxicam administration in food fines for 7 days to trigger colitis and then 16 additional days of symptom monitoring. b,c, Robust anti-PC IgG (b) and IgM (c) antibody responses were generated in Il10-/- mice. d, IgG subclasses elicited showed the same biasing as in wild-type C57BL/6 mice immunized with PCM-Q11/CpG. e,f, There was no significant difference in weight loss (e) or fecal consistency (f) scores between piroxicam-administered groups. g, PCM-Q11/CpG immunization significantly improved bleeding scores compared to disease controls. h, Both PCM-Q11/CpG and PBS-administered mice given piroxicam developed moderate colitis levels as indicated by total histology scores. i-k, Hematoxylin and eosin-stained sections showed similar colon mucosal hyperplasia and inflammation in Il10-/- mice exposed to piroxicam, whether immunized with PCM-Q11/CpG (i) or with PBS alone (j). Mice immunized with PCM-Q11/CpG but not exposed to piroxicam had minimal to no colitis (k). Distal colon is shown for all mice. Scale bar indicates 200 µm. Mean +, - or ± s.e.m. are shown. Statistical significance determined by mixed-effects analysis with Tukey’s multiple comparison test for b,c and e-g, two-way ANOVA with Tukey’s multiple comparison test for d, and one-way ANOVA with Tukey’s multiple comparison test for h. n = 10 mice for PCM-Q11/CpG with piroxicam group or n = 9 mice for other groups.
Extended Data Fig. 7 Levels of B cells, B1a cells and plasma cells in the colons of mice after immunization, after DSS-induced colitis, and after piroxicam-triggered colitis.
a-d, CD19+ B cells isolated from the colon after: (a) immunization, (b) therapeutic DSS-induced colitis (c) chronic DSS-induced colitis and (d) Il10-/- piroxicam-triggered colitis. e-h, B220+ B cells isolated from the colon after: (e) immunization, (f) therapeutic DSS-induced colitis (g) chronic DSS-induced colitis and (h) Il10-/- piroxicam-triggered colitis. i-l, CD19+CD5+ B1a cells isolated from the colon after: (i) immunization, (j) therapeutic DSS-induced colitis (k) chronic DSS-induced colitis and (l) Il10-/- piroxicam-triggered colitis. m-p, B220+CD138+ plasma cells isolated from the colon after: (m) immunization, (n) therapeutic DSS-induced colitis (o) chronic DSS-induced colitis and (p) Il10-/- piroxicam-triggered colitis. Percentages are reported as the percent of the parent population. Mean ± s.e.m. are shown. Statistical significance determined by one-way ANOVA with Tukey’s multiple comparison test. For immunization group, n = 5 mice, for chronic DSS-induced colitis, n = 10 mice except for the PBS with DSS group where n = 9 mice, for therapeutic colitis, n = 10 mice, and for Il10-/- piroxicam-induced colitis, n = 9 mice except for the PCM-Q11/CpG with piroxicam group where n = 10 mice.
Extended Data Fig. 8 Levels of the tight-junction proteins ZO-1 and occludin in colon sections after immunization and after chronic DSS-induced colitis.
a-c, Immunization with PCM-Q11 co-assembled with PADRE-Q11 and with or without CpG adjuvant at weeks 0, 2 and 4, does not significantly alter ZO-1 or occludin levels in the colon at week 5. a, Representative images showing colon section overviews (top row, scale bar = 500 μm) and region of interest ZO-1 and occludin staining (bottom two rows, scale bar = 50 μm). b,c, ZO-1 (b) and occludin (c) levels are not significantly different in immunized mice versus healthy controls. d-f, ZO-1 and occludin levels in the colon after chronic colitis experiment 2. d, Representative images showing colon section overviews (top row, scale bar = 500 μm) and region of interest ZO-1 and occludin staining (bottom two rows, scale bar = 50 μm). e,f, ZO-1 (e) and occludin (f) levels varied slightly among groups with PCM-Q11/CpG administered mice have significantly less ZO-1 and occludin than some other groups. Mean ± s.e.m. are shown. Statistical significance determined by one-way ANOVA with Tukey’s multiple comparison test. n = 5 mice. DAPI = blue, ZO-1 = green, and occludin = red.
Supplementary information
Main Supplementary Information
Supplementary tables, schemes and figures.
Supplementary Dataset
Source data for the supplementary figures.
Source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Curvino, E.J., Roe, E.F., Freire Haddad, H. et al. Engaging natural antibody responses for the treatment of inflammatory bowel disease via phosphorylcholine-presenting nanofibres. Nat. Biomed. Eng (2023). https://doi.org/10.1038/s41551-023-01139-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41551-023-01139-6