Abstract
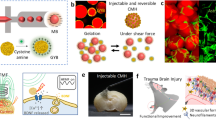
In the adult brain, neural stem cells are largely restricted into spatially discrete neurogenic niches, and hence areas of neuron loss during neurodegenerative disease or following a stroke or traumatic brain injury do not typically repopulate spontaneously. Moreover, understanding neural activity accompanying the neural repair process is hindered by a lack of minimally invasive devices for the chronic measurement of the electrophysiological dynamics in damaged brain tissue. Here we show that 32 individually addressable platinum microelectrodes integrated into laminin-coated branched polymer scaffolds stereotaxically injected to span a hydrogel-filled cortical lesion and deeper regions in the brains of mice promote neural regeneration while allowing for the tracking of migrating host brain cells into the lesion. Chronic measurements of single-unit activity and neural-circuit analyses revealed the establishment of spiking activity in new neurons in the lesion and their functional connections with neurons deeper in the brain. Electronic implants mimicking the topographical and surface properties of brain vasculature may aid the stimulation and tracking of neural-circuit restoration following injury.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data supporting the results in this study are available within the paper and its Supplementary Information. Source data are available in the Harvard Dataverse with the identifier https://doi.org/10.7910/DVN/4HIWCA.
Code availability
The custom code used in this study is available at https://github.com/XiaoYangPhD/Yang_2023_NBME.
References
Barker, R. A., Götz, M. & Parmar, M. New approaches for brain repair—from rescue to reprogramming. Nature 557, 329–334 (2018).
Martino, G. & Pluchino, S. The therapeutic potential of neural stem cells. Nat. Rev. Neurosci. 7, 395–406 (2006).
Carlson, A. L. et al. Generation and transplantation of reprogrammed human neurons in the brain using 3D microtopographic scaffolds. Nat. Commun. 7, 10862 (2016).
Nih, L. R., Gojgini, S., Carmichael, S. T. & Segura, T. Dual-function injectable angiogenic biomaterial for the repair of brain tissue following stroke. Nat. Mater. 17, 642–651 (2018).
Falkner, S. et al. Transplanted embryonic neurons integrate into adult neocortical circuits. Nature 539, 248–253 (2016).
Trounson, A. & McDonald, C. Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell 17, 11–22 (2015).
Green, J. J. & Elisseeff, J. H. Mimicking biological functionality with polymers for biomedical applications. Nature 540, 386–394 (2016).
Hussey, G. S., Dziki, J. L. & Badylak, S. F. Extracellular matrix-based materials for regenerative medicine. Nat. Rev. Mater. 3, 159–173 (2018).
Koffler, J. et al. Biomimetic 3D-printed scaffolds for spinal cord injury repair. Nat. Med. 25, 263–269 (2019).
Feiner, R. & Dvir, T. Tissue–electronics interfaces: from implantable devices to engineered tissues. Nat. Rev. Mater. 3, 17076 (2017).
Frank, J. A., Antonini, M.-J. & Anikeeva, P. Next-generation interfaces for studying neural function. Nat. Biotechnol. 37, 1013–1023 (2019).
Chen, X., Rogers, J. A., Lacour, S. P., Hu, W. & Kim, D.-H. Materials chemistry in flexible electronics. Chem. Soc. Rev. 48, 1431–1433 (2019).
Luan, L. et al. Recent advances in electrical neural interface engineering: minimal invasiveness, longevity, and scalability. Neuron 108, 302–321 (2020).
Tian, B. Z. et al. Macroporous nanowire nanoelectronic scaffolds for synthetic tissues. Nat. Mater. 11, 986–994 (2012).
Kalmykov, A. et al. Organ-on-e-chip: three-dimensional self-rolled biosensor array for electrical interrogations of human electrogenic spheroids. Sci. Adv. 5, eaax0729 (2019).
Floch, P. L. et al. Stretchable mesh nanoelectronics for three-dimensional single-cell chronic electrophysiology from developing brain organoids. Adv. Mater. 34, 2106829 (2022).
Yang, X. et al. Bioinspired neuron-like electronics. Nat. Mater. 18, 510–517 (2019).
Wang, L. et al. Functionalized helical fibre bundles of carbon nanotubes as electrochemical sensors for long-term in vivo monitoring of multiple disease biomarkers. Nat. Biomed. Eng. 4, 159–171 (2020).
Woodington, B. J. et al. Electronics with shape actuation for minimally invasive spinal cord stimulation. Sci. Adv. 7, eabg7833 (2021).
Chen, J. C. et al. A wireless millimetric magnetoelectric implant for the endovascular stimulation of peripheral nerves. Nat. Biomed. Eng. 6, 706–716 (2022).
Zhao, Z. et al. Ultraflexible electrode arrays for months-long high-density electrophysiological mapping of thousands of neurons in rodents. Nat. Biomed. Eng. 7, 520–532 (2023).
Goldman, S. A. & Chen, Z. Perivascular instruction of cell genesis and fate in the adult brain. Nat. Neurosci. 14, 1382–1389 (2011).
Ming, G.-L. & Song, H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 70, 687–702 (2011).
Fujioka, T. et al. β1 integrin signaling promotes neuronal migration along vascular scaffolds in the post-stroke brain. EBioMedicine 16, 195–203 (2017).
Todorov, M. I. et al. Machine learning analysis of whole mouse brain vasculature. Nat. Methods 17, 442–449 (2020).
Jeffries, E. M. & Wang, Y. Incorporation of parallel electrospun fibers for improved topographical guidance in 3D nerve guides. Biofabrication 5, 035015 (2013).
Wei, H., George, C. M. & Ravi, V. B. Nanoscale laminin coating modulates cortical scarring response around implanted silicon microelectrode arrays. J. Neural Eng. 3, 316 (2006).
Aisenbrey, E. A. & Murphy, W. L. Synthetic alternatives to Matrigel. Nat. Rev. Mater. 5, 539–551 (2020).
Magavi, S. S., Leavitt, B. R. & Macklis, J. D. Induction of neurogenesis in the neocortex of adult mice. Nature 405, 951–955 (2000).
Zhao, C., Deng, W. & Gage, F. H. Mechanisms and functional implications of adult neurogenesis. Cell 132, 645–660 (2008).
Sultan, S. et al. Synaptic integration of adult-born hippocampal neurons is locally controlled by astrocytes. Neuron 88, 957–972 (2015).
Wellman, S. M., Li, L., Yaxiaer, Y., McNamara, I. & Kozai, T. D. Y. Revealing spatial and temporal patterns of cell death, glial proliferation, and blood–brain barrier bysfunction around implanted intracortical neural interfaces. Front. Neurosci. 13, 493 (2019).
Villacampa, N., Almolda, B., González, B. & Castellano, B. in Microglia: Methods and Protocols (eds Joseph, B. & Venero, J. L.) 261–279 (Humana, 2013).
Lledo, P.-M., Alonso, M. & Grubb, M. S. Adult neurogenesis and functional plasticity in neuronal circuits. Nat. Rev. Neurosci. 7, 179–193 (2006).
Lerner, T. N., Ye, L. & Deisseroth, K. Communication in neural circuits: tools, opportunities, and challenges. Cell 164, 1136–1150 (2016).
Lindvall, O. & Kokaia, Z. Neurogenesis following stroke affecting the adult brain. Cold Spring Harb. Perspect. Biol. 7, a019034 (2015).
Kumar, A., Rotter, S. & Aertsen, A. Spiking activity propagation in neuronal networks: reconciling different perspectives on neural coding. Nat. Rev. Neurosci. 11, 615–627 (2010).
Khodagholy, D. et al. NeuroGrid: recording action potentials from the surface of the brain. Nat. Neurosci. 18, 310–315 (2015).
Barthó, P. et al. Characterization of neocortical principal cells and interneurons by network interactions and extracellular features. J. Neurophysiol. 92, 600–608 (2004).
Denoth-Lippuner, A. & Jessberger, S. Formation and integration of new neurons in the adult hippocampus. Nat. Rev. Neurosci. 22, 223–236 (2021).
Hua, Y. et al. Peripheral SMN restoration is essential for long-term rescue of a severe spinal muscular atrophy mouse model. Nature 478, 123–126 (2011).
Lichtman, J. W., Livet, J. & Sanes, J. R. A technicolour approach to the connectome. Nat. Rev. Neurosci. 9, 417–422 (2008).
Werner, C., Sauer, M. & Geis, C. Super-resolving microscopy in neuroscience. Chem. Rev. 121, 11971–12015 (2021).
Oh, S. W. et al. A mesoscale connectome of the mouse brain. Nature 508, 207 (2014).
Glover, G. H. Overview of functional magnetic resonance imaging. Neurosurg. Clin. N. Am. 22, 133–139 (2011).
Fu, T.-M. et al. Stable long-term chronic brain mapping at the single-neuron level. Nat. Methods 13, 875–882 (2016).
Grefkes, C. & Fink, G. R. Recovery from stroke: current concepts and future perspectives. Neurol. Res. Pract. 2, 17 (2020).
Lee, J. M. et al. Nanoenabled direct contact interfacing of syringe-injectable mesh electronics. Nano Lett. 19, 5818–5826 (2019).
Lee, J. M. et al. Scalable three-dimensional recording electrodes for probing biological tissues. Nano Lett. 22, 4552–4559 (2022).
Fu, T.-M., Hong, G., Viveros, R. D., Zhou, T. & Lieber, C. M. Highly scalable multichannel mesh electronics for stable chronic brain electrophysiology. Proc. Natl Acad. Sci. USA 114, E10046–E10055 (2017).
Jun, J. J. et al. Fully integrated silicon probes for high-density recording of neural activity. Nature 551, 232–236 (2017).
Ferdinand, W. et al. Stability of the hydrophilic behavior of oxygen plasma activated SU-8. J. Micromech. Microeng. 17, 524–531 (2007).
Fischer, M. J. E. Surface Plasmon Resonance: Methods and Protocols (Humana, 2010).
Bastiancich, C., Danhier, P., Préat, V. & Danhier, F. Anticancer drug-loaded hydrogels as drug delivery systems for the local treatment of glioblastoma. J. Control. Release 243, 29–42 (2016).
Hong, G. S. et al. Syringe injectable electronics: precise targeted delivery with quantitative input/output connectivity. Nano Lett. 15, 6979–6984 (2015).
Reza, M. et al. In vivo migration of endogenous brain progenitor cells guided by an injectable peptide amphiphile biomaterial. J. Tissue Eng. Regen. Med. 12, e2123–e2133 (2018).
Peden, C. S. et al. Striatal readministration of rAAV vectors reveals an immune response against AAV2 capsids that can be circumvented. Mol. Ther. 17, 524–537 (2009).
Murray, E. et al. Simple, scalable proteomic imaging for high-dimensional profiling of intact systems. Cell 163, 1500–1514 (2015).
Acknowledgements
The authors gratefully acknowledge S. Badylak, M. Murdock and Y.v.d. Merwe, who provided useful discussions and crucial insights for the generation and evaluation of the brain resection model at the initial stage of this work. We thank the assistance from J. Lee for the cross-correlation analysis, A. Zhang for the functionalization–stability characterization, T. Gao for electrophysiology recording instrumentation and T.-M. Fu for helpful discussions during the initial stages of this work. We thank Z. Ou, S. Zhao, X. Wu, G. Woods, and J. Shadrach for assistance with animal surgeries and vibratome sectioning. C.M.L. discloses support for the research described in this study from the National Institute on Drug Abuse (1R21DA043985-01) and the Director’s Pioneer Award (1DP1EB025835-01) of the National Institutes of Health. N.J.R. discloses support from a Stanford Bio-X Honorary Graduate Student Fellowship and the National Science Foundation Graduate Research Fellowship Program (DGE-1656518). This work was performed in part at the Harvard Center for Biological Imaging and Harvard University Center for Nanoscale Systems, a member of the National Nanotechnology Coordinated Infrastructure Network supported by the National Science Foundation.
Author information
Authors and Affiliations
Contributions
X.Y. and C.M.L. designed the experiments. X.Y. performed experiments including device fabrication, surface functionalization, animal surgeries, histology and electrophysiology. Y.Q. performed experiments including device fabrication, surface functionalization and histology at Harvard University. C.W. contributed to schematic illustrations and figure designs. T.J.Z. helped establish the surface functionalization and histology protocols. N.J.R. performed the finite-element simulations. G.H. helped establish the system and provided feedback. X.Y., Y.Q., C.W. and C.M.L. analysed the data. X.Y. and C.M.L. wrote the paper. All authors discussed the results, revised or commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Eric Glowacki, Jacob Robinson, Darren Svirskis and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Photographs and schematics illustrating the processes of brain resection surgery and VasES implantation.
In each panel, I, II and III show photographs, top view schematics and side view schematics, respectively. a, Generate a cranial window centered at bregma with a lateral dimension of 4 mm (mediolateral) by 2 mm (anteroposterior). b, Resect cortical tissue to the depth of 1 mm in each hemisphere, leaving behind the resection cavity and intact margins of dura. c, Fill the resection with Matrigel and let it sit for 10–15 min to solidify. d, Stereotaxically implant VasES throughout the cortical resection, remaining cortical tissue and SVZ. Bond the input-output interface of electronics to flat flexible cable for electrophysiology recording. e, Seal the cranial window with agarose, silicone adhesive and dental cement sequentially. Scale bars, 2 mm.
Extended Data Fig. 2 Efficacy of VasES in directing the migration of SVZ newborn neurons by histology assessment.
a, b, and c represent control probe, VasES without laminin, and VasES, respectively. I, II and III show topographical design, surface functionalization and fluorescence images, respectively, at 1 week post-implantation. The insets feature larger area images, with the SVZ and the volumes used for quantification of DCX intensity outlined by white and yellow contours, respectively. Scale bars, 100 µm. d, Comparison of normalized DCX fluorescence intensity within 60 µm from control probes (N = 4), VasES without laminin functionalization (N = 3), and VasES with laminin functionalization (N = 3) at 1 week post-injection (details in the Supplementary Note 3). Independent samples were collected from different mice for each group. Error bars represent ±1 s.d. **P = 1.02 × 10−3 (control versus VasES), *P = 1.61 × 10−2 (control versus VasES without laminin), *P = 1.70 × 10−2 (VasES without laminin versus VasES); two-tailed t-test.
Extended Data Fig. 3 Evaluation of VasES by electrophysiology recording.
Representative multiplexed electrophysiology recordings of control probe (a) and VasES (b) at 7 (I) and 14 (II) days post-implantation. Scale bars, 100 ms (lateral) and 100 µV (vertical). c, Bar chart of chronic electrophysiology recording signal-to-noise ratios (SNRs) of control probe and VasES (details in the Supplementary Note 2), showing that VasES has higher SNRs than control probe on 4–14 days post-implantation. Data includes 77 channels from 3 control probes and 69 channels from 3 VasES probes. Left to right, P = 0.316, **P = 1.63 × 10−3, **P = 8.28 × 10−3, **P = 4.51 × 10−3; two-tailed t-test. Bars represent mean values.
Extended Data Fig. 4 Histology characterization of migration of newborn neurons.
a, An overview fluorescence image of control brain resection of which Fig. 2a is derived from, showing the relative position of the cortical resection with respect to SVZ. Scale bar, 200 µm. b, DCX fluorescence intensity profile along VasES in the resection as a function of the distance from the resection boundary (normalized against the intensity at the resection border; details in the Supplementary Note 3). c, Fluorescence image shown in Fig. 2c is displayed at a larger scale (I) and the corresponding DCX channel of the same image (II). The DCX data shows that the leading processes of the newborn neurons are closely associated and aligned with the VasES structural elements. d, Close-up fluorescence image captured at a higher resolution on the sample shown in Fig. 2c, showing the characteristic DCX morphology. e, Distribution of angle between leading processes of DCX+ newborn neurons and VasES structures (N = 87). f, Fluorescence image of implantation of control probe into the resection at 1 week post-implantation. The gray curve delineates the resection boundary. g, Fluorescence image of VasES implantation into cortical resection without insertion into the SVZ at 1 week post-implantation. The gray curve delineates the resection boundary. h, Quantitative analysis of normalized DCX fluorescence intensity at 0–60 μm near control probes (N = 3 independent samples; ‘control probe resection’) and near VasES without insertion into the SVZ (N = 3 independent samples; ‘VasES resection - SVZ’) at 1 week post-implantation (details in the Supplementary Note 3). The quantification of VasES implantation in the resection (‘VasES resection’), as presented in Fig. 2e, is also replicated here for comparison. ***P = 7.84 × 10−4 (VasES resection versus control probe resection), ***P = 3.80 × 10−4 (VasES resection versus VasES resection - SVZ), two-tailed t-test. Error bars reflect ±1 s.d. Scale bars, 50 µm for c and d, 300 µm for f and g.
Extended Data Fig. 5 Migration of astrocytes along VasES into cortical resection.
Optical microscope image (a) and fluorescence image (b) of the same field of view of VasES-implanted tissue at 1 week post-implantation. c, Zoomed-in image of the magenta dashed box in b, showing the migration of astrocytes into the resection. d, Close-up image of the cyan dashed box in c. The white asterisks highlight the alignment of migrating astrocytes with VasES elements. The black and white curves delineate the resection boundary. Scale bars, 200 µm for a and b, 50 µm for c, 20 µm for d.
Extended Data Fig 6 Histology characterization of tomato lectin+ cells and neurofilaments.
a and b, Infiltration of tomato lectin (TL)-positive endothelial cells and microglia into the cortical resection at 1 week (a) and 2 weeks (b) post-implantation. c and d, Infiltration of neurofilaments into the cortical resection at 4 weeks (c) and 3 months (d) post-implantation. The white dashed curves delineate the resection boundary. Scale bars, 100 µm.
Extended Data Fig 7 Histology characterization of NeuN+ neuronal nuclei in the cortical resection.
a–c, Fluorescence images showing the development of newborn neurons at 4 weeks post-implantation. Scale bars, 100 µm. Colocalization quantification shows that 99.0% of NeuN+ cells in cortical resection are DAPI+, demonstrating that the NeuN immunostaining is highly specific, and that false positive staining is largely excluded. d, Quantitative analysis of normalized NeuN fluorescence intensity within 60 µm from VasES elements at 4 weeks post-implantation on N = 6 independent samples. Error bar represents ±1 s.d. The normalized NeuN intensity is calculated as the ratio of the average fluorescence intensity within 60 μm from VasES versus the baseline value as the average fluorescence intensity 340–400 μm away. e, Normalized NeuN intensity along VasES probes in the cortical resection versus NeuN intensity in the undamaged native tissue. This is calculated as the ratio of the average fluorescence intensity along the VasES versus the average fluorescence intensity in the undamaged native tissue. Error bar represents ±1 s.d.
Supplementary information
Supplementary Information
Supplementary Notes 1–4, Figs. 1–9, Table 1 and references.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, X., Qi, Y., Wang, C. et al. Laminin-coated electronic scaffolds with vascular topography for tracking and promoting the migration of brain cells after injury. Nat. Biomed. Eng 7, 1282–1292 (2023). https://doi.org/10.1038/s41551-023-01101-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-023-01101-6
This article is cited by
-
Kirigami electronics for long-term electrophysiological recording of human neural organoids and assembloids
Nature Biotechnology (2024)