Abstract
As a chronic autoinflammatory condition, ulcerative colitis is often managed via systemic immunosuppressants. Here we show, in three mouse models of established ulcerative colitis, that a subcutaneously injected colon-specific immunosuppressive niche consisting of colon epithelial cells, decellularized colon extracellular matrix and nanofibres functionalized with programmed death-ligand 1, CD86, a peptide mimic of transforming growth factor-beta 1, and the immunosuppressive small-molecule leflunomide, induced intestinal immunotolerance and reduced inflammation in the animals’ lower gastrointestinal tract. The bioengineered colon-specific niche triggered autoreactive T cell anergy and polarized pro-inflammatory macrophages via multiple immunosuppressive pathways, and prevented the infiltration of immune cells into the colon’s lamina propria, promoting the recovery of epithelial damage. The bioengineered niche also prevented colitis-associated colorectal cancer and eliminated immune-related colitis triggered by kinase inhibitors and immune checkpoint blockade.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. The raw and analysed datasets generated during the study are available for research purposes from the corresponding authors on reasonable request. Source data are provided with this paper.
References
Danese, S. & Fiocchi, C. Medical progress ulcerative colitis. N. Engl. J. Med. 365, 1713–1725 (2011).
Head, K. A. & Jurenka, J. S. Inflammatory bowel disease part 1: ulcerative colitis—pathophysiology and conventional and alternative treatment options. Alter. Med. Rev. 8, 247–283 (2003).
Ungaro, R., Colombel, J.-F., Lissoos, T. & Peyrin-Biroulet, L. A treat-to-target update in ulcerative colitis: a systematic review. Am. J. Gastroenterol. 114, 874–883 (2019).
Garduno, R. C. & Dabritz, J. New insights on CD8+ T cells in inflammatory bowel disease and therapeutic approaches. Front. Immunol. 12, 738762 (2021).
Na, Y. R., Stakenborg, M., Seok, S. H. & Matteoli, G. Macrophages in intestinal inflammation and resolution: a potential therapeutic target in IBD. Nat. Rev. Gastroenterol. Hepatol. 16, 531–543 (2019).
Isidro, R. A. & Appleyard, C. B. Colonic macrophage polarization in homeostasis, inflammation, and cancer. Am. J. Physiol. Gastrointest. Liver Physiol. 311, G59–G73 (2016).
Seyedian, S. S., Nokhostin, F. & Malamir, M. D. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J. Med. Life 12, 113–122 (2019).
Dahlgren, D., Agréus, L., Stålhammar, J. & Hellström, P. M. Ulcerative colitis progression: a retrospective analysis of disease burden using electronic medical records. Ups. J. Med. Sci. 127, e8833 (2022).
Loddo, I. & Romano, C. Inflammatory bowel disease: genetics, epigenetics, and pathogenesis. Front. Immunol. 6, 551 (2015).
Neuman, M. G. Immune dysfunction in inflammatory bowel disease. Transl. Res. 149, 173–186 (2007).
Zenlea, T. & Peppercorn, M. A. Immunosuppressive therapies for inflammatory bowel disease. World J. Gastroenterol. 20, 3146–3152 (2014).
Sands, B. E. Immunosuppressive drugs in ulcerative colitis: twisting facts to suit theories? Gut 55, 437–441 (2006).
Singh, J. A., Hossain, A., Kotb, A. & Wells, G. Risk of serious infections with immunosuppressive drugs and glucocorticoids for lupus nephritis: a systematic review and network meta-analysis. BMC Med. 14, 137 (2016).
Vial, T. Immunosuppressive drugs and cancer. Toxicology 185, 229–240 (2003).
Dantal, J. & Soulillou, J.-P. Immunosuppressive drugs and the risk of cancer after organ transplantation. N. Engl. J. Med. 352, 1371–1373 (2005).
Sabatos-Peyton, C. A., Verhagen, J. & Wraith, D. C. Antigen-specific immunotherapy of autoimmune and allergic diseases. Curr. Opin. Immunol. 22, 609–615 (2010).
Wraith, D. Autoimmunity: antigen-specific immunotherapy. Nature 530, 422–423 (2016).
Clemente-Casares, X. et al. Expanding antigen-specific regulatory networks to treat autoimmunity. Nature 530, 434–440 (2016).
Chen, X. et al. Modular immune-homeostatic microparticles promote immune tolerance in mouse autoimmune models. Sci. Transl. Med. 13, eaaw9668 (2021).
Fathallah, A. M., Bankert, R. B. & Balu-Iyer, S. V. Immunogenicity of subcutaneously administered therapeutic proteins—a mechanistic perspective. AAPS J. 15, 897–900 (2013).
Cook, I. F. Subcutaneous vaccine administration—an outmoded practice. Hum. Vaccin. Immunother. 17, 1329–1341 (2021).
Wang, Y. et al. Long-term culture captures injury–repair cycles of colonic stem cells. Cell 179, 1144–1159.e15 (2019).
Neal, M. D., Richardson, W. M., Sodhi, C. P., Russo, A. & Hackam, D. J. Intestinal stem cells and their roles during mucosal injury and repair. J. Surg. Res. 167, 1–8 (2011).
Francisco, L. M., Sage, P. T. & Sharpe, A. H. The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 236, 219–242 (2010).
Au, K. M., Medik, Y., Ke, Q., Tisch, R. & Wang, A. Z. Immune checkpoint-bioengineered beta cell vaccine reverses early-onset type 1 diabetes. Adv. Mater. 33, e2101253 (2021).
Au, K. M., Tisch, R. & Wang, A. Z. Immune checkpoint ligand bioengineered schwann cells as antigen-specific therapy for experimental autoimmune encephalomyelitis. Adv. Mater. 34, e2107392 (2021).
Wei, Y. et al. PD-L1 induces macrophage polarization toward the M2 phenotype via Erk/Akt/mTOR. Exp. Cell. Res. 402, 112575 (2021).
Dahlen, E., Hedlund, G. & Dawe, K. Low CD86 expression in the nonobese diabetic mouse results in the impairment of both T cell activation and CTLA-4 up-regulation. J. Immunol. 164, 2444–2456 (2000).
Kovarik, J. M. & Burtin, P. Immunosuppressants in advanced clinical development for organ transplantation and selected autoimmune diseases. Expert Opin. Emerg. Drugs 8, 47–62 (2003).
Cutolo, M. et al. Anti-inflammatory effects of leflunomide on cultured synovial macrophages from patients with rheumatoid arthritis. Ann. Rheum. Dis. 62, 297–302 (2003).
Johnston, C. J. C. et al. A structurally distinct TGF-beta mimic from an intestinal helminth parasite potently induces regulatory T cells. Nat. Commun. 8, 1741 (2017).
Vaz, E. R. et al. A short peptide that mimics the binding domain of TGF-beta 1 presents potent anti-inflammatory activity. PLoS ONE 10, e0136116 (2015).
Zhang, F. et al. TGF-beta induces M2-like macrophage polarization via SNAIL-mediated suppression of a pro-inflammatory phenotype. Oncotarget 7, 52294–52306 (2016).
Gong, D. et al. TGF beta signaling plays a critical role in promoting alternative macrophage activation. BMC Immunol. 13, 31 (2012).
Cao, C., Chen, F., Garvey, C. J. & Stenzel, M. H. Drug-directed morphology changes in polymerization-induced self-assembly (PISA) influence the biological behavior of nanoparticles. ACS Appl. Mater. Interfaces 12, 30221–30233 (2020).
Lowe, A. B. RAFT alcoholic dispersion polymerization with polymerization-induced self-assembly. Polymer 106, 161–181 (2016).
Sugihara, S., Blanazs, A., Armes, S. P., Ryan, A. J. & Lewis, A. L. Aqueous dispersion polymerization: a new paradigm for in situ block copolymer self-assembly in concentrated solution. J. Am. Chem. Soc. 133, 15707–15713 (2011).
Ilavsky, J. & Jemian, P. R. Irena: tool suite for modeling and analysis of small-angle scattering. J. Appl. Crystallogr. 42, 347–353 (2009).
Chen, H. J. et al. A recellularized human colon model identifies cancer driver genes. Nat. Biotechnol. 34, 845–851 (2016).
Giobbe, G. G. et al. Extracellular matrix hydrogel derived from decellularized tissues enables endodermal organoid culture. Nat. Commun. 10, 5658 (2019).
Mannon, P. J. et al. Successful granulocyte-colony stimulating factor treatment of Crohn’s disease is associated with the appearance of circulating interleukin-10-producing T cells and increased lamina propria plasmacytoid dendritic cells. Clin. Exp. Immunol. 155, 447–456 (2009).
Beck, P. L. & Podolsky, D. K. Growth factors in inflammatory bowel disease. Inflamm. Bowel Dis. 5, 44–60 (1999).
Sainathan, S. K. et al. Granulocyte macrophage colony-stimulating factor ameliorates DSS-induced experimental colitis. Inflamm. Bowel Dis. 14, 88–99 (2008).
Meran, L. et al. Engineering transplantable jejunal mucosal grafts using patient-derived organoids from children with intestinal failure. Nat. Med. 26, 1593–1601 (2020).
Singh, R., Singh, D. & Singh, A. Radiation sterilization of tissue allografts: a review. World J. Radiol. 8, 355–369 (2016).
Spang, M. T. et al. Intravascularly infused extracellular matrix as a biomaterial for targeting and treating inflamed tissues. Nat. Biomed. Eng. 7, 94–109 (2023).
Alvarez, Z. et al. Artificial extracellular matrix scaffolds of mobile molecules enhance maturation of human stem cell-derived neurons. Cell Stem Cell 30, 219–238.e14 (2023).
Trickett, A. & Kwan, Y. L. T cell stimulation and expansion using anti-CD3/CD28 beads. J. Immunol. Methods 275, 251–255 (2003).
Anderson, A. C., Joller, N. & Kuchroo, V. K. Lag-3, Tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity 44, 989–1004 (2016).
Lyons, A. B. Analysing cell division in vivo and in vitro using flow cytometric measurement of CFSE dye dilution. J. Immunol. Methods 243, 147–154 (2000).
Shearer, G. M. Cell-mediated cytotoxicity to trinitrophenyl-modified syngeneic lymphocytes. Eur. J. Immunol. 4, 527–533 (1974).
Nancey, S. et al. CD8+ cytotoxic T cells induce relapsing colitis in normal mice. Gastroenterology 131, 485–496 (2006).
Chassaing, B., Aitken, J. D., Malleshappa, M. & Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 104, 15.25.1–15.25.14 (2014).
Eichele, D. D. & Kharbanda, K. K. Dextran sodium sulfate colitis murine model: an indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis. World J. Gastroenterol. 23, 6016–6029 (2017).
Kuhn, R., Lohler, J., Rennick, D., Rajewsky, K. & Muller, W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75, 263–274 (1993).
Flannigan, K. L. et al. An intact microbiota is required for the gastrointestinal toxicity of the immunosuppressant mycophenolate mofetil. J. Heart Lung Transpl. 37, 1047–1059 (2018).
Johnson, B. M. et al. STING agonist mitigates experimental autoimmune encephalomyelitis by stimulating type I IFN-dependent and -independent immune-regulatory pathways. J. Immunol. 206, 2015–2028 (2021).
Hong, J. Y. et al. Decellularized brain matrix enhances macrophage polarization and functional improvements in rat spinal cord injury. Acta Biomater. 101, 357–371 (2020).
Roth, L., MacDonald, J. K., McDonald, J. W. & Chande, N. Sargramostim (GM-CSF) for induction of remission in Crohn’s disease: a cochrane inflammatory bowel disease and functional bowel disorders systematic review of randomized trials. Inflamm. Bowel Dis. 18, 1333–1339 (2012).
Giuffrida, E. et al. Risk of colorectal cancer in inflammatory bowel disease: prevention and monitoring strategies according with risk factors. Clin. Manag. Issues https://doi.org/10.7175/cmi.v15i1.1464 (2021).
Lakatos, P. L. & Lakatos, L. Risk for colorectal cancer in ulcerative colitis: changes, causes and management strategies. World J. Gastroenterol. 14, 3937–3947 (2008).
De Robertis, M. et al. The AOM/DSS murine model for the study of colon carcinogenesis: from pathways to diagnosis and therapy studies. J. Carcinog. 10, 9 (2011).
Parang, B., Barrett, C. W. & Williams, C. S. AOM/DSS model of colitis-associated cancer. Methods Mol. Biol. 1422, 297–307 (2016).
Mensah, F. A., Blaize, J. P. & Bryan, L. J. Spotlight on copanlisib and its potential in the treatment of relapsed/refractory follicular lymphoma: evidence to date. Onco Targets Ther. 11, 4817–4827 (2018).
Killock, D. Copanlisib safe and active in combination. Nat. Rev. Clin. Oncol. 18, 322 (2021).
Eschweiler, S. et al. Intermittent PI3K delta inhibition sustains anti-tumour immunity and curbs irAEs. Nature 605, 741–746 (2022).
Yan, J., Yang, S., Tian, H., Zhang, Y. & Zhao, H. Copanlisib promotes growth inhibition and apoptosis by modulating the AKT/FoxO3a/PUMA axis in colorectal cancer. Cell Death Dis. 11, 943 (2020).
Som, A. et al. Immune checkpoint inhibitor-induced colitis: a comprehensive review. World J. Clin. Cases 7, 405–418 (2019).
Westdorp, H. et al. Mechanisms of immune checkpoint inhibitor-mediated colitis. Front. Immunol. 12, 768957 (2021).
Wolchok, J. D., Rollin, L. & Larkin, J. Nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 377, 2503–2504 (2017).
Motzer, R. J. et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N. Engl. J. Med. 378, 1277–1290 (2018).
Hellmann, M. D. et al. Nivolumab plus ipilimumab in lung cancer with a high tumour mutational burden. N. Engl. J. Med. 378, 2093–2104 (2018).
Bury, M. I. et al. Self‐assembling nanofibers inhibit inflammation in a murine model of Crohn’s‐disease‐like ileitis. Adv. Ther. 4, 2000274 (2021).
Okamoto, R. et al. Organoid-based regenerative medicine for inflammatory bowel disease. Regen. Ther. 13, 1–6 (2020).
Sharma, P., Gangopadhyay, D., Mishra, P. C., Mishra, H. & Singh, R. K. Detection of in vitro metabolite formation of leflunomide: a fluorescence dynamics and electronic structure study. J. Med. Chem. 59, 3418–3426 (2016).
Neri, S., Mariani, E., Meneghetti, A., Cattini, L. & Facchini, A. Calcein-acetyoxymethyl cytotoxicity assay: standardization of a method allowing additional analyses on recovered effector cells and supernatants. Clin. Diagn. Lab. Immunol. 8, 1131–1135 (2001).
Genin, M., Clement, F., Fattaccioli, A., Raes, M. & Michiels, C. M1 and M2 macrophages derived from THP-1 cells differentially modulate the response of cancer cells to etoposide. BMC Cancer 15, 577 (2015).
Orecchioni, M., Ghosheh, Y., Pramod, A. B. & Ley, K. Macrophage polarization: different gene signatures in M1(LPS+) vs. classically and M2(LPS-) vs. alternatively activated macrophages. Front. Immunol. 10, 1084 (2019).
Garrett, W. S., Gordon, J. I. & Glimcher, L. H. Homeostasis and inflammation in the intestine. Cell 140, 859–870 (2010).
Acknowledgements
We thank the UTSW Animal Resource Center, UTSW ARC Diagnostic Laboratory, UTSW ARC Veterinary Services, UTSW Electron Microscopy Facility, UTSW Quantitative Light Microscopy Core, UTSW Flow Cytometry Core, UTSW Histo Pathology Core, UTSW Proteomics Core, UTSW Microarray and Immune Phenotyping Core, UTSW Microbiome Research Lab., UTSW Preclinical Radiation Core Facility and UTSW Whole Brain Microscopy Facility at the UTSW, UT Austin Texas Materials Institute, University of North Carolina at Chapel Hill Pathology Services Core, Akina, Inc. (West Lafayette, IN), RayBiotech Life, Inc. (Corners, GA), IDEXX BioAnalytics (Columbia, MI) and iHisto, Inc. (Salem, MA) for their assistance with the procedures in this article. UTSW Electron Microscopy Facility is supported by the NIH grant 1S10OD021685-01A1. UTSW Quantitative Light Microscopy Core is supported by the NIH grant 1S10OD021684-01. UT Austin Texas Materials Institute is supported by the National Science Foundation Major Research Instrument Grant CBET-1624659. Pathology Services Core at the University of North Carolina-Chapel Hill is supported in part by an NCI Centre Core Support Grant 5P30CA016080-42. A.Z.W. was supported by the NIH grants R01GM130590 and R01EB025651.
Author information
Authors and Affiliations
Contributions
K.M.A., A.Z.W., J.P.-Y.T. and J.E.W. conceived and designed the experiments. K.M.A. conceived the experiments. K.M.A. and A.Z.W. analysed the data. K.M.A. and A.Z.W. co-wrote the paper. All authors discussed the results and edited the manuscript at all stages.
Corresponding authors
Ethics declarations
Competing interests
The University of Texas Southwestern Medical Center filed a patent application on the technology and intellectual property reported in this work.
Peer review
Peer review information
Nature Biomedical Engineering thanks Paolo De Coppi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
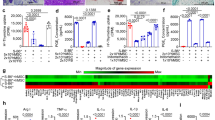
Extended Data Fig. 1 Combinational immunosuppressive nanofibers effectively inhibit antigen-specific CD8+ T cell activation in vitro.
a, T cell inhibition (exhaustion) markers and proliferation indices of CD8+ T cells cultured with different functionalized NFs in the presence of anti-CD3/anti-CD28-functionalized T cell activation beads (Dynabeads) at a 1:1 ratio for 48 h, as quantified through fluorescence-activated cell sorting. The proliferation study was performed on CFSE-labelled CD8+ T cells. (n = 4). b, T cell activation and exhaustion markers on naïve and trinitrophenol-sensitized CD8+ T cells (in splenocytes) after co-culture with trinitrophenol-modified colon epithelial cells in the presence or absence of combinational immunosuppressive molecules or combinational immunosuppressive nanofibers (48 h). (n = 4). c, ELISpot assay of IFN-gamma in splenocytes after culture with unmodified or trinitrophenol-modified colon epithelial cells in the presence or absence of combinational immunosuppressive molecules or combinational immunosuppressive nanofibers (n = 4). d, Cytotoxicity of splenocytes against colon epithelial cells in the presence or absence of combinational immunosuppressive molecules or combinational immunosuppressive nanofibers (n = 7). Combinational nanofibers, the combination of PD-L1/CD86-functionalized nanofibers, leflunomide-encapsulated nanofibers and TGF-β1 mimetic peptide-encapsulated nanofibers; combinational immunosuppressive molecules, the combination of PD-L1/CD86-functionalized nanofibers, leflunomide-encapsulated nanofibers and TGF-β1 mimetic peptide-encapsulated nanofibers. Data are presented as the mean ± s.e.m. All P values were analysed using two-way ANOVA with Tukey’s HSD multiple comparisons post-hoc test.
Extended Data Fig. 2 Therapeutic treatment with immune checkpoint molecule-bioengineered colon-specific immune niches prevents colitis-associated weight loss, and colon shortening and reduces immune cell infiltration into the lamina propria.
a, Bodyweight changes throughout the study. b, Digital photographs of colons preserved at the study endpoint, that is, 7 days after therapeutic treatments. c, Representative H&E-stained section of each colon preserved at the study endpoint after different treatments. The inserted label in each image represents the corresponding colon epithelial damage score and inflammatory cell infiltration score. P values were analysed using one-way ANOVA (a) with Tukey’s HSD multiple comparisons post-hoc test.
Extended Data Fig. 3 Treatment with subcutaneously inoculated colon-specific immune niches or intraperitoneally administered combination of immunosuppressive molecule-functionalized nanofibers inhibits the production of colitis-associated myeloperoxidase and proinflammatory cytokines in the colon.
a-c, Colonic myeloperoxidase activity (a), pro-inflammatory cytokine (b) and pro-regulatory cytokine (c) expression in the preserved colon specimens at the study endpoint (5 days after different therapeutic treatments) after different therapeutic treatments (n = 5). d, Representative immunofluorescence images of colons preserved from dextran sodium sulfate-induced colitis mice, captured 5 days after different therapeutic treatments, reveal lamina propria-infiltrated IFN-gamma+ CD8+ T cells. Data are presented as the mean ± s.e.m. All P values were analysed using one-way ANOVA with Tukey’s HSD multiple comparisons post-hoc test.
Extended Data Fig. 4 Therapeutic treatment with non-immune checkpoint molecule-functionalized colon-specific immune niches and colon-specific immune niches prevents colitis-associated colon shortening and reduces immune cell infiltration into the lamina propria.
a, Bodyweight changes throughout the study. b, Digital photographs of colons preserved at the study endpoint, that is, 7 days after different therapeutic treatments. c, Representative H&E-stained section of each colon preserved at the study endpoint after different treatments. The inserted label in each image represents the corresponding colon epithelial damage score and inflammatory cell infiltration score. P values were analysed using one-way ANOVA (a) with Tukey’s HSD multiple comparisons post-hoc test.
Extended Data Fig. 5 Colon-specific immune niches effectively ameliorate DSS-induced colitis in male C57BL/6 mice.
a-d, Evaluation of the therapeutic treatment efficiency of combinational colon-specific immune niches in the dextran sodium sulfate-induced-induced colitis mouse model. Therapeutic treatment schedule (a): colitis mice received a single subcutaneous treatment with the combo immune niche at day 6. b-c, Bodyweight change (b) and disease activity index score (c) were monitored for up to 12 days. d, Digital images and length of colon preserved at the study endpoint. e, H&E-stained sections of each colon preserved at the study endpoint and their disease scores after therapeutic treatment with the combo immune niche. The inserted label in each image represents the corresponding colon epithelial damage score and inflammatory cell infiltration score. Data are presented as the mean ± standard error of the mean (s.e.m.). All P values were analysed using one-way (d,e) or two-way (b,c) ANOVA with Tukey’s HSD multiple comparisons post-hoc test.
Extended Data Fig. 6 The combination of colon epithelial cells and combinational immunosuppressive nanofibers, or colon extracellular matrix alone are not as effective as combinational colon-specific immune niche (the combination of colon epithelial cells, colon extracellular matrix, combinational immunosuppressive nanofibers) in relieving the colitis symptoms in a dextran sodium sulfate-induced colitis model.
Bodyweight changes (a,c,e) and DAI scores (b,d,f) after different control colitis treatments with different control colon-specific immune niches. Statistical analysis was performed 4 days after different colitis treatments. (n = 6 or 7) Data are presented as the mean ± s.e.m. All P values were analysed using two-way ANOVA with Tukey’s HSD multiple comparisons post-hoc test.
Extended Data Fig. 7 The combination of colon epithelial cells and combinational immunosuppressive nanofibers, or colon extracellular matrix alone are not as effective as colon-specific immune niches (the combination of colon epithelial cells, colon extracellular matrix, combinational immunosuppressive nanofibers) in preventing inflammation-associated colon shortening, colitis-induced colon epithelial damage and immune cell infiltration into the colon in the dextran sodium sulfate colitis model.
a, Digital photographs and the lengths of colons preserved at the study endpoint after different colitis treatments. b, Representative H&E-stained sections of each colon and colon damage scores after different colitis treatments. The inserted label in each image represents the corresponding colon epithelial damage score and inflammatory cell infiltration score. (n = 6 or 7) All P values were analysed using one-way (a) or two-way (b) ANOVA with Tukey’s HSD multiple comparisons post-hoc test.
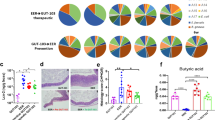
Extended Data Fig. 8 Pancreas-specific combinational immune niche cannot relieve colitis symptoms in dextran sodium sulfate colitis mice.
a, Analysis of growth factors and cytokines present in decellularized colon extracellular matrix and decellularized mouse pancreas via sandwich-type antibody microarray assay. (n = 4 biologically independent samples) b-c, Bodyweight change (b) and disease activity index score (c) after colitis treatment with combinational colon-specific immune niche or combinational pancreas-specific immune niche. d, Digital photographs of colons preserved at the study endpoint (5 days after therapeutic treatment), and their lengths (d). (n = 7 or 8) All P values were analysed using one-way (d) or two-way (a,b,c) ANOVA with Tukey’s HSD multiple comparisons post-hoc test.
Extended Data Fig. 9 Intraperitoneal-administered combinational immunosuppressive nanofibers are as effective as subcutaneously inoculated combinational colon-specific immune niche to treat dextran sodium sulfate-induced colitis.
a, b, Bodyweight change (a) and disease activity index score (b) after colitis treatment with subcutaneously administered combinational colon-specific immune niche or intraperitoneally-administrated combinational immunosuppressive nanofibers. c, Digital photographs of colons preserved at the study endpoint (5 days after therapeutic treatment), and their lengths. d, Representative H&E-stained sections of each colon and colon damage scores after different colitis treatments. The inserted label in each image represents the corresponding colon epithelial damage score and inflammatory cell infiltration score. (n = 8) All P values were analysed using one-way (c) or two-way (a,b,d) ANOVA with Tukey’s HSD multiple comparisons post-hoc test.
Extended Data Fig. 10 Single therapeutic treatment with combinational colon-specific immune niches effectively ameliorates colitis symptoms in a chronic dextran sodium sulfate-induced colitis model.
a, b, Disease activity index score (a) and bodyweight (b) change after treatment(s) with combinational colon-specific immune niche. c, Digital photographs and length of colons preserved at the study endpoint (48 days after the initial colitis induction) after one or three therapeutic treatments with the combo immune niche. d, Representative H&E-stained images, colon epithelial damage score, inflammatory cell infiltration score, and colonic damage score of colons preserved at the study endpoint (48 days after the first induction of colitis) after one or three therapeutic treatments with combinational colon-specific immune niche.
Supplementary information
Supplementary Information
Supplementary figures and tables.
Supplementary Table 3
Proteomic analysis of native colon samples versus decellularized COL ECM samples.
Supplementary Table 4
Statistical analysis of faecal microbiome compositions after different colitis treatments.
Source data
Source Data Figs. 1–8 and Extended Data Figs. 1–10
Source data for all figures.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Au, K.M., Wilson, J.E., Ting, J.PY. et al. An injectable subcutaneous colon-specific immune niche for the treatment of ulcerative colitis. Nat. Biomed. Eng (2023). https://doi.org/10.1038/s41551-023-01136-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41551-023-01136-9