Abstract
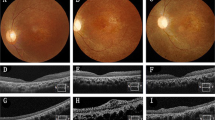
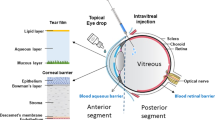
The therapeutic benefits of many cell types involve paracrine mechanisms. Inspired by the paracrine functions of exosomes and the sustained degradation properties of microcapsules, here we report the therapeutic benefits of exosome-loaded degradable poly(lactic-co-glycolic acid) microcapsules with micrometric pores for the treatment of vitreoretinal diseases. On intravitreal injection in a mouse model of retinal ischaemia-reperfusion injury, microcapsules encapsulating mouse mesenchymal-stem-cell-derived exosomes settled in the inferior vitreous cavity, released exosomes for over one month as they underwent degradation and led to the restoration of retinal thickness to nearly that of the healthy retina. In mice and non-human primates with primed mycobacterial uveitis, intravitreally injected microcapsules loaded with exosomes from monkey regulatory T cells resulted in a substantial reduction in the levels of inflammatory cells. The exosome-encapsulating microcapsules, which can be lyophilised, may offer alternative treatment options for vitreoretinal diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. The RNA-sequencing data are available from the NCBI BioProject via the accession code PRJNA1000442. The mass spectrometry and proteomics data are available from the ProteomeXchange Consortium (through the iProX partner repository) via the dataset identifier PXD044401. Source data for the figures are provided with this paper, and are also available from figshare at https://doi.org/10.6084/m9.figshare.23849181.v1.
References
Berger, W., Kloeckener-Gruissem, B. & Neidhardt, J. The molecular basis of human retinal and vitreoretinal diseases. Prog. Retin. Eye Res. 29, 335–375 (2010).
Chodnicki, K. et al. Stroke risk before and after central retinal artery occlusion: a population-based analysis. Ophthalmology 129, 203–208 (2022).
Russo, R. et al. Rapamycin and fasting sustain autophagy response activated by ischemia/reperfusion injury and promote retinal ganglion cell survival. Cell Death Dis. 9, 981 (2018).
Shosha, E. et al. Arginase 2 promotes neurovascular degeneration during ischemia/reperfusion injury. Cell Death Dis. 7, e2483 (2016).
Szabadfi, K. et al. Novel neuroprotective strategies in ischemic retinal lesions. Int. J. Mol. Sci. 11, 544–561 (2010).
Tang, Y. et al. Therapeutic targeting of retinal immune microenvironment with CSF-1 receptor antibody promotes visual function recovery after ischemic optic neuropathy. Front. Immunol. 11, 585918 (2020).
Stitt, A. et al. Vascular stem cells and ischaemic retinopathies. Prog. Retin. Eye Res. 30, 149–166 (2011).
Nguyen, Q. D. et al. Intravitreal sirolimus for the treatment of noninfectious uveitis: evolution through preclinical and clinical studies. Ophthalmology 125, 1984–1993 (2018).
Myles, M., Neumann, D. & Hill, J. Recent progress in ocular drug delivery for posterior segment disease: emphasis on transscleral iontophoresis. Adv. Drug Deliv. Rev. 57, 2063–2079 (2005).
Slabaugh, M. A., Herlihy, E., Ongchin, S. & van Gelder, R. N. Efficacy and potential complications of difluprednate use for pediatric uveitis. Am. J. Ophthalmol. 153, 932–938 (2012).
Gillies, M. C. et al. Intravitreal triamcinolone-induced elevated intraocular pressure is associated with the development of posterior subcapsular cataract. Ophthalmology 112, 139–143 (2005).
Roth, D. B. et al. Long-term incidence and timing of intraocular hypertension after intravitreal triamcinolone acetonide injection. Ophthalmology 116, 455–460 (2009).
Han, J. et al. Iron oxide nanoparticle-mediated development of cellular gap junction crosstalk to improve mesenchymal stem cells’ therapeutic efficacy for myocardial infarction. ACS Nano 9, 2805–2819 (2015).
Romano, M., Fanelli, G., Albany, C. J., Giganti, G. & Lombardi, G. Past, present, and future of regulatory T cell therapy in transplantation and autoimmunity. Front. Immunol. 10, 43 (2019).
Okoye, I. et al. MicroRNA-containing T-regulatory-cell-derived exosomes suppress pathogenic T helper 1 cells. Immunity 41, 89–103 (2014).
Li, L. et al. Overexpression of heme oxygenase-1 in mesenchymal stem cells augments their protection on retinal cells in vitro and attenuates retinal ischemia/reperfusion injury in vivo against oxidative stress. Stem Cells Int. 2017, 4985323 (2017).
Zhang, X. et al. Effects of mesenchymal stem cells and their exosomes on the healing of large and refractory macular holes. Graefes Arch. Clin. Exp. Ophthalmol 256, 2041–2052 (2018).
Singh, M. S. et al. Retinal stem cell transplantation: balancing safety and potential. Prog. Retin. Eye Res. 75, 100779 (2020).
Park, S. S. et al. Advances in bone marrow stem cell therapy for retinal dysfunction. Prog. Retin. Eye Res. 56, 148–165 (2017).
He, C., Zheng, S., Luo, Y. & Wang, B. Exosome theranostics: biology and translational medicine. Theranostics 8, 237–255 (2018).
Mathieu, M., Martin-Jaular, L., Lavieu, G. & Thery, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 21, 9–17 (2019).
Zhang, Y. et al. Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int. J. Nanomedicine 15, 6917–6934 (2020).
Xi, X. et al. Self-healing microcapsules synergetically modulate immunization microenvironments for potent cancer vaccination. Sci. Adv. 6, eaay7735 (2020).
Reinhold Samuel, E. et al. Self-healing microencapsulation of biomacromolecules without organic solvents. Angew. Chem. Int. Ed. Engl. 51, 10800–10803 (2012).
Desai Kashappa-Goud, H. et al. Active self-healing encapsulation of vaccine antigens in PLGA microspheres. J. Control. Release 165, 62–74 (2013).
Li, S. et al. Protective effect of HINT2 on mitochondrial function via repressing MCU complex activation attenuates cardiac microvascular ischemia-reperfusion injury. Basic Res. Cardiol. 116, 65 (2021).
Liu, Y., Tang, L. & Chen, B. Effects of antioxidant gene therapy on retinal neurons and oxidative stress in a model of retinal ischemia/reperfusion. Free Radic. Biol. Med. 52, 909–915 (2012).
Sayyad, Z. et al. Human primary retinal cells as an in-vitro model for investigating defective signalling caused by OPTN mutants associated with glaucoma. Neurochem. Int. 148, 105075 (2021).
Mahrouf-Yorgov, M. et al. Mesenchymal stem cells sense mitochondria released from damaged cells as danger signals to activate their rescue properties. Cell Death Differ. 24, 1224–1238 (2017).
Eastlake, K. et al. Prospects for the application of Müller glia and their derivatives in retinal regenerative therapies. Prog. Retin. Eye Res. 85, 100970 (2021).
Hu, M. et al. The effect of miRNA-modified exosomes in animal models of spinal cord injury: a meta-analysis. Front. Bioeng. Biotechnol. 9, 819651 (2022).
Yang, B. et al. Mucin 17 inhibits the progression of human gastric cancer by limiting inflammatory responses through a MYH9-p53-RhoA regulatory feedback loop. J. Exp. Clin. Cancer Res. 38, 283 (2019).
Fudalej, E. et al. Neuroprotective factors of the retina and their role in promoting survival of retinal ganglion cells: a review. Ophthalmic Res. 64, 345–355 (2021).
Sivilia, S. et al. Intravitreal NGF administration counteracts retina degeneration after permanent carotid artery occlusion in rat. BMC Neurosci. 10, 52 (2009).
Weymouth, A. E. & Vingrys, A. J. Rodent electroretinography: methods for extraction and interpretation of rod and cone responses. Prog. Retin. Eye Res. 27, 1–44 (2008).
Chatila, T. & Williams, C. Regulatory T cells: exosomes deliver tolerance. Immunity 41, 3–5 (2014).
Gutowski, M., Wilson, L., van Gelder, R. & Pepple, K. In vivo bioluminescence imaging for longitudinal monitoring of inflammation in animal models of uveitis. Invest. Ophthalmol. Vis. Sci. 58, 1521–1528 (2017).
Jonas, J., Kreissig, I. & Degenring, R. Intravitreal triamcinolone acetonide for treatment of intraocular proliferative, exudative, and neovascular diseases. Prog. Retin. Eye Res. 24, 587–611 (2005).
Tian, Y. et al. Reduction of choroidal neovascularization via cleavable VEGF antibodies conjugated to exosomes derived from regulatory T cells. Nat. Biomed. Eng. 5, 968–982 (2021).
Pepple, K., Wilson, L. & van Gelder, R. Comparison of aqueous and vitreous lymphocyte populations from two rat models of experimental uveitis. Invest. Ophthalmol. Vis. Sci. 59, 2504–2511 (2018).
Yang, H., Tyagi, P., Kadam, R., Holden, C. & Kompella, U. Hybrid dendrimer hydrogel/PLGA nanoparticle platform sustains drug delivery for one week and antiglaucoma effects for four days following one-time topical administration. ACS Nano 6, 7595–7606 (2012).
Jaissle, G. B. et al. Epiretinal deposit of triamcinolone acetonide at the posterior pole after intravitreal injection. Ophthalmic Surg. Lasers Imaging 38, 238–241 (2007).
Raia Nicole, R. et al. Characterization of silk-hyaluronic acid composite hydrogels towards vitreous humor substitutes. Biomaterials 233, 119729 (2020).
Wang, Y. et al. Stem/progenitor cell-based transplantation for retinal degeneration: a review of clinical trials. Cell Death Dis. 11, 793 (2021).
Zhou, X. et al. Rescue the retina after the ischemic injury by polymer-mediated intracellular superoxide dismutase delivery. Biomaterials 268, 120600 (2021).
Mandal, A. et al. Ocular delivery of proteins and peptides: challenges and novel formulation approaches. Adv. Drug Deliv. Rev. 126, 67–95 (2018).
Jirarattanasopa, P. et al. Results of intravitreal dexamethasone implant (Ozurdex®) for retinal vascular diseases with macular edema: an observational study of real-life situations. Medicine 101, e29807 (2022).
Leinonen, S. et al. Fluocinolone acetonide intravitreal implant (Retisert) in the treatment of sight threatening macular oedema of juvenile idiopathic arthritis-related uveitis. Acta Ophthalmol. 96, 648–651 (2018).
Kim, G. U. et al. Therapeutic potential of mesenchymal stem cells (MSCs) and MSC-derived extracellular vesicles for the treatment of spinal cord injury. Int. J. Mol. Sci. 22, 13672 (2021).
Kwon, H. et al. Surgical and tissue engineering strategies for articular cartilage and meniscus repair. Nat. Rev. Rheumatol. 15, 550–570 (2019).
Huang, K., Ozpinar, E. W., Su, T., Tang, J. & Cheng, K. An off-the-shelf artificial cardiac patch improves cardiac repair after myocardial infarction in rats and pigs. Sci. Transl. Med. 12, eaat9683 (2020).
Xie, X. et al. Therapeutic vaccination against leukaemia via the sustained release of co-encapsulated anti-PD-1 and a leukaemia-associated antigen. Nat. Biomed. Eng. 5, 414–428 (2021).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (T2225021 and U2001224 to W.W., 82070948 to Y. Tao, 32030062 to G.M. and 82101138 to Y. Tian), the Beijing Natural Science Foundation (JQ21027 to W.W.), the Shunyi District ‘Beijing science and technology achievements transformation coordination and service platform’ construction fund (SYGX202010 to Y. Tao) and the Beijing Hospitals Authority’s Ascent Plan (DFL20220301 to Y. Tao).
Author information
Authors and Affiliations
Contributions
W.W., Y. Tao and G.M. conceived and designed the study. H.B. and Y. Tian performed most of the experiments and analysed the data. T.Y. and H.W. assisted with microcapsule preparation. S.W., J.Z. and J.L. provided suggestions about the project design and data presentation. Y.Q. and C.P. assisted with the experiment on cynomolgus monkeys. W.W., H.B. and Y. Tian wrote the original draft manuscript, and W.W., Y. Tao and G.M. revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Ke Cheng and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Main Supplementary Information
Supplementary figures and tables.
Source data
SD for Fig. 1
Source data.
SD for Fig. 2
Source data.
SD for Fig. 3
Source data.
SD for Fig. 4
Source data.
SD for Fig. 5
Source data.
SD for Fig. 6
Source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bao, H., Tian, Y., Wang, H. et al. Exosome-loaded degradable polymeric microcapsules for the treatment of vitreoretinal diseases. Nat. Biomed. Eng (2023). https://doi.org/10.1038/s41551-023-01112-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41551-023-01112-3
This article is cited by
-
Enhancing aortic valve drug delivery with PAR2-targeting magnetic nano-cargoes for calcification alleviation
Nature Communications (2024)
-
Hypoxic preconditioned MSCs-derived small extracellular vesicles for photoreceptor protection in retinal degeneration
Journal of Nanobiotechnology (2023)