Abstract
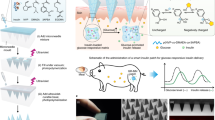
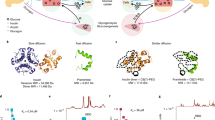
Glucose-responsive formulations of insulin can increase its therapeutic index and reduce the burden of its administration. However, it has been difficult to develop single-dosage formulations that can release insulin in both a sustained and glucose-responsive manner. Here we report the development of a subcutaneously injected glucose-responsive formulation that nearly does not trigger the formation of a fibrous capsule and that leads to week-long normoglycaemia and negligible hypoglycaemia in mice and minipigs with type 1 diabetes. The formulation consists of gluconic acid-modified recombinant human insulin binding tightly to poly-l-lysine modified by 4-carboxy-3-fluorophenylboronic acid via glucose-responsive phenylboronic acid–diol complexation and electrostatic attraction. When the insulin complex is exposed to high glucose concentrations, the phenylboronic acid moieties of the polymers bind rapidly to glucose, breaking the complexation and reducing the polymers’ positive charge density, which promotes the release of insulin. The therapeutic performance of this long-acting single-dose formulation supports its further evaluation and clinical translational studies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The authors declare that all of the data supporting the findings of this study are available within the paper and its Supplementary Information. Source data are provided with this paper.
References
Sun, H. et al. IDF diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 183, 109119 (2022).
Garg, S. K., Rewers, A. H. & Akturk, H. K. Ever-increasing insulin-requiring patients globally. Diabetes Technol. Ther. 20, S21–S24 (2018).
Cefalu, W. T. et al. Insulin access and affordability working group: conclusions and recommendations. Diabetes Care 41, 1299–1311 (2018).
Katsarou, A. et al. Type 1 diabetes mellitus. Nat. Rev. Dis. Primers 3, 17016 (2017).
McCall, A. L. Insulin therapy and hypoglycemia. Endocrinol. Metab. Clin. North Am. 41, 57–87 (2012).
Gordijo, C. R. et al. Nanotechnology-enabled closed loop insulin delivery device: in vitro and in vivo evaluation of glucose-regulated insulin release for diabetes control. Adv. Funct. Mater. 21, 73–82 (2011).
Veiseh, O., Tang, B., Whitehead, K. A., Anderson, D. G. & Langer, R. Managing diabetes with nanomedicine: challenges and opportunities. Nat. Rev. Drug Discov. 14, 45–57 (2015).
Lu, Y., Aimetti, A. A., Langer, R. & Gu, Z. Bioresponsive materials.Nat. Rev. Mater. 2, 16075 (2017).
Bakh, N. A. et al. Glucose-responsive insulin by molecular and physical design. Nat. Chem. 9, 937–943 (2017).
Wang, J. et al. Glucose-responsive insulin and delivery systems: innovation and translation. Adv. Mater. 32, e1902004 (2020).
Jarosinski, M. A., Dhayalan, B., Rege, N., Chatterjee, D. & Weiss, M. A. ‘Smart’ insulin-delivery technologies and intrinsic glucose-responsive insulin analogues. Diabetologia 64, 1016–1029 (2021).
Wang, Z. J., Wang, J. Q., Kahkoska, A. R., Buse, J. B. & Gu, Z. Developing insulin delivery devices with glucose responsiveness. Trends Pharmacol. Sci. 42, 31–44 (2021).
Kitano, S., Koyama, Y., Kataoka, K., Okano, T. & Sakurai, Y. A novel drug delivery system utilizing a glucose responsive polymer complex between poly(vinyl alcohol) and poly(N-vinyl-2-pyrrolidone) with a phenylboronic acid moiety. J. Control. Release 19, 161–170 (1992).
Shiino, D. et al. Amine containing phenylboronic acid gel for glucose-responsive insulin release under physiological pH. J. Control. Release 37, 269–276 (1995).
Hisamitsu, I., Kataoka, K., Okano, T. & Sakurai, Y. Glucose-responsive gel from phenylborate polymer and poly(vinyl alcohol): prompt response at physiological pH through the interaction of borate with amino group in the gel. Pharm. Res. 14, 289–293 (1997).
Kataoka, K., Miyazaki, H., Bunya, M., Okano, T. & Sakurai, Y. Totally synthetic polymer gels responding to external glucose concentration: their preparation and application to on–off regulation of insulin release. J. Am. Chem. Soc. 120, 12694–12695 (1998).
Shen, D. et al. Recent progress in design and preparation of glucose-responsive insulin delivery systems. J. Control. Release 321, 236–258 (2020).
Matsumoto, A., Yoshida, R. & Kataoka, K. Glucose-responsive polymer gel bearing phenylborate derivative as a glucose-sensing moiety operating at the physiological pH. Biomacromolecules 5, 1038–1045 (2004).
Zhao, Y., Trewyn, B. G., Slowing, I. I. & Lin, V. S. Mesoporous silica nanoparticle-based double drug delivery system for glucose-responsive controlled release of insulin and cyclic AMP. J. Am. Chem. Soc. 131, 8398–8400 (2009).
Liang, L. & Liu, Z. A self-assembled molecular team of boronic acids at the gold surface for specific capture of cis-diol biomolecules at neutral pH. Chem. Commun. (Camb.) 47, 2255–2257 (2011).
Matsumoto, A. et al. A synthetic approach toward a self-regulated insulin delivery system. Angew. Chem. Int. Ed. Engl. 51, 2124–2128 (2012).
Chou, D. H. et al. Glucose-responsive insulin activity by covalent modification with aliphatic phenylboronic acid conjugates. Proc. Natl Acad. Sci. USA 112, 2401–2406 (2015).
Dong, Y. et al. Injectable and glucose-responsive hydrogels based on boronic acid–glucose complexation. Langmuir 32, 8743–8747 (2016).
Brooks, W. L. A. & Sumerlin, B. S. Synthesis and applications of boronic acid-containing polymers: from materials to medicine. Chem. Rev. 116, 1375–1397 (2016).
Matsumoto, A. et al. Synthetic ‘smart gel’ provides glucose-responsive insulin delivery in diabetic mice. Sci. Adv. 3, eaaq0723 (2017).
Yu, J. C. et al. Glucose-responsive oral insulin delivery for postprandial glycemic regulation. Nano Res. 12, 1539–1545 (2019).
Wang, J. et al. Charge-switchable polymeric complex for glucose-responsive insulin delivery in mice and pigs. Sci. Adv. 5, eaaw4357 (2019).
Yu, J. et al. Glucose-responsive insulin patch for the regulation of blood glucose in mice and minipigs. Nat. Biomed. Eng. 4, 499–506 (2020).
Xiao, Y., Sun, H. & Du, J. Sugar-breathing glycopolymersomes for regulating glucose level. J. Am. Chem. Soc. 139, 7640–7647 (2017).
Wang, C. et al. Red blood cells for glucose-responsive insulin delivery. Adv. Mater. 29, 1606617 (2017).
Yang, R. et al. A glucose-responsive insulin therapy protects animals against hypoglycemia. JCI Insight 3, e97476 (2018).
Gu, Z. et al. Glucose-responsive microgels integrated with enzyme nanocapsules for closed-loop insulin delivery. ACS Nano 7, 6758–6766 (2013).
Gu, Z. et al. Injectable nano-network for glucose-mediated insulin delivery. ACS Nano 7, 4194–4201 (2013).
Yu, J. C. et al. Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. Proc. Natl Acad. Sci. USA 112, 8260–8265 (2015).
Yu, J. et al. Hypoxia and H2O2 dual-sensitive vesicles for enhanced glucose-responsive insulin delivery. Nano Lett. 17, 733–739 (2017).
Wang, J. et al. Core–shell microneedle gel for self-regulated insulin delivery. ACS Nano 12, 2466–2473 (2018).
Chen, Z. et al. Synthetic beta cells for fusion-mediated dynamic insulin secretion. Nat. Chem. Biol. 14, 86–93 (2018).
Podual, K., Doyle, F. J. & Peppas, N. A. Glucose-sensitivity of glucose oxidase-containing cationic copolymer hydrogels having poly(ethylene glycol) grafts. J. Control. Release 67, 9–17 (2000).
Podual, K., Doyle, F. J. & Peppas, N. A. Dynamic behavior of glucose oxidase-containing microparticles of poly(ethylene glycol)-grafted cationic hydrogels in an environment of changing pH. Biomaterials 21, 1439–1450 (2000).
Podual, K., Doyle, F. J. & Peppas, N. A. Preparation and dynamic response of cationic copolymer hydrogels containing glucose oxidase. Polymer 41, 3975–3983 (2000).
Shiino, D. et al. Preparation and characterization of a glucose-responsive insulin-releasing polymer device. Biomaterials 15, 121–128 (1994).
Chang, R. et al. Nanoporous immunoprotective device for stem-cell-derived beta-cell replacement therapy. ACS Nano 11, 7747–7757 (2017).
Liu, W. et al. Macroencapsulation devices for cell therapy. Engineering 13, 53–70 (2022).
Qiu, Y. B. et al. Long-lasting designer insulin with glucose-dependent solubility markedly reduces risk of hypoglycemia. Adv. Ther. (Weinh) 2, 1900128 (2019).
Chinisaz, M. et al. Structure and function of anhydride-modified forms of human insulin: in silico, in vitro and in vivo studies. Eur. J. Pharm. Sci. 96, 342–350 (2017).
Wang, J. et al. Injectable biodegradable polymeric complex for glucose-responsive insulin delivery. ACS Nano 15, 4294–4304 (2021).
Zhang, D. et al. Dealing with the foreign-body response to implanted biomaterials: strategies and applications of new materials. Adv. Funct. Mater. 31, 2007226 (2021).
Zhang, D. et al. Bio-inspired poly-dl-serine materials resist the foreign-body response. Nat. Commun. 12, 5327 (2021).
Chung, L. et al. Interleukin 17 and senescent cells regulate the foreign body response to synthetic material implants in mice and humans. Sci. Transl. Med. 12, eaax3799 (2020).
Acknowledgements
This work was supported by the grants from National Key R&D Program of China (grant no. 2022YFE0202200), Juvenile Diabetes Research Foundation (grant nos 2-SRA-2021-1064-M-B, 2-SRA-2022-1159-M-B), and Zhejiang University’s start-up packages, the Kunpeng Program grant and Fundamental Research Funds for the Central Universities (grant no. 2021FZZX001-46). A.R.K. is supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant no. KL2TR002490. The project was supported by grant no. UL1TR002489 from the Clinical and Translational Science Award programme of the National Center for Advancing Translational Sciences, National Institutes of Health. We appreciate the help from G. Zhu and Y. Zhang (Cryo-EM Centre, Zhejiang University) for processing of samples for electron microscopy, D. Guo (Division of Hepatobiliary and Pancreatic Surgery, Department of Surgery, The First Affiliated Hospital, Zhejiang University School of Medicine) for slicing and staining skin tissue, C. Sun (Analysis Center of Agrobiology and Environmental Sciences, Zhejiang University) for MALDI-TOF mass analysis and D. Xu (Animal Center, Zhejiang University) for taking care of the minipigs.
Author information
Authors and Affiliations
Contributions
Z.G., J.B.B. and J.W. conceived and designed the study. J.W., J.Z., X.W., W.L., Y.W., W.Z., T.S., Y.Z., Y.L., K.J. and J.X. conducted experiments and obtained data. X.Z. and P.Z. provided experimental and theoretical guidance, respectively. H.Z. and Y.X. conducted western blot experiments and provided theoretical support, respectively. J.W., J.Z., X.W., W.L., Y.Z., W.Z., T.S., J.B.B., A.R.K. and Z.G. analysed the data and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
Z.G. is the co-founder of Zenomics Inc., Zcapsule Inc. and μZen Inc. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
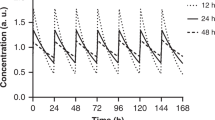
Extended Data Fig. 1 BG regulation by the insulin complex in diabetic mice.
The dose was set to 2.5 (a), 5 (b), 10 (c), and 20 (d) mg/kg. Insulin complex was subcutaneously injected. Data points are means ± SD (n = 5).
Extended Data Fig. 2 Time proportion in the range of hyperglycaemia, normoglycaemia and hypoglycaemia in diabetic minipigs after subcutaneous injection of the complex or of insulin glargine.
Hyperglycaemia, normoglycaemia, and hypoglycaemia were defined as BG more than 200 mg/dL, 50-200 mg/dL, and less than 50 mg/dL. Data points are means ± SD (n = 3). a, Both insulin glargine (0.4 to 0.6 U/kg) and complex (0.2 to 0.3 mg/kg) were injected once at the beginning of treatment. As BG was monitored for 3 days for the insulin-treated group, BG within three days was included in the statistical analysis. b, Insulin glargine (0.4 to 0.6 U/kg) was injected daily for 7 days, and complex (0.2 to 0.3 mg/kg) was injected once at the beginning of treatment. BG within 7 days was included in the statistical analysis. Two-tailed Student’s t-test was used for statistical analysis.
Supplementary information
Supplementary Information
Supplementary Figs. 1–13.
Supplementary Data
Source data for Supplementary Figs. 2 and 3 and 6–9.
Supplementary Data
Unprocessed western blots for Supplementary Fig. 2d.
Source data
Source Data
Statistical source data for Figs. 1–5 and Extended Data Figs. 1–2.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, J., Wei, X., Liu, W. et al. Week-long normoglycaemia in diabetic mice and minipigs via a subcutaneous dose of a glucose-responsive insulin complex. Nat. Biomed. Eng (2023). https://doi.org/10.1038/s41551-023-01138-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41551-023-01138-7