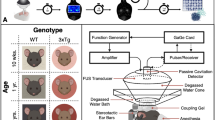
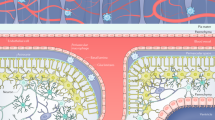
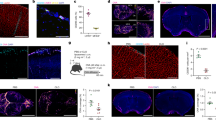
Abstract
The opening of the blood–brain barrier (BBB) by focused ultrasound (FUS) coupled with intravenously injected microbubbles can be leveraged as a form of immunotherapy for the treatment of neurodegenerative disorders. However, how FUS BBB opening affects brain macrophages is not well understood. Here by using single-cell sequencing to characterize the distinct responses of microglia and central nervous system-associated macrophages (CAMs) to FUS-mediated BBB opening in mice, we show that the treatment remodels the immune landscape via the recruitment of CAMs and the proliferation of microglia and via population size increases in disease-associated microglia. Both microglia and CAMs showed early and late increases in population sizes, yet only the proliferation of microglia increased at both timepoints. The population of disease-associated microglia also increased, accompanied by the upregulation of genes associated with gliogenesis and phagocytosis, with the depletion of brain macrophages significantly decreasing the duration of BBB opening.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All sequencing data is available in both raw FASTQ and processed-table form on Gene Expression Omnibus via the accession code 22573264. The GRCm38 genome dataset is publicly available at https://www.ncbi.nlm.nih.gov/assembly/GCF_000001635.20.
Code availability
All custom code for analysis is available at https://github.com/ark2173/Seq1-2022.
References
Konofagou, E. et al. Ultrasound-induced blood–brain barrier opening. Curr. Pharm. Biotechnol. 13, 1332–1345 (2012).
Stockwell, J. et al. Novel central nervous system drug delivery systems. Chem. Biol. Drug Des. 83, 507–520 (2014).
Karakatsani, M. et al. Unilateral focused ultrasound-induced blood–brain barrier opening reduces phosphorylated tau from the rtg4510 mouse model. Theranostics 9, 5396–5411 (2019).
Leinenga, G. & Götz, J. Scanning ultrasound removes amyloid-β and restores memory in an Alzheimer’s disease mouse model. Sci. Transl. Med. 7, 278ra33 (2015).
Burgess, A. et al. Alzheimer disease in a mouse model: MR imaging-guided focused ultrasound targeted to the hippocampus opens the blood–brain barrier and improves pathologic abnormalities and behavior. Radiology 7, 736–745 (2014).
Shen, Y. et al. Ultrasound with microbubbles improves memory, ameliorates pathology and modulates hippocampal proteomic changes in a triple transgenic mouse model of Alzheimer’s disease. Theranostics 10, 11794–11819 (2020).
Kugelman, T. et al. Analysis of Focused Ultrasound with Microbubbles Induced BBB Disruption on Tight Junction Morphology (IEEE International Ultrasonics Symposium, 2017).
Ji, R. et al. Cavitation-modulated inflammatory response following focused ultrasound blood–brain barrier opening. J. Control. Release 337, 458–471 (2021).
Choi, J., Pernot, M., Small, S. & Konofagou, E. Non-invasive, transcranial and localized opening of the blood–brain barrier using focused ultrasound in mice. Ultrasound Med. Biol. 33, 95–104 (2007).
Wu, S. et al. Efficient blood–brain barrier opening in primates with neuronavigation-guided ultrasound and real-time acoustic mapping. Sci. Rep. 8, 7978–7989 (2018).
Haut, M. et al. Non-invasive hippocampal blood–brain barrier opening in Alzheimer’s disease with focused ultrasound. Proc. Natl Acad. Sci. USA 117, 9180–9182 (2020).
Shin, J. et al. Focused ultrasound-induced blood–brain barrier opening improves adult hippocampal neurogenesis and cognitive function in a cholinergic degeneration dementia rat model. Alzheimers Res. Ther. 11, 1–15 (2019).
Mooney, S. et al. Antidepressant effects of focused ultrasound induced blood–brain barrier opening. Behav. Brain Res. 16, 57–61 (2018).
Choi, J. et al. Non-invasive and transient blood–brain barrier opening in the hippocampus of Alzheimer’s double transgenic mice using focused ultrasound. Ultrason. Imaging 30, 189–200 (2008).
Burgess, A. et al. Alzheimer disease in a mouse model: MR imaging–guided focused ultrasound targeted to the hippocampus opens the blood–brain barrier and improves pathologic abnormalities and behavior. Radiology 273, 736–745 (2014).
Mooney, S. et al. Focused ultrasound-induced neurogenesis requires an increase in blood–brain barrier permeability. PLoS ONE 11, 0159892 (2016).
McMahon, D., Bendayan, R. & Hynynen, K. Acute effects of focused ultrasound-induced increases in blood–brain barrier permeability on rat microvascular transcriptome. Sci. Rep. 7, 1–15 (2017).
Downs, M. et al. Long-term safety of repeated blood–brain barrier opening via focused ultrasound with microbubbles in non-human primates performing a cognitive task. PLoS ONE 10, 0125911 (2015).
Wu, Y. et al. Microglia: dynamic mediators of synapse development and plasticity. Trends Immunol. 36, 605–613 (2015).
Chintamen, S., Imessadouene, F. & Kernie, S. Immune regulation of adult neurogenic niches in health and disease. Front. Cell. Neurosci. 14, 571071 (2020).
Rogers, J. et al. Cx3cr1 deficiency leads to impairment of hippocampal cognitive function and synaptic plasticity. J. Neurosci. 31, 16241–16250 (2011).
Pascal, A. et al. Histologic evaluation of activation of acute inflammatory response in a mouse model following ultrasound-mediated blood–brain barrier using different acoustic pressures and microbubble doses. Nanotheranostics 4, 210–223 (2020).
Hove, H., Martens, L. & Scheyltjens, I. A single-cell atlas of mouse brain macrophages reveals unique transcriptional identities shaped by ontogeny and tissue environment. Nat. Neurosci. 22, 1021–1035 (2019).
Kierdorf, K. et al. Macrophages at CNS interfaces: ontogeny and function in health and disease. Nat. Rev. Neurosci. 20, 547–562 (2019).
Sevenich, L. Brain-resident microglia and blood-borne macrophages orchestrate central nervous system inflammation in neurodegenerative disorders and brain cancer. Front. Immunol. 9, 697 (2018).
Tan, Y., Yuan, Y. & Tian, L. Microglial regional heterogeneity and its role in the brain. Mol. Psychiatry 25, 351–367 (2020).
Keren-Shaul, H. et al. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 169, 1276–1290 (2017).
Li, Q. et al. Developmental heterogeneity of microglia and brain myeloid cells revealed by deep single-cell RNA sequencing. Neuron 101, 207–223.e10 (2019).
Masuda, T. et al. Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature 566, 388–392 (2019).
Matcovitch-Natan, O. et al. Microglia development follows a stepwise program to regulate brain homeostasis. Science 353, 789 (2016).
Réu, P. et al. The lifespan and turnover of microglia in the human brain. Cell Rep. 20, 779–784 (2017).
Mildner, A. et al. Distinct and non-redundant roles of microglia and myeloid subsets in mouse models of Alzheimer’s disease. J. Neurosci. 31, 11159–11171 (2011).
Kim, J. et al. A binary cre transgenic approach dissects microglia and CNS border-associated macrophages. Immunity 54, 176–190 (2021).
Goldmann, T. et al. Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat. Immunol. 17, 797–805 (2016).
Mrdjen, D. et al. High-dimensional single-cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease. Immunity 48, 380–395 (2018).
Deczkowska, A. et al. Disease-associated microglia: a universal immune sensor of neurodegeneration. Cell 173, 1073–1081 (2018).
McDonough, A., Lee, R. & Weinstein, J. Microglial interferon signaling and white matter. Neurochem. Res. 42, 2625–2638 (2017).
Vukovic, J. et al. Microglia modulate hippocampal neural precursor activity in response to exercise and aging. J. Neurosci. 32, 6435–6443 (2012).
Hammond, T. et al. Single-cell RNA sequencing of microglia throughout the mouse lifespan and in the injured brain reveals complex cell-state changes. Immunity 50, 253–271 (2019).
Zhao, N. et al. Microglia-targeting nanotherapeutics for neurodegenerative diseases. APL Bioeng. 4, 030902 (2020).
Zhao, R. et al. Phosphatidylserine-microbubble targeting-activated microglia/macrophage in inflammation combined with ultrasound for breaking through the blood–brain barrier. J. Neuroinflammation 15, 334 (2018).
Hafemeister, C. & Satija, R. Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol. 20, 296 (2019).
Menon, V. Clustering single cells: a review of approaches on high-and low-depth single-cell RNA-seq data. Brief. Funct. Genomics 17, 240–245 (2019).
Jung, S. et al. Analysis of fractalkine receptor cx3cr1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell. Biol. 20, 4106–4114 (2000).
Pedragosa, J. et al. CNS-border associated macrophages respond to acute ischemic stroke attracting granulocytes and promoting vascular leakage. Acta Neuropathol. Commun. 6, 76 (2018); https://doi.org/10.1186/s40478-018-0581-6
Rajan, W. et al. Defining molecular identity and fates of CNS-border associated macrophages after ischemic stroke in rodents and humans. Neurobiol. Dis. 137, 104722 (2020).
Zelco, A. et al. Single-cell atlas reveals meningeal leukocyte heterogeneity in the developing mouse brain. Genes & Dev. 35, 1190–1207 (2021); http://www.genesdev.org/cgi/doi/10.1101/gad.348190.120
Han, J., Harris, R. & Zhang, X. An updated assessment of microglia depletion: current concepts and future directions. Mol. Brain 10, 25 (2017).
Han, J. et al. Underestimated peripheral effects following pharmacological and conditional genetic microglial depletion. Int. J. Mol. Sci. 21, 8603 (2020).
Kovacs, Z. et al. Disrupting the blood–brain barrier by focused ultrasound induces sterile inflammation. Proc. Natl Acad. Sci. USA 114, 75–84 (2017).
Willis, E. et al. Repopulating microglia promote brain repair in an IL-6-dependent manner. Cell 180, 833–846 (2020).
Lee, S. et al. Microglia depletion increase brain injury after acute ischemic stroke in aged mice. Exp. Neurol. 336, 113530 (2020).
Mathew, A., Gorick, C. & Price, R. Single-cell mapping of focused ultrasound-transfected brain. Gene Ther. 30, 255–263 (2021).
Mathew, A., Gorick, C. M. & Price, R. J. Multiple regression analysis of a comprehensive transcriptomic data assembly elucidates mechanically- and biochemically-driven responses to focused ultrasound blood-brain barrier disruption. Theranostics 11, 9847–9858 (2021).
Leinenga, G. et al. Transcriptional signature in microglia isolated from an Alzheimer’s disease mouse model treated with scanning ultrasound. Bioeng. Transl. Med. 8, e10329 (2022).
Eguchi, K. et al. Whole-brain low-intensity pulsed ultrasound therapy markedly improves cognitive dysfunctions in mouse models of dementia—crucial roles of endothelial nitric oxide synthase. Brain Stimul. 11, 959–973 (2018).
Bobola, M. et al. Transcranial focused ultrasound, pulsed at 40 hz, activates microglia acutely and reduces aβ load chronically, as demonstrated in vivo. Brain Stimul. 13, 1014–1023 (2020).
Blackmore, D. G. et al. Low-intensity ultrasound restores long-term potentiation and memory in senescent mice through pleiotropic mechanisms including NMDAR signaling. Mol. Psychiatry 26, 6975–6991 (2021).
Elmore, M. et al. Replacement of microglia in the aged brain reverses cognitive, synaptic, and neuronal deficits in mice. Aging Cell 17, e12832 (2018).
Lloyd, A. et al. Central nervous system regeneration is driven by microglia necroptosis and repopulation. Nat. Neurosci. 22, 1046–1052 (2019).
Wang, S. et al. Microbubble type and distribution dependence of focused ultrasound-induced blood–brain barrier opening. Ultrasound Med. Biol. 40, 103–107 (2014).
Macosko, E. et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell 161, 1202–1214 (2015).
Acknowledgements
This research was funded by the National Institutes of Health R01AG038961 (EEK), R01NS095803 (SGK) and National Science Foundation DGE-2036197 (RLN).
Author information
Authors and Affiliations
Contributions
The original scRNA-seq experiments were executed, designed and performed by A.R.K.-S., S.C., S.G.K. and E.E.K., with input from V.M. Bioinformatics analyses were performed by A.R.K.-S. and S.C., with input from V.M. and oversight from E.E.K. S.G.K. and E.E.K. performed the data interpretation. IHC validation experiments were performed by A.R.K.-S., with support from M.J.W., M.R.D., R.L.N., A.J.B. and N.K. Flow cytometry validation experiments were performed by A.R.K.-S., M.J.W. and M.R.D. Manuscript preparation was performed by A.R.K.-S., with input and editing from V.M., S.G.K. and E.E.K. E.E.K., S.Z., S.G.K. and C.-C.W. obtained funding for this work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Evandro Fang, Jurgen Gotz, Rao P. Gullapalli, Kazuyuki Takata and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary figs. 1 and 2.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kline-Schoder, A.R., Chintamen, S., Willner, M.J. et al. Characterization of the responses of brain macrophages to focused ultrasound-mediated blood–brain barrier opening. Nat. Biomed. Eng (2023). https://doi.org/10.1038/s41551-023-01107-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41551-023-01107-0
This article is cited by
-
Focused ultrasound-mediated blood–brain barrier opening is safe and feasible with moderately hypofractionated radiotherapy for brainstem diffuse midline glioma
Journal of Translational Medicine (2024)