Abstract
Retinal prostheses could restore image-forming vision in conditions of photoreceptor degeneration. However, contrast sensitivity and visual acuity are often insufficient. Here we report the performance, in mice and monkeys with induced photoreceptor degeneration, of subretinally implanted gold-nanoparticle-coated titania nanowire arrays providing a spatial resolution of 77.5 μm and a temporal resolution of 3.92 Hz in ex vivo retinas (as determined by patch-clamp recording of retinal ganglion cells). In blind mice, the arrays allowed for the detection of drifting gratings and flashing objects at light-intensity thresholds of 15.70–18.09 μW mm–2, and offered visual acuities of 0.3–0.4 cycles per degree, as determined by recordings of visually evoked potentials and optomotor-response tests. In monkeys, the arrays were stable for 54 weeks, allowed for the detection of a 10-μW mm–2 beam of light (0.5° in beam angle) in visually guided saccade experiments, and induced plastic changes in the primary visual cortex, as indicated by long-term in vivo calcium imaging. Nanomaterials as artificial photoreceptors may ameliorate visual deficits in patients with photoreceptor degeneration.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The raw and analysed datasets generated during the study are available for research purposes from the corresponding authors on reasonable request. The study using stimuli of natural scenes used 30 natural images from the van Hateren Natural Image Dataset (https://github.com/hunse/vanhateren) and the McGill Calibrated Colour Image Database (http://tabby.vision.mcgill.ca.). Source data are provided with this paper.
Code availability
Custom-written codes used to analyse data from this study are available from the corresponding authors on request.
References
Maguire, A. M. et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N. Engl. J. Med. 358, 2240–2248 (2008).
Cascella, R. et al. Towards the application of precision medicine in age-related macular degeneration. Prog. Retin. Eye Res. 63, 132–146 (2018).
Zhou, H. et al. Glia-to-neuron conversion by CRISPR–CasRx alleviates symptoms of neurological disease in mice. Cell 181, 590–603 (2020).
Mahato, B. et al. Pharmacologic fibroblast reprogramming into photoreceptors restores vision. Nature 581, 83–88 (2020).
Hoang, T. et al. Gene regulatory networks controlling vertebrate retinal regeneration. Science 370, eabb8598 (2020).
Lu, Y. et al. Reprogramming to recover youthful epigenetic information and restore vision. Nature 588, 124–129 (2020).
Maeder, M. L. et al. Development of a gene-editing approach to restore vision loss in Leber congenital amaurosis type 10. Nat. Med. 25, 229–233 (2019).
Sahel, J. A. et al. Partial recovery of visual function in a blind patient after optogenetic therapy. Nat. Med. 27, 1223–1229 (2021).
Roska, B. & Sahel, J. A. Restoring vision. Nature 557, 359–367 (2018).
Sahel, J. A., Bennett, J. & Roska, B. Depicting brighter possibilities for treating blindness. Sci. Transl. Med. 11, eaax2324 (2019).
Humayun, M. S. et al. Visual perception elicited by electrical stimulation of retina in blind humans. Arch. Ophthalmol. 114, 40–46 (1996).
da Cruz, L. et al. Five-year safety and performance results from the argus ii retinal prosthesis system clinical trial. Ophthalmology 123, 2248–2254 (2016).
Wilke, R. et al. Spatial resolution and perception of patterns mediated by a subretinal 16-electrode array in patients blinded by hereditary retinal dystrophies. Invest. Ophthalmol. Vis. Sci. 52, 5995–6003 (2011).
Mills, J. O., Jalil, A. & Stanga, P. E. Electronic retinal implants and artificial vision: journey and present. Eye 31, 1383–1398 (2017).
Stingl, K. et al. Interim results of a multicenter trial with the new electronic subretinal implant alpha AMS in 15 patients blind from inherited retinal degenerations. Front. Neurosci. 11, 445 (2017).
Edwards, T. L. et al. Assessment of the electronic retinal implant alpha ams in restoring vision to blind patients with end-stage retinitis pigmentosa. Ophthalmology 125, 432–443 (2018).
Palanker, D., Le Mer, Y., Mohand-Said, S., Muqit, M. & Sahel, J. A. Photovoltaic restoration of central vision in atrophic age-related macular degeneration. Ophthalmology 127, 1097–1104 (2020).
Palanker, D., Le Mer, Y., Mohand-Said, S. & Sahel, J. A. Simultaneous perception of prosthetic and natural vision in AMD patients. Nat. Commun. 13, 513 (2022).
Lorach, H. et al. Photovoltaic restoration of sight with high visual acuity. Nat. Med. 21, 476–482 (2015).
Wang, B. Y. et al. Electronic photoreceptors enable prosthetic visual acuity matching the natural resolution in rats. Nat. Commun. 13, 6627 (2022).
Huang, T. W. et al. Vertical-junction photodiodes for smaller pixels in retinal prostheses. J. Neural Eng. 18, 036015 (2021).
Ho, E. et al. Temporal structure in spiking patterns of ganglion cells defines perceptual thresholds in rodents with subretinal prosthesis. Sci. Rep. 8, 3145 (2018).
Prevot, P. H. et al. Behavioural responses to a photovoltaic subretinal prosthesis implanted in non-human primates. Nat. Biomed. Eng. 4, 172–180 (2020).
Beauchamp, M. S. et al. Dynamic stimulation of visual cortex produces form vision in sighted and blind humans. Cell 181, 774–783 (2020).
Chen, X., Wang, F., Fernandez, E. & Roelfsema, P. R. Shape perception via a high-channel-count neuroprosthesis in monkey visual cortex. Science 370, 1191–1196 (2020).
Gu, L. et al. A biomimetic eye with a hemispherical perovskite nanowire array retina. Nature 581, 278–282 (2020).
Nelidova, D. et al. Restoring light sensitivity using tunable near-infrared sensors. Science 368, 1108–1113 (2020).
Maya-Vetencourt, J. F. et al. Subretinally injected semiconducting polymer nanoparticles rescue vision in a rat model of retinal dystrophy. Nat. Nanotechnol. 15, 698–708 (2020).
Acaron Ledesma, H. et al. An atlas of nano-enabled neural interfaces. Nat. Nanotechnol. 14, 645–657 (2019).
Tang, J. et al. Nanowire arrays restore vision in blind mice. Nat. Commun. 9, 786 (2018).
Li, J. et al. Nanoscale stacking fault-assisted room temperature plasticity in flash-sintered TiO2. Sci. Adv. 5, eaaw5519 (2019).
Wang, G., Wang, H., Ling, Y., Tang, Y. & Li, Y. Hydrogen-treated TiO2 nanowire arrays for photoelectrochemical water splitting. Nano Lett. 11, 3026–3033 (2011).
Stirman, J., Townsend, L. B. & Smith, S. A touchscreen based global motion perception task for mice. Vis. Res 127, 74–83 (2016).
Delori, F. C., Webb, R. H., Sliney, D. H., & American National Standards Institute. Maximum permissible exposures for ocular safety (ANSI 2000), with emphasis on ophthalmic devices. J. Opt. Soc. Am. A 24, 1250–1265 (2007).
Goetz, G. A. & Palanker, D. V. Electronic approaches to restoration of sight. Rep. Prog. Phys. 79, 096701 (2016).
Xiong, Y.-Z. et al. Relationship between acuity and contrast sensitivity: differences due to eye disease. Invest. Ophthalmol. Vis. Sci. 61, 40–40 (2020).
Schmucker, C. & Schaeffel, F. In vivo biometry in the mouse eye with low coherence interferometry. Vis. Res 44, 2445–2456 (2004).
Ganjawala, T. H., Lu, Q., Fenner, M. D., Abrams, G. W. & Pan, Z. H. Improved CoChR variants restore visual acuity and contrast sensitivity in a mouse model of blindness under ambient light conditions. Mol. Ther. 27, 1195–1205 (2019).
Prusky, G. T., West, P. W. & Douglas, R. M. Behavioral assessment of visual acuity in mice and rats. Vis. Res 40, 2201–2209 (2000).
Sinex, D. G., Burdette, L. J. & Pearlman, A. L. A psychophysical investigation of spatial vision in the normal and reeler mutant mouse. Vis. Res 19, 853–857 (1979).
Prusky, G. T., Alam, N. M., Beekman, S. & Douglas, R. M. Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Invest. Ophthalmol. Vis. Sci. 45, 4611–4616 (2004).
Tomita, H. et al. Channelrhodopsin-2 gene transduced into retinal ganglion cells restores functional vision in genetically blind rats. Exp. Eye Res 90, 429–436 (2010).
Histed, M. H., Carvalho, L. A. & Maunsell, J. H. Psychophysical measurement of contrast sensitivity in the behaving mouse. J. Neurophysiol. 107, 758–765 (2012).
Harvey, B. M. & Dumoulin, S. O. The relationship between cortical magnification factor and population receptive field size in human visual cortex: constancies in cortical architecture. J. Neurosci. 31, 13604–13612 (2011).
Wang, Q. & Burkhalter, A. Area map of mouse visual cortex. J. Comp. Neurol. 502, 339–357 (2007).
Sterratt, D. C., Lyngholm, D., Willshaw, D. J. & Thompson, I. D. Standard anatomical and visual space for the mouse retina: computational reconstruction and transformation of flattened retinae with the Retistruct package. PLoS Comput. Biol. 9, e1002921 (2013).
Roth, M. M. et al. Thalamic nuclei convey diverse contextual information to layer 1 of visual cortex. Nat. Neurosci. 19, 299–307 (2016).
Samonds, J. M., Feese, B. D., Lee, T. S. & Kuhlman, S. J. Nonuniform surround suppression of visual responses in mouse V1. J. Neurophysiol. 118, 3282–3292 (2017).
Schrater, P. R., Knill, D. C. & Simoncelli, E. P. Mechanisms of visual motion detection. Nat. Neurosci. 3, 64–68 (2000).
Fisher, Y. E., Silies, M. & Clandinin, T. R. Orientation selectivity sharpens motion detection in Drosophila. Neuron 88, 390–402 (2015).
Galletti, C. & Fattori, P. Neuronal mechanisms for detection of motion in the field of view. Neuropsychologia 41, 1717–1727 (2003).
Wei, W. Neural mechanisms of motion processing in the mammalian retina. Annu. Rev. Vis. Sci. 4, 165–192 (2018).
Huang, X. et al. Visual stimulation induces distinct forms of sensitization of On–Off direction-selective ganglion cell responses in the dorsal and ventral retina. J. Neurosci. 42, 4449–4469 (2022).
Henschke, J. U. et al. Reward association enhances stimulus-specific representations in primary visual cortex. Curr. Biol. 30, 1866–1880 (2020).
Perez-Ortega, J., Alejandre-Garcia, T. & Yuste, R. Long-term stability of cortical ensembles. eLife 10, e64449 (2021).
Gdalyahu, A. et al. Associative fear learning enhances sparse network coding in primary sensory cortex. Neuron 75, 121–132 (2012).
Kalia, A. et al. Development of pattern vision following early and extended blindness. Proc. Natl Acad. Sci. USA 111, 2035–2039 (2014).
Rolls, E. T. & Tovee, M. J. Sparseness of the neuronal representation of stimuli in the primate temporal visual cortex. J. Neurophysiol. 73, 713–726 (1995).
Vinje, W. E. & Gallant, J. L. Sparse coding and decorrelation in primary visual cortex during natural vision. Science 287, 1273–1276 (2000).
de Vries, S. E. J. et al. A large-scale standardized physiological survey reveals functional organization of the mouse visual cortex. Nat. Neurosci. 23, 138–151 (2020).
Kalloniatis, M., Nivison-Smith, L., Chua, J., Acosta, M. L. & Fletcher, E. L. Using the rd1 mouse to understand functional and anatomical retinal remodelling and treatment implications in retinitis pigmentosa: a review. Exp. Eye Res. 150, 106–121 (2016).
Spoerl, E., Mrochen, M., Sliney, D., Trokel, S. & Seiler, T. Safety of UVA-riboflavin cross-linking of the cornea. Cornea 26, 385–389 (2007).
Watanabe, Y. et al. Development of an optogenetic gene sensitive to daylight and its implications in vision restoration. NPJ Regen. Med. 6, 64 (2021).
Stingl, K. et al. Artificial vision with wirelessly powered subretinal electronic implant alpha-IMS. Proc. Biol. Sci. 280, 20130077 (2013).
Goo, Y. S. et al. Spontaneous oscillatory rhythm in retinal activities of two retinal degeneration (rd1 and rd10) mice. Korean J. Physiol. Pharm. 15, 415–422 (2011).
Stutzki, H., Helmhold, F., Eickenscheidt, M. & Zeck, G. Subretinal electrical stimulation reveals intact network activity in the blind mouse retina. J. Neurophysiol. 116, 1684–1693 (2016).
Ahn, J. et al. Correlated activity in the degenerate retina inhibits focal response to electrical stimulation. Front. Cell Neurosci. 16, 889663 (2022).
Foik, A. T. et al. Detailed visual cortical responses generated by retinal sheet transplants in rats with severe retinal degeneration. J. Neurosci. 38, 10709–10724 (2018).
Muralidharan, M. et al. Neural activity of functionally different retinal ganglion cells can be robustly modulated by high-rate electrical pulse trains. J. Neural Eng. 17, 045013 (2020).
Kasi, H. et al. Simulations to study spatial extent of stimulation and effect of electrode–tissue gap in subretinal implants. Med. Eng. Phys. 33, 755–763 (2011).
Linderholm, P. et al. Long-term in vivo impedance changes of subretinal microelectrodes implanted in dystrophic P23H rats. Int. J. Artif. Organs 36, 612–619 (2013).
Boinagrov, D. et al. Upper threshold of extracellular neural stimulation. J. Neurophysiol. 108, 3233–3238 (2012).
Li, X. et al. Serotonin receptor 2c-expressing cells in the ventral CA1 control attention via innervation of the Edinger–Westphal nucleus. Nat. Neurosci. 21, 1239–1250 (2018).
Peirce, J. W. PsychoPy—Psychophysics software in Python. J. Neurosci. Methods 162, 8–13 (2007).
Ho, E. et al. Characteristics of prosthetic vision in rats with subretinal flat and pillar electrode arrays. J. Neural Eng. 16, 066027 (2019).
van Hateren, J. H. & van der Schaaf, A. Independent component filters of natural images compared with simple cells in primary visual cortex. Proc. Biol. Sci. 265, 359–366 (1998).
Olmos, A. & Kingdom, F. A. A biologically inspired algorithm for the recovery of shading and reflectance images. Perception 33, 1463–1473 (2004).
Giovannucci, A. et al. CaImAn an open source tool for scalable calcium imaging data analysis. eLife 8, e38173 (2019).
Pnevmatikakis, E. A. & Giovannucci, A. NoRMCorre: an online algorithm for piecewise rigid motion correction of calcium imaging data. J. Neurosci. Methods 291, 83–94 (2017).
Pnevmatikakis, E. A. et al. Simultaneous denoising, deconvolution, and demixing of calcium imaging data. Neuron 89, 285–299 (2016).
Matsuo, T. et al. Visual evoked potential recovery by subretinal implantation of photoelectric dye-coupled thin film retinal prosthesis in monkey eyes with macular degeneration. Artif. Organs 42, E186–E203 (2018).
Tu, H. Y. et al. Medium- to long-term survival and functional examination of human iPSC-derived retinas in rat and primate models of retinal degeneration. EBioMedicine 39, 562–574 (2019).
Acknowledgements
This work was supported by the MOST of China (2022ZD0208604 and 2022ZD0208605 to J.Z.; 2022ZD0210000 to B.Y.), NSF of China (31771195, 81790640, 81773513 and 820712002 to J.Z.; 32100803 to B.Y.), Shanghai Municipal Science and Technology Major Project (no. 2018SHZDZX01 to J.Z.) and ZJLab, the Key Research and Development Program of Ningxia (no. 2022BEG02046 to J.Z.), Sanming Project of Medicine in Shenzhen (SZSM202011015) to J.Z., Key Scientific Technological Innovation Research Project by Ministry of Education to J.Z., Research and Development Fund of Zhongshan Hospital (2020ZSFZ19 to Y.Y.).
Author information
Authors and Affiliations
Contributions
J.Z., B.Y. and Y.Y. conceived the study. J.Z. and B.Y. wrote the paper. R.Y. performed the behavioural experiments of mice and monkeys, performed ex vivo patch-clamp experiments, refined the techniques and analysed the data, with help from P.Z., Z.W, K.S., X.C. and T.Z. C.P. and G.Z. synthesized the AuTiO2-x NW arrays. P.Z., R.Y. and Y.C. developed two-photon microscope setups and chronic calcium imaging. R.Y., X.C., T.Z. and S.W. cut and polished the AuTiO2-x NW arrays. Q.C., L.L., Chengyong J. and G.W. analysed the VEP data. Z.W., Shanshan J., J.L., Z.Y., S.W. and G.W. performed data analysis about patch-clamp experiments. L.W., M.S., C.D., Y.L., X.Z., X.M., Chunhui J. and Y.Y. conducted the surgical procedures for NW array implantation in monkey. C.F., Y.L, Y.W., Y.Z., A.C., F.Z., F.W., Su J. and W.X. worked on monkeys’ eyes examinations. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
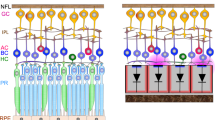
Extended Data Fig. 1 Characterization of the photocurrent in AuTiO2-x NW arrays.
a, Schematic of AuTiO2-x NW arrays photocurrent measurement with UV (375/28 nm, 292.99 μW·mm−2), blue (470/20 nm, 318.47 μW·mm−2) or green (546/12 nm, 420.38 μW·mm−2). The photocurrents were measured by Ag/AgCl electrodes attached to the surface of the NW arrays in normal saline solution. b, Photocurrents generated by illumination of UV, blue, green and red light, receptively with an AuTiO2-x NW arrays. c, UV-visible absorbance spectra of original TiO2 and AuTiO2-x. d, Compared with the published results30, the photocurrent of improved NW arrays increased by 760.08%, 559.48% and 556.80% under UV, blue and green light, receptively. e,f, Example of photocurrent measures obtained with UV light stimulus (DMD, wavelength: 400 nm) at 10 μW·mm−2 (e) and 3 μW·mm−2 (f) for 0.005–1.0 sec pulse duration. g, Photocurrent at 10 μW·mm−2 (top) and 3 μW·mm−2 (bottom) for 1.0, 0.5, 0.2, 0.1, 0.05, 0.025 and 0.005 sec in e and f. h, Representative photocurrent recording with UV stimulus (DMD, wavelength: 400 nm, light intensity: 10 μW·mm−2) at different flicker frequency (Magenta, 1.0 Hz; blue, 2.5 Hz; cyan, 5.0 Hz; green, 10.0 Hz; red, 20 Hz). i, Zoom in photocurrent of gray rectangle in h. Dashed black line indicate the stimuli on. j, Mean latency of NW arrays to light stimulus (n = 31 measurements from 4 NW arrays). Latency was defined as the time difference between the onset of the visual stimuli and the time that photocurrent reaches the peak value. All data are expressed as mean ± SEM.
Extended Data Fig. 2 Miss rate of training trials and choice-box-based light localization test with flashing light at 20 Hz flicker frequency.
a, Miss rates of normal mice (red, n = 3 mice), blind mice with glass implant (black, n = 3 mice) and blind mice with NW arrays implant (blue, n = 4 mice) during training trials. Green line indicates 50% miss rate. b, Correct rates of normal mice (n = 3 mice), blind mice with glass implant (n = 3 mice) and blind mice with NW arrays-implanted (n = 5 mice) in the choice-box-based behavioural test of light localization with flashing light at 20 Hz flicker frequency (pulse duration: 25 msec). c, Correct rates of normal mice (85.02%, red, n = 3 mice), blind mice with glass implant (15.68%, black, n = 3 mice) and blind mice with NW arrays implant (82.07%, blue, n = 5 mice). The correct rates of blind mice with NW arrays implant had no significant difference between flash light at 20 Hz flicker frequency lasted for 10 sec (filled blue column) and 10-sec-long light (hollow blue column). All data are expressed as mean ± SEM. Statistical significance was determined using one-way ANOVA with Tukey’s multiple comparisons in c (flash light), and two-sided unpaired t-test in c (10-sec-long light). NS non-significant. *** P < 0.001.
Extended Data Fig. 3 Choice-box-based static object discrimination test, light localization test, flashing detection test and moving bar detection test of blind mice with the NW arrays implant.
a, Schematic of static object discrimination test. Black dashed line indicates the range where the object was placed. b, Correct rate of normal mice (red, n = 3 mice), blind mice with glass implant (n = 3 mice) and blind mice with NW arrays implant (n = 5 mice). c, Correct rate of 4-week group and 1-week group in choice-box-based light localization test using a UV LED (375/15 nm, 6.24 μW·mm−2). d, Correct rates of normal mice (n = 3 mice), blind mice with glass implant (n = 3 mice) and blind mice with NW arrays implant for 1 week (n = 4 mice) in the choice-box-based behavioural test using flashing light. e, Correct rates of normal mice (n = 3 mice), blind mice with glass implant (n = 3 mice) and blind mice with NW arrays implant for 1 week (n = 4 mice) in the choice-box-based behavioural test using moving light bars (5.25 degrees, 7.85 degrees/sec, 13.38 μW·mm−2). All data are expressed as mean ± SEM. Statistical significance was determined using one-way ANOVA with Tukey’s multiple comparisons in b, two-way RM ANOVA with Tukey’s multiple comparisons in c. * P < 0.05, *** P < 0.001.
Extended Data Fig. 4 Responses of RGCs to flashing light in the normal retina.
a, Schematic of flashing light stimuli. b, Examples responses of RGC to flashing light stimuli in normal retina (Temporal frequency: 0.50-20.0 Hz).
Extended Data Fig. 5 Responses of RGCs to moving light bars in NW arrays-attached blind retina ex vivo, and choice-box-based behavioural test using moving light bars.
a, Schematics of ex vivo patch-clamp recording with moving light bars stimuli. b-d, Responses of representative RGCs to moving light bars stimulus (DMD, wavelength: 400 nm, light intensity: 10 μW·mm−2, width: 120 μm, speed: 200 μm/sec) in normal, blind and NW-array-attached blind retinas. e, Responses of a representative RGC to moving light bars stimuli with different width (width: 20–180 μm). f-g, Average firing rates and fraction of responses of RGCs in normal (n = 9 RGCs from 6 retinas), blind (n = 10 RGCs from 8 retinas) and NW-array-attached blind retinas (16 RGCs from 10 retinas) using moving light bars with width ranging from 0.64 to 5.76 degrees. h, Schematics of the choice-box-based moving bar detection test using moving and static light bar stimuli. i, Correct rate of normal mice (n = 6 mice), blind mice with glass implant (n = 6 mice) and blind mice with NW arrays implant (n = 8 mice) in moving bar detection test (5.25 degrees in width at 7.85 degrees/sec). j-k, Correct rate of normal mice (n = 3 mice), blind mice with glass implant (n = 3 mice) and blind mice with NW arrays implant (n = 4 mice) in moving bar detection test with moving light bars of different widths and speeds (width: 2.63–7.88 degrees, speed: 5.23–10.47 degrees/sec). All data are expressed as mean ± SEM. Statistical significance was determined using one-way ANOVA with Tukey’s multiple comparisons in g and i, two-way RM ANOVA with Tukey’s multiple comparisons in j and k. * indicates a significant difference between blind mice with NW arrays and blind mice with glass implant, # indicates a significant difference between blind mice with NW arrays and normal mice. * and # P < 0.05, ** and ## P < 0.01, *** P < 0.001.
Extended Data Fig. 6 Long-term light-evoked responses using two-photon calcium imaging in V1 of NW-array-implanted blind mice in vivo.
a, Schematic of the position of the cranial window in V1 and the close-up of a completed cranial window by the day of surgery. b, The number of neurons in imaging site (n = 4 sites from 3 mice) recorded at different time points throughout the experiment. c,e, Grayscale images of recording fields of two blind mice before implantation (c) and 7 days after implantation (e). 14 representative light-responsive neurons were marked by red contours. Scale bars, 50 μm. d,f, The fluorescence changes of neurons in c and e in response to visual stimulations (Blue LED, 465/25 nm, 6.75 μW·mm−2, duration: 1 second). Dashed blue lines indicate the onset time of the visual stimulations.
Extended Data Fig. 7 Responses to natural scenes in V1 neurons of NW-array-implanted blind mice.
a, Schematics of two-photon calcium imaging with natural scenes, which were presented for 0.5 sec followed by 5 sec inter-image gray periods. b, Examples of natural scenes and heatmaps of cellular responses to the natural scenes in normal mice (left, n = 3 mice), blind mice (middle, n = 4 mice) and blind mice with NW arrays implant (right, n = 4 mice). Each axial column represented responses of 30 neurons to one natural scene, with the strongest response positioned at the core. c, Fraction of neurons that responded to at least one natural scene in normal mice (72.36%, n = 3 mice), blind mice (16.69%, n = 4 mice) and blind mice with NW arrays implant (56.71%, n = 4 mice). d, Average lifetime sparseness of neurons in normal mice (0.61, n = 3 mice) and blind mice with NW arrays implant (0.54, n = 4 mice). All data are expressed as mean ± SEM. Statistical significance was determined using one-way ANOVA with Tukey’s multiple comparisons in c, two-sided unpaired t-test in d. NS non-significant, * P < 0.05, ** P < 0.01.
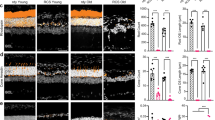
Extended Data Fig. 8 Biocompatibility and dendritic structural analysis of implants in mice.
a, A three-view diagram showing the 3-D outline of an implanted region and ROIs chosen for quantitative analysis. The shape of the NW arrays was represented by the convex of retina since it didn’t emit detectable fluorescence signals. I: Interface, O: Outskirts. b, Example slices of z-stack images cropped by the ROIs in a, cross-dot markers illustrating identification of cells in these slices from corresponding fluorescence signals. Scale bar, 20 μm. c, 3-D reconstructed models of retinas at the borders of the NW arrays at 3 timepoints. Models were presented in x-y view (upper row) and x-z view with y axis rotated for 40 degrees towards z axis (lower row). The dark areas on the lower right side of the models were the edges of NW arrays. Scale bar, 40 μm. d,e, Quantitative analysis of RGC numbers (d) and RBP numbers (e) at 3 timepoints (n = 5 sites for 2 weeks, n = 3 sites for 4 weeks and 6 months). Cell numbers of RGCs and RBPs in each ROI at the interface were normalized to the average cell numbers of RGCs and RBPs of all ROIs at outskirt regions, respectively. f, Typical late-stage RP affected RBP structure from the intact eye of an implanted mouse. The mouse was implanted with NW arrays in another eye at P60 and was 10 months old upon perfusion. The upper graph showed the locations of two 30 μm × 30 μm ROIs (marked by red dashed squares) in a 200 μm × 200 μm window, generated by the maximum intensity projection of three consecutive slices in a z-stack. Two rows of zoomed-in ROIs showed the dendritic features of RBPs (marked by white dashed circles) in blind mice. Z-distances between two consecutive slices were 2 μm. Scale bars, 30 μm for top panels, 10 μm for bottom panels. g,h, Dendritic structures of interface RBPs from the retina of implanted eyes after 2 weeks (g) and 8 months (h). Subfigures were aligned as in f. Red arrows indicated the dendritic bifurcations. Scale bars, 30 μm for top panels, 10 μm for bottom panels. The experiments in a, b, c, f and g were carried out at least three times. The experiments in h were carried out two times. All data are expressed as mean ± SEM.
Extended Data Fig. 9 VGS performance in Monkey A.
a, Standard deviation of Monkey A’s horizontal and vertical eye traces during fixation before photocoagulation (n = 64 trials), 2 weeks (n = 5 trials) and 11 months (n = 218 trials) after photocoagulation. b, Schematic of VGS task. The gray area indicates target area. c, Example traces of eye position and velocity in the VGS task. Dashed black lines indicate value of 0. d, Distribution of normalized saccadic endpoints in normal retinal regions (eccentricities: 19 and 21 degrees, polar angles: 40, 50 and 130 degrees) with red LED (642/18 nm) stimulus. e-h, Distribution of normalized saccadic endpoints using red LED in NW-array-implanted region (eccentricities: 19 and 21 degrees, polar angles: 200–250 degrees) before implantation, 5–7 weeks, 10–12 weeks and 14–15 weeks after implantation. i, Distribution of normalized saccadic endpoints in normal retinal regions (eccentricities: 19 and 21 degrees, polar angles: 40, 50 and 130 degrees) with blue (465/25 nm) and UV (360/15 nm) LED stimulus. j, Illustration of mean hit rates in VGS task of Monkey A with blue LED stimuli (0.5°, polar angle: 190 to 270 degrees, eccentricity: 19 and 21 degrees) 14–15 weeks after implantation. k, Example saccadic trace of Monkey A in response to blue stimuli in the target points (polar angle: 190 to 270 degrees, eccentricity: 21 degrees), corresponding to j. l, Mean hit rates of VGS task in d-i. Normal region: n = 40 sessions for Red LED stimuli, n = 23 sessions for Blue LED stimuli. NW arrays implanted region: n = 20 sessions for Before, n = 12 sessions for 5–7 weeks, n = 14 sessions for 10–12 weeks, n = 18 sessions for 14–15 weeks. All data are expressed as mean ± SEM. Statistical significance was determined using one-way ANOVA with Tukey’s multiple comparisons in l. *** P < 0.001.
Extended Data Fig. 10 Saccade latency, hit rate and distance between saccadic endpoint and target point in VGS task.
a, Saccade latency of monkey A (left) and E (right). Left: saccade latency of monkey A to UV LED stimuli in normal regions and NW arrays implanted regions at 14–15 weeks after implantation. Right: saccade latency of monkey E to UV LED stimuli in normal regions and NW arrays implanted regions at 18–20 weeks after implantation. Monkey A: normal, n = 285 trials, implant, n = 68 trials, Monkey E: normal, n = 44 trials, implant, n = 72 trials. b, Saccade latency for 1000 msec and 250 msec illuminations with UV LED stimuli in NW-array-implanted region (left) and red LED stimuli in normal region (right). UV LED stimuli: 360/15 nm, 10.00 μW·mm−2. Red LED stimuli: 642/18 nm, 10.00 μW·mm−2. NW-array-implanted region: 1000 msec, n = 60 trials; 250 msec, n = 60 trials. Normal region: 1000 msec, n = 60 trials; 250 msec, n = 40 trials. Dashed black line indicates 250 msec of saccade latency. c, Hit rate for 1000 msec and 250 msec illuminations with UV LED stimuli in NW-array-implanted region (left) and red LED stimuli in normal region (right). NW-array-implanted region: 1000 msec, n = 19 sessions; 250 msec, n = 13 sessions. Normal region: 1000 msec, n = 14 sessions; 250 msec, n = 13 sessions. d, Distance between saccadic endpoint and target point for 1000 msec and 250 msec illuminations with UV LED stimuli in NW-array-implanted region (left) and red LED stimuli in normal region (right). NW-array-implanted region: 1000 msec, n = 60 trials; 250 msec, n = 60 trials. Normal region: 1000 msec, n = 60 trials; 250 msec, n = 40 trials. All data are expressed as mean ± SEM. Statistical significance was determined using two-sided unpaired t-test in b, c and d. NS non-significant.
Supplementary information
Supplementary Information
Supplementary figures, tables and video captions.
Supplementary Video 1
Choice-box-based light localization test with green-LED-light stimulus.
Supplementary Video 2
Choice-box-based static-object-discrimination test.
Supplementary Video 3
Choice-box-based static-flashing-detection test.
Supplementary Video 4
Visual-acuity and contrast-sensitivity tests.
Supplementary Video 5
Choice-box-based orientation-discrimination test.
Supplementary Video 6
Choice-box-based moving-bar-detection test.
Supplementary Video 7
Light-evoked responses in V1 neurons through two-photon calcium-imaging recording.
Supplementary Video 8
VGS task with red LED stimulus.
Supplementary Video 9
VGS task with blue LED stimulus.
Source data
Source Data Fig. 1
Source data and statistics.
Source Data Fig. 2
Source data and statistics.
Source Data Fig. 3
Source data and statistics.
Source Data Fig. 4
Source data and statistics.
Source Data Fig. 5
Source data and statistics.
Source Data Fig. 7
Source data and statistics.
Source Data Extended Data Fig. 1
Source data and statistics.
Source Data Extended Data Fig. 2
Source data and statistics.
Source Data Extended Data Fig. 3
Source data and statistics.
Source Data Extended Data Fig. 5
Source data and statistics.
Source Data Extended Data Fig. 6
Source data and statistics.
Source Data Extended Data Fig. 7
Source data and statistics.
Source Data for Extended Data Fig. 8
Source data and statistics.
Source Data for Extended Data Fig. 9
Source data and statistics.
Source Data for Extended Data Fig. 10
Source data and statistics.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, R., Zhao, P., Wang, L. et al. Assessment of visual function in blind mice and monkeys with subretinally implanted nanowire arrays as artificial photoreceptors. Nat. Biomed. Eng (2023). https://doi.org/10.1038/s41551-023-01137-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41551-023-01137-8