Abstract
Multimodal sensory feedback from upper-limb prostheses can increase their function and usability. Here we show that intuitive thermal perceptions during cold-object grasping with a prosthesis can be restored in a phantom hand through targeted nerve stimulation via a wearable thin-film thermoelectric device with high cooling power density and speed. We found that specific regions of the residual limb, when thermally stimulated, elicited thermal sensations in the phantom hand that remained stable beyond 48 weeks. We also found stimulation sites that selectively elicited sensations of temperature, touch or both, depending on whether the stimulation was thermal or mechanical. In closed-loop functional tasks involving the identification of cold objects by amputees and by non-amputee participants, and compared with traditional bulk thermoelectric devices, the wearable thin-film device reliably elicited cooling sensations that were up to 8 times faster and up to 3 times greater in intensity while using half the energy and 1/600th the mass of active thermoelectric material. Wearable thin-film thermoelectric devices may allow for the non-invasive restoration of thermal perceptions during touch.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All source data generated or analysed during the study and needed to interpret and verify the findings are available within the paper and its Supplementary Information. Source data are provided with this paper.
Code availability
The code used for controlling the virtual and physical prosthetic limb is available at https://bitbucket.org/rarmiger/minivie. The custom Arduino code used for monitoring and controlling thermal stimulation and the custom MATLAB code used for running the thermal-reaction-time experiment and for analysing the data are available for research purposes from the corresponding authors on reasonable request.
References
Abraira, V. E. & Ginty, D. D. The sensory neurons of touch. Neuron 79, 618–639 (2013).
Schepers, R. J. & Ringkamp, M. Thermoreceptors and thermosensitive afferents. Neurosci. Biobehav. Rev. 34, 177–184 (2010).
Graczyk, E. L. et al. The neural basis of perceived intensity in natural and artificial touch. Sci. Transl. Med. 8, 362ra142 (2016).
Oddo, C. M. et al. Intraneural stimulation elicits discrimination of textural features by artificial fingertip in intact and amputee humans. eLife 5, e09148 (2016).
Osborn, L. E. et al. Prosthesis with neuromorphic multilayered e-dermis perceives touch and pain. Sci. Robot. 3, eaat3818 (2018).
Kuiken, T. A., Marasco, P. D., Lock, B. A., Harden, R. N. & Dewald, J. P. A. Redirection of cutaneous sensation from the hand to the chest skin of human amputees with targeted reinnervation. Proc. Natl Acad. Sci. USA 104, 20061–20066 (2007).
Ortiz-Catalan, M., Mastinu, E., Sassu, P., Aszmann, O. & Brånemark, R. Self-contained neuromusculoskeletal arm prostheses. N. Engl. J. Med. 382, 1732–1738 (2020).
Srinivasan, S. S. & Herr, H. M. A cutaneous mechanoneural interface for neuroprosthetic feedback. Nat. Biomed. Eng. 6, 731–740 (2022).
Bensmaia, S. J., Tyler, D. J. & Micera, S. Restoration of sensory information via bionic hands. Nat. Biomed. Eng. 7, 443–455 (2023).
Raspopovic, S., Valle, G. & Petrini, F. M. Sensory feedback for limb prostheses in amputees. Nat. Mater. 20, 925–939 (2021).
Bohic, M. & Abraira, V. E. Wired for social touch: the sense that binds us to others. Curr. Opin. Behav. Sci. 43, 207–215 (2022).
Zbinden, J., Lendaro, E. & Ortiz-Catalan, M. A multi-dimensional framework for prosthetic embodiment: a perspective for translational research. J. Neuroeng. Rehabil. 19, 122 (2022).
Srinivasan, S. S. et al. On prosthetic control: a regenerative agonist–antagonist myoneural interface. Sci. Robot. 2, eaan2971 (2017).
Valle, G. et al. Biomimetic intraneural sensory feedback enhances sensation naturalness, tactile sensitivity, and manual dexterity in a bidirectional prosthesis. Neuron 100, 37–45 (2018).
Osborn, L. E. et al. Sensory stimulation enhances phantom limb perception and movement decoding. J. Neural Eng. 17, 056006 (2020).
Christie, B. P. et al. Ambulatory searching task reveals importance of somatosensation for lower-limb amputees. Sci. Rep. 10, 1–11 (2020).
George, J. A. et al. Biomimetic sensory feedback through peripheral nerve stimulation improves dexterous use of a bionic hand. Sci. Robot. 4, eaax2352 (2019).
Preatoni, G., Valle, G., Petrini, F. M. & Raspopovic, S. Lightening the perceived prosthesis weight with neural embodiment promoted by sensory feedback. Curr. Biol. 31, 1065–1071 (2021).
Graczyk, E. L., Resnik, L., Schiefer, M. A., Schmitt, M. S. & Tyler, D. J. Home use of a neural-connected sensory prosthesis provides the functional and psychosocial experience of having a hand again. Sci. Rep. 8, 9866 (2018).
Merrill, D. R., Bikson, M. & Jefferys, J. G. R. Electrical stimulation of excitable tissue: design of efficacious and safe protocols. J. Neurosci. Methods 141, 171–198 (2005).
Venkatasubramanian, R., Siivola, E., Colpitts, T. & O’Quinn, B. Thin-film thermoelectric devices with high room-temperature figures of merit. Nature 413, 597–602 (2001).
Jin, Q. et al. Flexible layer-structured Bi2Te3 thermoelectric on a carbon nanotube scaffold. Nat. Mater. 18, 62–68 (2019).
Sun, T. et al. Stretchable fabric generates electric power from woven thermoelectric fibers. Nat. Commun. 11, 1–10 (2020).
Venkatasubramanian, R., Pierce, J. M. & Dezsi, G. Superlattice structures for thermoelectric devices. US patent 10,903,139 (2021).
Venkatasubramanian, R., Pierce, J. M., Himmtann, M., Dezsi, G. & Rhim, Y.-R. Thin-film thermoelectric conversion devices for direct thermal-to-electric conversion for DC and pulse power. Johns Hopkins APL Tech. Dig. 35, 448–452 (2021).
Osborn, L. et al. Phantom hand activation during physical touch and targeted transcutaneous electrical nerve stimulation. In MEC Symposium Conference (2020).
Osborn, L. et al. Targeted transcutaneous electrical nerve stimulation for phantom limb sensory feedback. In IEEE Trans. Biomed. Circuits Syst. 1–4 (2017); https://doi.org/10.1109/BIOCAS.2017.8325200
Luo, M. et al. High-density thermal sensitivity maps of the human body. Build. Environ. 167, 106435 (2020).
Green, B. G. & Akirav, C. Threshold and rate sensitivity of low-threshold thermal nociception. Eur. J. Neurosci. 31, 1637–1645 (2010).
Adair, R. K. A model of the detection of warmth and cold by cutaneous sensors through effects on voltage-gated membrane channels. Proc. Natl Acad. Sci. USA 96, 11825–11829 (1999).
Chowdhury, I. et al. On-chip cooling by superlattice-based thin-film thermoelectrics. Nat. Nanotechnol. 4, 235–238 (2009).
Venkatasubramanian, R., Osborn, L. E., Armiger, R. S., Himmtann, M. & Pierce, J. M. Fast-rate thermoelectric device. US patent 11,227,988 (2022).
Johannes, M. S. et al. In Wearable Robotics (eds Rosen, J. & Ferguson, P. W.) 393–444 (Academic, 2020).
Izawa, J. & Shadmehr, R. On-line processing of uncertain information in visuomotor control. J. Neurosci. 28, 11360–11368 (2008).
Wei, K. & Koerding, K. Uncertainty of feedback and state estimation determines the speed of motor adaptation. Front. Comput. Neurosci. 4, 11 (2010).
Zhu, K., Perrault, S., Chen, T., Cai, S. & Lalintha Peiris, R. A sense of ice and fire: exploring thermal feedback with multiple thermoelectric-cooling elements on a smart ring. Int. J. Hum. Comput. Stud. 130, 234–247 (2019).
Gallo, S. et al. Encoded and crossmodal thermal stimulation through a fingertip-sized haptic display. Front. Robot. AI 2, 25 (2015).
Kim, S. et al. Two-dimensional thermal haptic module based on a flexible thermoelectric device. Soft Robot. 7, 736–742 (2020).
Kishore, R. A., Nozariasbmarz, A., Poudel, B., Sanghadasa, M. & Priya, S. Ultra-high performance wearable thermoelectric coolers with less materials. Nat. Commun. 10, 1765 (2019).
Lee, J. et al. Stretchable skin-like cooling/heating device for reconstruction of artificial thermal sensation in virtual reality. Adv. Funct. Mater. 30, 1909171 (2020).
Chatt, A. B. & Kenshalo, D. R. Cerebral evoked responses to skin warming recorded from human scalp. Exp. Brain Res 28, 449–455 (1977).
Li, G. et al. Integrated microthermoelectric coolers with rapid response time and high device reliability. Nat. Electron. 1, 555–561 (2018).
Ren, W. et al. High-performance wearable thermoelectric generator with self-healing, recycling, and Lego-like reconfiguring capabilities. Sci. Adv. 7, eabe0586 (2021).
Dunham, M. T. et al. Experimental characterization of microfabricated thermoelectric energy harvesters for smart sensor and wearable applications. Adv. Mater. Technol. 3, 1700383 (2018).
Kim, F. et al. 3D printing of shape-conformable thermoelectric materials using all-inorganic Bi2Te3-based inks. Nat. Energy 3, 301–309 (2018).
Jo, S., Choo, S., Kim, F., Heo, S. H. & Son, J. S. Ink processing for thermoelectric materials and power‐generating devices. Adv. Mater. 31, 1804930 (2019).
Beukema, P. et al. TrpM8-mediated somatosensation in mouse neocortex. J. Comp. Neurol. 526, 1444–1456 (2018).
Naver, H. et al. Autonomic and thermal sensory symptoms and dysfunction after stroke. Stroke 26, 1379–1385 (1995).
Xue, M. & Jackson, C. J. Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv. Wound Care 4, 119–136 (2015).
Osborn, L. E. et al. Extended home use of an advanced osseointegrated prosthetic arm improves function, performance, and control efficiency. J. Neural Eng. 18, 026020 (2021).
Hebert, J. S. et al. Novel targeted sensory reinnervation technique to restore functional hand sensation after transhumeral amputation. IEEE Trans. Neural Syst. Rehabil. Eng. 22, 765–773 (2014).
Roh, I. J. et al. Harman measurements for thermoelectric materials and modules under non-adiabatic conditions. Sci. Rep. 6, 1–10 (2016).
Harman, T. C. Special techniques for measurement of thermoelectric properties. J. Appl. Phys. 29, 1373–1374 (1958).
Cook, B. A. et al. High-performance three-stage cascade thermoelectric devices with 20% efficiency. J. Electron. Mater. 44, 1936–1942 (2015).
Geng, Q. et al. Temperature limit values for touching cold surfaces with the fingertip. Ann. Occup. Hyg. 50, 851–862 (2006).
McKemy, D. D. The molecular and cellular basis of cold sensation. ACS Chem. Neurosci. 4, 238–247 (2012).
Simone, D. A. & Kajander, K. C. Responses of cutaneous A-fiber nociceptors to noxious cold. J. Neurophysiol. 77, 2049–2060 (1997).
Osborn, L. E. et al. Monitoring at-home prosthesis control improvements through real-time data logging. J. Neural Eng. 19, 036021 (2022).
Handelman, D. A. et al. Shared control of bimanual robotic limbs with a brain-machine interface for self-feeding. Front. Neurorobot. 16, 918001 (2022).
Perry, B. N. et al. Virtual integration environment as an advanced prosthetic limb training platform. Front. Neurol. 9, 785 (2018).
Englehart, K. & Hudgins, B. A robust, real-time control scheme for multifunction myoelectric control. IEEE Trans. Biomed. Eng. 50, 848–854 (2003).
Armiger, R. S. et al. A real-time virtual integration environment. Johns Hopkins APL Tech. Dig. 30, 198–206 (2011).
Wester, B. A. et al. CONVEY: connecting STEM outreach now using VIE education for youth. Johns Hopkins APL Tech. Dig. 35, 259–266 (2020).
Shapiro, S. S. & Wilk, M. B. An analysis of variance test for normality (complete samples). Biometrika 52, 591–611 (1965).
Goldsmid, H. J. In CRC Handbook of Thermoelectrics (ed. Rowe, D. M.) 19–26 (CRC, 1995).
Semenyuk, V. In Thermoelectrics Handbook (ed. Rowe, D. M.) 58-1-58-21 (Taylor & Francis, 2006).
von Gunten, K. Thermal cycler optimized for real-time DNA analysis. EE Times (2012).
Acknowledgements
The authors thank the participants who contributed their time to improving the technology for those with upper-extremity impairment. The authors thank B. Christie for reviewing results and analysis, M. Iskarous for assistance with managing ethical-approval documentation, J. Forsberg and P. Pasquina for programmatic support, B. Wester for data-visualization guidance and C. Carneal for programmatic guidance and support. R.S.A. acknowledges support from the Uniformed Services University of Health Sciences and the Henry M. Jackson Foundation for the Advancement of Military Medicine (HJF) under federal awards HU00011520028 and HU00012020062. L.E.O. acknowledges internal research support from the Johns Hopkins University Applied Physics Laboratory. R.V. acknowledges support for the original development of CHESS thin-film thermoelectric materials and technology from the Defense Advanced Research Projects Agency (DARPA) under contract HR0011-16-C-0011. The MPL and vMPL were previously developed as part of the DARPA Revolutionizing Prosthetics Program. The views expressed in this article are those of the authors and do not reflect the official policy of HJF, the Department of Army/Navy/Air Force, Department of Defense or the US Government. The opinions and assertions expressed herein are those of the authors and do not necessarily reflect the official policy or position of the Uniformed Services University or the Department of Defense.
Author information
Authors and Affiliations
Contributions
L.E.O., R.V., M.H., A.C.G.C., C.W.M., J.M.W., H.H.N., M.S.F. and R.S.A. designed, implemented and conducted the thermotactile experiments. R.V., M.H., P.G. and R.J.U. designed, fabricated and tested the TFTEC devices and their integration into suitable heat sinks for human participant testing. J.M.P. performed epitaxial thin-film growth needed for the TFTEC devices. L.E.O. performed data visualization. L.E.O. and R.V. wrote the manuscript. All authors contributed to editing and reviewing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
R.V., L.E.O., M.H., J.M.P. and R.S.A. are inventors on intellectual property pertaining to thin-film thermoelectric devices and US patents 11,227,988 and 11,532,778 and application 18/071,789. R.V. and J.M.P. are inventors on US patent 10,903,139. The Johns Hopkins University is the applicant on these patents. The other authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Kornelius Nielsch and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
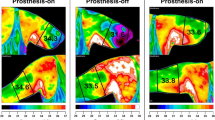
Extended Data Fig. 1 Thermal sensations are stable over 11 months.
a, Noninvasive thermal stimulation of the skin was used to restore thermal sensations in the phantom hand using thin-film thermoelectric cooling (TFTEC) devices and enable perception of cold objects during grasping with a prosthesis. b, The activated regions of the phantom hand remained similar after 11 months (48 weeks) for participant A2, showing long-term stability of restored thermal perceptions. This stability aligns with previously documented stability in phantom hand sensory maps for other stimulation modalities15. c, Participant A1 underwent an unrelated surgery on the amputated arm after the initial sensory mapping, which affected the phantom hand sensory maps. Sensory sites were mapped again 29 months (128 weeks) after the initial mapping session. Although activated regions changed due to the unrelated surgery, we were able to convey thermal sensations to the phantom hand. With the new sensory sites, we observed similarities from previous sites in that mechanical and thermal perceptions did not always project to the same region of the phantom hand despite the same site of stimulation.
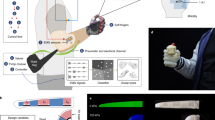
Extended Data Fig. 2 TFTEC device fabrication.
a, Key steps in the fabrication of a TFTEC module, used in this study, utilizing the Controlled Hierarchically Engineered Superlattice Structures (CHESS) materials. 1) 25 μm p-type and n-type CHESS thin-films are grown on GaAs substrates and a metallization layer (Cu/Au/Sn, 51 μm) is placed on top of the thin-films. 2) The p-type and n-type materials are cut into strips and 3) are bonded onto an AlN substrate (Header 1, 380 μm). An additional metallization layer (Ni/Cu/Au, 30 μm) is placed, which is used to bond the CHESS thin-films onto Au-plated Cu traces (30 μm) with an In alloy solder (25 μm) to form a single p-n couple module. 4) A 3 × 4 array of the p-n coupled modules is assembled on a common AlN substrate (Header 2, 380 μm), which acts as a heat collector, and an additional SiC common header (Header 3, 330 μm) is placed on top of the module array, connecting the p-n couples in parallel and enabling contact with the skin. b, Physical dimensions of the thin-film thermoelectric cooling (TFTEC) and bulk devices, showing the benefits of the TFTEC for wearable applications. The total mass of the TFTEC module is about 1/2 of a small Band-Aid or 1/5th of a rubber band. It is worth noting that the TFTEC module is 1/28th the total mass of the bulk module, and uses ~1/600th the active TE material mass for better functionality. Future development of TFTEC devices can include lowering the weight of AlN (Headers 1, 2, and 3) enabling more lightweight thermotactile packages, while keeping the functionality of cooling and heating. The TFTEC technology for thermotactile applications presented here is a proof-of-concept demonstration in producing biologically relevant speeds of cooling. c, The three types of thermoelectric cooling device used in the thermotactile experiments.
Extended Data Fig. 3 Steady-state thermoelectric device cooling response, material properties, and figures of merit.
a, All three of the TEC devices remained stable and did not deviate once reaching the target temperature value. Differences in current used, compared to Fig. 2g, is because the input current to reach a target temperature can vary ±0.1 A across modules. b, Steady-state response of the thin-film module used with participant A1 for the cold object identification experiments. c, The figure of merit (ZT) was estimated using the Harman method to measure Ohmic (Vr) and Peltier (V0) voltage components when TEC device input current was switched off. d, Measured voltage values for each TEC device used to estimate the ZT. e, VT was estimated as the voltage at steady state before current was switched off (t0) and V0 was estimated by the voltage immediately after input current is removed. Measurements were taken at T = 300 K. f, Effective ZT estimated from thermal efficiency for thin-film (1 × 4 array) and bulk thermoelectric generator (TEG) devices. Data redrawn with permission from25. g, Inherent material properties of both p- and n-type materials were nominally the same in TFTEC modules and generally the same approach of comparable p- and n-type material properties are used by manufacturers of bulk modules65. Despite having a smaller active aspect ratio compared to bulkHC, the thin-film device has larger ZT and a slightly higher Seebeck coefficient, which leads to higher Peltier cooling. The material ZT were calculated from the three individual properties (that is, electrical resistivity, Seebeck coefficient and thermal conductivity) at T = 300 K. The observed module ZT of the CHESS TFTEC device is higher than both bulk devices – translating to less energy consumed in the cooling sensation in the present study and higher heat-to-electric conversion efficiency in a related study25.
Extended Data Fig. 4 Exemplar thin-film thermoelectric device cooling and repeatability.
a, Cooling profile as a function of current for two example TFTEC devices with a ΔTmax = 61.8 °C (305 K p-n couple Harman ZT of ~0.72) and ΔTmax = 68.7 °C (305 K p-n couple Harman ZT of ~0.96) for Module 1 and 2, respectively. Unlike thinned bulk TE materials which can achieve a ΔTmax up to 23 °C66, modules with thin-film TE materials can result in ΔTmax up to 68.7 °C. b, The temperature differential (ΔT) is the difference between the hot side (Thot) and the cold side (Tcold) of the TFTEC device during steady-state performance. c, Cooling reproducibility of the TFTEC device (Module 1) in (a) for 50 cycles over more than 400 min. Data points shown are the temperature differential between Thot and Tcold at steady state after input current to the device was turned off (ΔT = 0 °C) or on (ΔT ~ 62 °C). Previous TFTEC devices were reported to be stable over 500,000 cycles67. d, Perceptual data was collected with one participant wearing the TFTEC device on the index finger over 3 hr. The reaction and perceived intensity of the thermal stimulation did not significantly change over the experiment and the participant perceived thermal sensations on every trial (n = 45 independent trials from one TFTEC device). Trend lines were fit using linear regression and the fitted slopes were not significantly different from zero (Pslope > 0.05), suggesting perceptual and hardware stability (that is, no significant changes in perception) while wearing the TFTEC device for the extended duration. Data are presented as individual measurements. A one-sample t-test, using the estimated regression slope and its standard error, were used to calculate the statistical P values for the regression slopes.
Extended Data Fig. 5 Baseline visual reaction time.
a, The amputee and non-amputee participants performed a visual reaction time task using the same button used in the thermal stimulation task. Data represents independent trials; n = 30 for A2, A3, and A4; n = 90 for B1and B3; n = 60 for B2 and B4. b, Performance metrics. Participant A1 did not perform the visual reaction time task. The violin plot whiskers represent the minimal and maximal values, the vertical lines indicate the first and third quartiles, the horizontal lines are means, and the white dots are the medians. The average reaction time for each non-amputee participant was used to normalize the thermal stimulation reaction time results and compare across individuals.
Extended Data Fig. 6 Thermal reaction and perception in participants with limb amputation.
a, Thermal detection experiment at 23 °C for participant A1. Statistical comparisons were not performed because only one trial was detected for the bulk device at each stimulation site. b, Participant A4 also performed the thermal detection experiment with the bulkHC device and reported sensations of warming on some trials despite the target temperature being set to 16 °C (Supplementary Discussion). Data presented as performance per block of up to five independent trials, n = 20 trials (4 blocks, bulk), 22 trials (5 blocks, bulkHC), and 50 trials (10 blocks, TFTEC). c, Thermal stimulation on the residual limb with the thin-film device leads to faster reaction times; however, the bulkHC device was perceived faster on trials that were felt as cooling sensations in the phantom hand. There were no significant differences across the devices on trials that were perceived as warming. d, Perceived intensity was similar across all devices and stimulation sites for this participant, with the exception of the thin-film device eliciting slightly stronger cooling sensations on the arm and the bulkHC device eliciting slightly stronger sensations on trials perceived as warming. a, c, d, Number of independent trials for each condition that elicited thermal perception is given by n. Total number of independent trials, including those that did not elicit thermal perception, for each condition was 10 (A1 arm TFTEC); 5 (A1 bulk, phantom TFTEC); 10 (A4 bulk, arm bulkHC); 12 (A4 bulkHC phantom); and 25 (A4 TFTEC). Data represents independent trials and bars represent mean ± s.e.m of individual trials. P values were generated with a two-sided Mann-Whitney U test.
Extended Data Fig. 7 Detection, reaction, and perception of thermal stimulation for non-amputee participants.
a, Probability of detecting cooling sensations in individual intact limb participants. Data presented as performance per block of five independent trials, n = 20 independent trials (4 blocks) for all conditions except for n = 25 independent trials (5 blocks) for B3 with the bulk device. b, Reaction time and c, perceived intensity of thermal stimulation, n represents number of independent trials where cooling sensation was perceived. Data are presented from individual trials where cooling was perceived. The target temperature was set to 16 °C for all devices. In all instances, the thin-film device led to faster and more intense thermal perception during stimulation of the index fingertip. Bar plots are presented as mean ± s.e.m. of individual trials, P values were generated with a two-sided Mann-Whitney U test.
Extended Data Fig. 8 Cold object detection using thermal feedback for amputee and non-amputee participants.
a, Participants controlled the vMPL using EMG (A1) or motion tracking (A2, B5, B6) with a wireless armband. Thermal feedback was provided to the phantom hand (amputee) or tip of the index finger (non-amputee). b, EMG decoding (A1). Only elbow movements were used for the virtual task. Data presented as mean ± s.e.m. of n = 5 feature sets. c, The time spent touching each object during the virtual task for A2 was similar between bulkHC (n = 40 touches over 10 trials) and TFTEC (n = 54 touches, 15 trials) devices. d, The two non-amputee participants were more successful detecting virtual cold objects with the bulkHC (n = 24 trials, 5 blocks) and TFTEC (n = 34 trials, 7 blocks) device compared to the bulk device (n = 25 trials, 5 blocks). Bar plots represent mean ± s.e.m.; data points represent blocks with up to five trials. e, For the two non-amputee participants, the normalized time spent touching virtual objects was significantly shorter for the TFTEC (n = 112 touches, 34 trials) compared to the bulk (n = 133 touches, 25 trials) and bulkHC (n = 92 touches, 24 trials) devices. Data normalized using max-min normalization for each participant. f, Time spent touching each virtual object. For B5, n = 80, 58, and 46 touches, over 15 trials with each device, for bulk, bulkHC, and TFTEC, respectively. For B6, n = 53, 34, and 66 touches for bulk (10 trials), bulkHC (9 trials), and TFTEC (19 trials), respectively. c, e, f, Data represent independent virtual object touches and all trials are independent. Violin plot whiskers represent the minimal and maximal values, vertical lines indicate first and third quartiles, horizontal lines are means, and white dots are the medians. P values were generated with a two-sided Mann-Whitney U test.
Supplementary information
Supplementary Information
Supplementary methods, discussion, figures and references.
Supplementary Video 1
Description of thermal sensations in the phantom hand and comparison of bulk and TFTEC devices by participant A2.
Supplementary Video 2
Description of thermal and mechanical sensations in the phantom hand by participant A3.
Supplementary Video 3
Thermal reaction and perception experiment with participant A3.
Supplementary Video 4
Thermal reaction and perception experiment with non-amputee participants.
Supplementary Video 5
Cold-object detection experiment with amputee participant.
Supplementary Video 6
Cold-object detection and drinking with amputee participant.
Supplementary Video 7
Virtual cold-object detection experiment with amputee participant.
Supplementary Video 8
Virtual cold-object detection experiment with non-amputee participant.
Supplementary Video 9
Open-air liquid collection and freezing with thin-film thermoelectric cooling device.
Source data
Source Data for Figs. 2–5 and Extended Data Figs. 2–8
Source data and statistics.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Osborn, L.E., Venkatasubramanian, R., Himmtann, M. et al. Evoking natural thermal perceptions using a thin-film thermoelectric device with high cooling power density and speed. Nat. Biomed. Eng (2023). https://doi.org/10.1038/s41551-023-01070-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41551-023-01070-w
This article is cited by
-
Thermally sentient bionic limbs
Nature Biomedical Engineering (2024)
-
A Skin-Inspired Self-Adaptive System for Temperature Control During Dynamic Wound Healing
Nano-Micro Letters (2024)