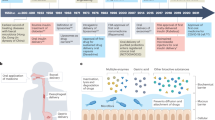
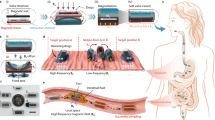
Abstract
Robotic pills leverage the advantages of oral pharmaceutical formulations—in particular, convenient encapsulation, high loading capacity, ease of manufacturing and high patient compliance—as well as the multifunctionality, increasing miniaturization and sophistication of microrobotic systems. In this Perspective, we provide an overview of major innovations in the development of robotic pills—specifically, oral pills embedded with robotic capabilities based on microneedles, microinjectors, microstirrers or microrockets—summarize current progress and applicational gaps of the technology, and discuss its prospects. We argue that the integration of multiple microrobotic functions within oral delivery systems alongside accurate control of the release characteristics of their payload provides a basis for realizing sophisticated multifunctional robotic pills that operate as closed-loop systems.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Feynman, R. P. There’s plenty of room at the bottom. Eng. Sci. 23, 22–36 (1960).
Wang, J. Nanomachines: Fundamentals and Applications (Wiley-VCH, 2013).
Wang, H. & Pumera, M. Fabrication of micro/nanoscale motors. Chem. Rev. 115, 8704–8735 (2015).
Peng, F., Tu, Y. & Wilson, D. A. Micro/nanomotors towards in vivo application: cell, tissue and biofluid. Chem. Soc. Rev. 46, 5289–5310 (2017).
Peyer, K. E., Zhang, L. & Nelson, B. J. Bio-inspired magnetic swimming microrobots for biomedical applications. Nanoscale 5, 1259–1272 (2013).
Soto, F. et al. Smart materials for microrobots. Chem. Rev. 122, 5365–5403 (2022).
de Ávila, B. E.-F. et al. Micromotor-enabled active drug delivery for in vivo treatment of stomach infection. Nat. Commun. 8, 272 (2017).
Li, J., Esteban-Fernández de Ávila, B., Gao, W., Zhang, L. & Wang, J. Micro/nanorobots for biomedicine: delivery, surgery, sensing, and detoxification. Sci. Robot. 2, eaam6431 (2017).
Wang, B., Kostarelos, K., Nelson, B. J. & Zhang, L. Trends in micro-/nanorobotics: materials development, actuation, localization, and system integration for biomedical applications. Adv. Mater. 33, 2002047 (2021).
Walker, D., Käsdorf, B. T., Jeong, H.-H., Lieleg, O. & Fischer, P. Enzymatically active biomimetic micropropellers for the penetration of mucin gels. Sci. Adv. 1, e1500501 (2015).
Wu, Z. et al. Self-propelled polymer-based multilayer nanorockets for transportation and drug release. Angew. Chem. Int. Ed. 52, 7000–7003 (2013).
Breger, J. C. et al. Self-folding thermo-magnetically responsive soft microgrippers. ACS Appl. Mater. Interfaces 7, 3398–3405 (2015).
Mushtaq, F. et al. Magnetoelectrically driven catalytic degradation of organics. Adv. Mater. 31, 1901378 (2019).
Karshalev, E. et al. Micromotor pills as a dynamic oral delivery platform. ACS Nano 12, 8397–8405 (2018).
Mundaca-Uribe, R. et al. Zinc microrocket pills: fabrication and characterization toward active oral delivery. Adv. Healthc. Mater. 9, 2000900 (2020).
Liu, K. et al. Micromotor based mini-tablet for oral delivery of insulin. ACS Nano 17, 300–311 (2023).
Mohammed, S. & Alqahtani, M. K. Mohammad A. Alsenaidy & Muhammad Z. Ahmad. Advances in Oral Drug Delivery. Front. Pharmacol. 19, 618411 (2021).
Ingersoll, K. S. & Cohen, J. The impact of medication regimen factors on adherence to chronic treatment: a review of literature. J. Behav. Med. 31, 213–224 (2008).
Homayun, B., Lin, X. & Choi, H.-J. Challenges and recent progress in oral drug delivery systems for biopharmaceuticals. Pharmaceutics 11, 129 (2019).
Waterman, K. C. et al. Osmotic capsules: a universal oral, controlled-release drug delivery dosage form. J. Control. Release 152, 264–269 (2011).
Arora, S., Ali, J., Ahuja, A., Khar, R. K. & Baboota, S. Floating drug delivery systems: a review. AAPS PharmSciTech 6, E372–E390 (2005).
Kirtane, A. R. et al. Development of an oral once-weekly drug delivery system for HIV antiretroviral therapy. Nat. Commun. 9, 2 (2018).
Abramson, A. et al. An ingestible self-orienting system for oral delivery of macromolecules. Science 363, 611–615 (2019).
Wang, J. et al. Novel nanomicelles based on rebaudioside A: a potential nanoplatform for oral delivery of honokiol with enhanced oral bioavailability and antitumor activity. Int. J. Pharm. 590, 119899 (2020).
Sze, L. P. et al. Oral delivery of paclitaxel by polymeric micelles: a comparison of different block length on uptake, permeability and oral bioavailability. Colloids Surf. B 184, 110554 (2019).
Siu FYK, Y. S., Lin, H. & Li, S. Galactosylated PLGA nanoparticles for the oral delivery of resveratrol: enhanced bioavailability and in vitro anti-inflammatory activity. Int. J. Nanomed. 13, 4133–4144 (2018).
Zhu, Y. et al. Nanostructured lipid carriers as oral delivery systems for improving oral bioavailability of nintedanib by promoting intestinal absorption. Int. J. Pharm. 586, 119569 (2020).
Mundaca-Uribe, R. et al. A microstirring pill enhances bioavailability of orally administered drugs. Adv. Sci. 8, 2100389 (2021).
Chen, W. et al. Dynamic omnidirectional adhesive microneedle system for oral macromolecular drug delivery. Sci. Adv. 8, eabk1792 (2022).
Zhang, X., Chen, G., Fu, X., Wang, Y. & Zhao, Y. Magneto-responsive microneedle robots for intestinal macromolecule delivery. Adv. Mater. 33, 2104932 (2021).
Abramson, A. et al. Oral mRNA delivery using capsule-mediated gastrointestinal tissue injections. Matter 5, 975–987 (2022).
Scudellari, M. Shot to the gut: “robotic” pill sails through human safety study. IEEE Spectrum (14 March 2019).
Yu, M. M2A™ capsule endoscopy. A breakthrough diagnostic tool for small intestine imaging. Gastroenterol. Nurs. 25, 24–27 (2002).
Pandolfino, J. E. Bravo capsule pH monitoring. Am. J. Gastroenterol. 100, 8–10 (2005).
Min, J., Yang, Y., Wu, Z. & Gao, W. Robotics in the Gut. Adv. Ther. 3, 1900125 (2020).
Wang, W., Duan, W., Ahmed, S., Mallouk, T. E. & Sen, A. Small power: autonomous nano- and micromotors propelled by self-generated gradients. Nano Today 8, 531–554 (2013).
Nelson, B. J., Kaliakatsos, I. K. & Abbott, J. J. Microrobots for minimally invasive medicine. Annu. Rev. Biomed. Eng. 12, 55–85 (2010).
Wei, X. et al. Biomimetic micromotor enables active delivery of antigens for oral vaccination. Nano Lett. 19, 1914–1921 (2019).
Hou, K. et al. A multifunctional magnetic red blood cell-mimetic micromotor for drug delivery and image-guided therapy. ACS Appl. Mater. Interfaces 14, 3825–3837 (2022).
Hortelao, A. C. et al. Swarming behavior and in vivo monitoring of enzymatic nanomotors within the bladder. Sci. Robot. 6, eabd2823 (2021).
Gao, W. et al. Artificial micromotors in the mouse’s stomach: a step toward in vivo use of synthetic motors. ACS Nano 9, 117–123 (2015).
Karshalev, E. et al. Micromotors for active delivery of minerals toward the treatment of iron deficiency anemia. Nano Lett. 19, 7816–7826 (2019).
Li, J., Rozen, I. & Wang, J. Rocket science at the nanoscale. ACS Nano 10, 5619–5634 (2016).
Wu, Z. et al. A microrobotic system guided by photoacoustic computed tomography for targeted navigation in intestines in vivo. Sci. Robot. 4, eaax0613 (2019).
Sun, L. et al. Biohybrid robotics with living cell actuation. Chem. Soc. Rev. 49, 4043–4069 (2020).
Zhang, F. et al. ACE2 receptor-modified algae-based microrobot for removal of SARS-CoV-2 in wastewater. J. Am. Chem. Soc. 143, 12194–12201 (2021).
Rajabasadi, F. et al. Multifunctional 4D-printed sperm-hybrid microcarriers for assisted reproduction. Adv. Mater. 34, 2204257 (2022).
Xu, H., Medina-Sánchez, M., Maitz, M. F., Werner, C. & Schmidt, O. G. Sperm micromotors for cargo delivery through flowing blood. ACS Nano 14, 2982–2993 (2020).
Ricotti, L. et al. Biohybrid actuators for robotics: a review of devices actuated by living cells. Sci. Robot. 2, eaaq0495 (2017).
Xu, H. et al. Human spermbots for patient-representative 3D ovarian cancer cell treatment. Nanoscale 12, 20467–20481 (2020).
Zhang, F. et al. Gastrointestinal tract drug delivery using algae motors embedded in a degradable capsule. Sci. Robot. 7, eabo4160 (2022).
Katzung, B. G. (ed.) Basic & Clinical Pharmacology, 14th edn (McGraw Hill, 2017).
Parmar, J., Vilela, D., Villa, K., Wang, J. & Sánchez, S. Micro- and nanomotors as active environmental microcleaners and sensors. J. Am. Chem. Soc. 140, 9317–9331 (2018).
Soler, L., Magdanz, V., Fomin, V. M., Sanchez, S. & Schmidt, O. G. Self-propelled micromotors for cleaning polluted water. ACS Nano 7, 9611–9620 (2013).
Orozco, J., Mercante, L. A., Pol, R. & Merkoçi, A. Graphene-based Janus micromotors for the dynamic removal of pollutants. J. Mater. Chem. A 4, 3371–3378 (2016).
Mundaca-Uribe, R. et al. A microstirring oral pill for improving the glucose-lowering effect of metformin. ACS Nano 17, 9272–9279 (2023).
Srinivasan, S. S. et al. RoboCap: robotic mucus-clearing capsule for enhanced drug delivery in the gastrointestinal tract. Sci. Robot. 7, eabp9066 (2022).
Traverso, G. et al. Microneedles for drug delivery via the gastrointestinal tract. J. Pharm. Sci. 104, 362–367 (2015).
Abramson, A. et al. A luminal unfolding microneedle injector for oral delivery of macromolecules. Nat. Med. 25, 1512–1518 (2019).
Abramson, A. et al. Oral delivery of systemic monoclonal antibodies, peptides and small molecules using gastric auto-injectors. Nat. Biotech. 40, 103–109 (2022).
Kriegel, C., Attarwala, H. & Amiji, M. Multi-compartmental oral delivery systems for nucleic acid therapy in the gastrointestinal tract. Adv. Drug Deliv. Rev. 65, 891–901 (2013).
Elzatahry, A. A., Eldin, M. S. M., Soliman, E. A. & Hassan, E. A. Evaluation of alginate–chitosan bioadhesive beads as a drug delivery system for the controlled release of theophylline. J. Appl. Polym. Sci. 111, 2452–2459 (2009).
Guan, J. et al. A novel gastric-resident osmotic pump tablet: in vitro and in vivo evaluation. Int. J. Pharm. 383, 30–36 (2010).
Liu, J. et al. Triggerable tough hydrogels for gastric resident dosage forms. Nat. Commun. 8, 124 (2017).
Liu, X. et al. Ingestible hydrogel device. Nat. Commun. 10, 493 (2019).
Felfoul, O. et al. Magneto-aerotactic bacteria deliver drug-containing nanoliposomes to tumour hypoxic regions. Nat. Nanotech. 11, 941–947 (2016).
Maric, T. et al. Self-propelled Janus micromotors for pH-responsive release of small molecule drug. Appl. Mater. Today 27, 101418 (2022).
Dhalla, A. K. et al. A robotic pill for oral delivery of biotherapeutics: safety, tolerability, and performance in healthy subjects. Drug Deliv. Transl. Res. 12, 294–305 (2022).
Jin, Q., Yang, Y., Jackson, J. A., Yoon, C. & Gracias, D. H. Untethered single cell grippers for active biopsy. Nano Lett. 20, 5383–5390 (2020).
Gultepe, E. et al. Biologic tissue sampling with untethered microgrippers. Gastroenterology 144, 691–693 (2013).
Beardslee, L. A. et al. Ingestible sensors and sensing systems for minimally invasive diagnosis and monitoring: the next frontier in minimally invasive screening. ACS Sens. 5, 891–910 (2020).
Kalantar-Zadeh, K. et al. A human pilot trial of ingestible electronic capsules capable of sensing different gases in the gut. Nat. Electron. 1, 79–87 (2018).
Nejati, S. et al. Small intestinal sampling capsule for inflammatory bowel disease type detection and management. Lab Chip 22, 57–70 (2022).
Soto, F. et al. Robotic pill for biomarker and fluid sampling in the gastrointestinal tract. Adv. Intell. Syst. 4, 2200030 (2022).
van der Merwe, J., Steenekamp, J., Steyn, D. & Hamman, J. The role of functional excipients in solid oral dosage forms to overcome poor drug dissolution and bioavailability. Pharmaceutics 12, 393 (2020).
Velligan, D. I. & Kamil, S. H. Enhancing patient adherence: introducing smart pill devices. Ther. Deliv. 5, 611–613 (2014).
Hafezi, H. et al. An ingestible sensor for measuring medication adherence. IEEE Trans. Biomed. Eng. 62, 99–109 (2015).
De la Paz, E. et al. A self-powered ingestible wireless biosensing system for real-time in situ monitoring of gastrointestinal tract metabolites. Nat. Commun. 13, 7405 (2022).
Lu, Y., Aimetti, A. A., Langer, R. & Gu, Z. Bioresponsive materials. Nat. Rev. Mater. 2, 16075 (2016).
Adler, S. N. & Metzger, Y. C. PillCam COLON capsule endoscopy: recent advances and new insights. Ther. Adv. Gastroenterol. 4, 265–268 (2011).
Jones, A. A. D. III, Mi, G. & Webster, T. J. A status report on FDA approval of medical devices containing nanostructured materials. Trends Biotechnol. 37, 117–120 (2019).
Acknowledgements
This work was supported by the National Institutes of Health, under award number R21AI159492, and by the Defense Threat Reduction Agency Joint Science and Technology Office for Chemical and Biological Defense, under award number HDTRA1‐21‐1‐0010.
Author information
Authors and Affiliations
Contributions
All authors contributed to researching the literature and to the writing and editing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Samuel Sanchez and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mundaca-Uribe, R., Askarinam, N., Fang, R.H. et al. Towards multifunctional robotic pills. Nat. Biomed. Eng (2023). https://doi.org/10.1038/s41551-023-01090-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41551-023-01090-6