Abstract
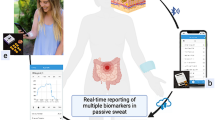
The quantification of protein biomarkers in blood at picomolar-level sensitivity requires labour-intensive incubation and washing steps. Sensing proteins in sweat, which would allow for point-of-care monitoring, is hindered by the typically large interpersonal and intrapersonal variations in its composition. Here we report the design and performance of a wearable and wireless patch for the real-time electrochemical detection of the inflammatory biomarker C-reactive (CRP) protein in sweat. The device integrates iontophoretic sweat extraction, microfluidic channels for sweat sampling and for reagent routing and replacement, and a graphene-based sensor array for quantifying CRP (via an electrode functionalized with anti-CRP capture antibodies-conjugated gold nanoparticles), ionic strength, pH and temperature for the real-time calibration of the CRP sensor. In patients with chronic obstructive pulmonary disease, with active or past infections or who had heart failure, the elevated concentrations of CRP measured via the patch correlated well with the protein’s levels in serum. Wearable biosensors for the real-time sensitive analysis of inflammatory proteins in sweat may facilitate the management of chronic diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. Source data for Figs. 3 and 5 are provided with this paper. All raw and analysed datasets generated during the study are available from the corresponding author on request.
References
The top 10 causes of death. World Health Organization https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (2020).
Mannino, D. M. et al. Economic burden of COPD in the presence of comorbidities. Chest 148, 138–150 (2015).
Ferrucci, L. & Fabbri, E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 15, 505–522 (2018).
Ashley, N. T., Weil, Z. M. & Nelson, R. J. Inflammation: mechanisms, costs, and natural variation. Annu. Rev. Ecol. Evol. Syst. 43, 385–406 (2012).
Schett, G. & Neurath, M. F. Resolution of chronic inflammatory disease: universal and tissue-specific concepts. Nat. Commun. 9, 3261 (2018).
Furman, D. et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 25, 1822–1832 (2019).
Wylezinski, L. S., Gray, J. D., Polk, J. B., Harmata, A. J. & Spurlock, C. F. Illuminating an invisible epidemic: a systemic review of the clinical and economic benefits of early diagnosis and treatment in inflammatory disease and related syndromes. J. Clin. Med. 8, 493 (2019).
Emerging Risk Factors Collaboration et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet 375, 132–140 (2010).
Ridker, P. M. A test in context: high-sensitivity C-reactive protein. J. Am. Coll. Cardiol. 67, 712–723 (2016).
Proctor, M. J. et al. Systemic inflammation predicts all-cause mortality: a Glasgow inflammation outcome study. PLoS ONE 10, e0116206 (2015).
Prins, B. P. et al. Investigating the causal relationship of C-reactive protein with 32 complex somatic and psychiatric outcomes: a large-scale cross-consortium Mendelian randomization study. PLoS Med. 13, e1001976 (2016).
Balayan, S., Chauhan, N., Rosario, W. & Jain, U. Biosensor development for C-reactive protein detection: a review. Appl. Surf. Sci. Adv. 12, 100343 (2022).
Young, B., Gleeson, M. & Cripps, A. W. C-reactive protein: a critical review. Pathology 23, 118–124 (1991).
Lobo, S. M. Sequential C-reactive protein measurements in patients with serious infections: does it help? Crit. Care 16, 130 (2012).
Guo, S., Mao, X. & Liang, M. The moderate predictive value of serial serum CRP and PCT levels for the prognosis of hospitalized community-acquired pneumonia. Respir. Res. 19, 193 (2018).
Gao, W. et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 529, 509–514 (2016).
Yang, Y. & Gao, W. Wearable and flexible electronics for continuous molecular monitoring. Chem. Soc. Rev. 48, 1465–1491 (2019).
Ray, T. R. et al. Soft, skin-interfaced sweat stickers for cystic fibrosis diagnosis and management. Sci. Transl. Med. 13, eabd8109 (2021).
Kim, J., Campbell, A. S., de Ávila, B. E.-F. & Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 37, 389–406 (2019).
Lee, H. et al. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nat. Nanotechol. 11, 566–572 (2016).
Koh, A. et al. A soft, wearable microfluidic device for the capture, storage, and colorimetric sensing of sweat. Sci. Transl. Med. 8, 366ra165 (2016).
Ates, H. C. et al. On-site therapeutic drug monitoring. Trends Biotechnol. 38, 1262–1277 (2020).
Bariya, M., Nyein, H. Y. Y. & Javey, A. Wearable sweat sensors. Nat. Electron. 1, 160–171 (2018).
Parlak, O., Keene, S. T., Marais, A., Curto, V. F. & Salleo, A. Molecularly selective nanoporous membrane-based wearable organic electrochemical device for noninvasive cortisol sensing. Sci. Adv. 4, eaar2904 (2018).
Tu, J., Torrente‐Rodríguez, R. M., Wang, M. & Gao, W. The era of digital health: a review of portable and wearable affinity biosensors. Adv. Funct. Mater. 30, 1906713 (2020).
Heikenfeld, J. et al. Accessing analytes in biofluids for peripheral biochemical monitoring. Nat. Biotechnol. 37, 407–419 (2019).
Yang, Y. et al. A laser-engraved wearable sensor for sensitive detection of uric acid and tyrosine in sweat. Nat. Biotechnol. 38, 217–224 (2020).
Yu, Y. et al. Biofuel-powered soft electronic skin with multiplexed and wireless sensing for human-machine interfaces. Sci. Robot. 5, eaaz7946 (2020).
Sempionatto, J. R. et al. An epidermal patch for the simultaneous monitoring of haemodynamic and metabolic biomarkers. Nat. Biomed. Eng. 5, 737–748 (2021).
Emaminejad, S. et al. Autonomous sweat extraction and analysis applied to cystic fibrosis and glucose monitoring using a fully integrated wearable platform. Proc. Natl Acad. Sci. USA 114, 4625–4630 (2017).
Bandodkar, A. J., Jeang, W. J., Ghaffari, R. & Rogers, J. A. Wearable sensors for biochemical sweat analysis. Annual Rev. Anal. Chem. 12, 1–22 (2019).
Xuan, X., Pérez-Ràfols, C., Chen, C., Cuartero, M. & Crespo, G. A. Lactate biosensing for reliable on-body sweat analysis. ACS Sens. 6, 2763–2771 (2021).
Pirovano, P. et al. A wearable sensor for the detection of sodium and potassium in human sweat during exercise. Talanta 219, 121145 (2020).
Garcia-Cordero, E. et al. Three-dimensional integrated ultra-low-volume passive microfluidics with ion-sensitive field-effect transistors for multiparameter wearable sweat analyzers. ACS Nano 12, 12646–12656 (2018).
He, W. et al. Integrated textile sensor patch for real-time and multiplex sweat analysis. Sci. Adv. 5, eaax0649 (2019).
Choi, D.-H., Kitchen, G. B., Jennings, M. T., Cutting, G. R. & Searson, P. C. Out-of-clinic measurement of sweat chloride using a wearable sensor during low-intensity exercise. npj Digit. Med. 3, 49 (2020).
Zhong, B., Jiang, K., Wang, L. & Shen, G. Wearable sweat loss measuring devices: from the role of sweat loss to advanced mechanisms and designs. Adv. Sci. 9, 2103257 (2022).
Lin, H. et al. A programmable epidermal microfluidic valving system for wearable biofluid management and contextual biomarker analysis. Nat. Commun. 11, 4405 (2020).
Saldanha, D. J., Cai, A. & Dorval Courchesne, N.-M. The evolving role of proteins in wearable sweat biosensors. ACS Biomater. Sci. Eng. 9, 2020–2047 (2021).
Mishra, R. K. et al. Continuous opioid monitoring along with nerve agents on a wearable microneedle sensor array. J. Am. Chem. Soc. 142, 5991–5995 (2020).
Wang, B. et al. Wearable aptamer-field-effect transistor sensing system for noninvasive cortisol monitoring. Sci. Adv. 8, eabk0967 (2022).
An, J. E. et al. Wearable cortisol aptasensor for simple and rapid real-time monitoring. ACS Sens. 7, 99–108 (2022).
Torrente-Rodríguez, R. M. et al. Investigation of cortisol dynamics in human sweat using a graphene-based wireless mHealth system. Matter 2, 921–937 (2020).
Jagannath, B. et al. Novel approach to track the lifecycle of inflammation from chemokine expression to inflammatory proteins in sweat using electrochemical biosensor. Adv. Mater. Technol. 7, 2101356 (2022).
Gao, Y. et al. A flexible multiplexed immunosensor for point-of-care in situ wound monitoring. Sci. Adv. 7, eabg9614 (2021).
Barkas, F., Liberopoulos, E., Kei, A. & Elisaf, M. Electrolyte and acid-base disorders in inflammatory bowel disease. Ann. Gastroenterol. 26, 23–28 (2013).
Majewski, S. et al. Skin condition and its relationship to systemic inflammation in chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 12, 2407–2415 (2017).
Yang, X. et al. Graphene uniformly decorated with gold nanodots: in situ synthesis, enhanced dispersibility and applications. J. Mater. Chem. 21, 8096–8103 (2011).
Yu, Y. et al. All-printed soft human-machine interface for robotic physicochemical sensing. Sci. Robot. 7, eabn0495 (2022).
Harshman, S. W. et al. The proteomic and metabolomic characterization of exercise-induced sweat for human performance monitoring: a pilot investigation. PLoS ONE 13, e0203133 (2018).
Pinto-Plata, V. M. et al. C-reactive protein in patients with COPD, control smokers and non-smokers. Thorax 61, 23–28 (2006).
Retamales, I. et al. Amplification of inflammation in emphysema and its association with latent adenoviral infection. Am. J. Respir. Crit. Care Med. 164, 469–473 (2001).
Tonstad, S. & Cowan, J. L. C-reactive protein as a predictor of disease in smokers and former smokers: a review. Int. J. Clin. Pract. 63, 1634–1641 (2009).
Simmonds, S. J., Cuijpers, I., Heymans, S. & Jones, E. A. V. Cellular and molecular differences between HFpEF and HFrEF: a step ahead in an improved pathological understanding. Cells 9, 242 (2020).
Lakhani, I. et al. Diagnostic and prognostic value of serum C-reactive protein in heart failure with preserved ejection fraction: a systematic review and meta-analysis. Heart Fail. Rev. 26, 1141–1150 (2021).
Butler, J. et al. Clinical and economic burden of chronic heart failure and reduced ejection fraction following a worsening heart failure event. Adv. Ther. 37, 4015–4032 (2020).
Albar, Z. et al. Inflammatory markers and risk of heart failure with reduced to preserved ejection fraction. Am. J. Cardiol. 167, 68–75 (2022).
Xin, Y., Zhou, J., Nesser, H. & Lubineau, G. Design strategies for strain‐insensitive wearable healthcare sensors and perspective based on the Seebeck coefficient. Adv. Electron. Mater. 9, 2200534 (2023).
Wang, M. et al. A wearable electrochemical biosensor for the monitoring of metabolites and nutrients. Nat. Biomed. Eng. 6, 1225–1235 (2022).
Bandodkar, A. J. et al. Battery-free, skin-interfaced microfluidic/electronic systems for simultaneous electrochemical, colorimetric, and volumetric analysis of sweat. Sci. Adv. 5, eaav3294 (2019).
Torrente-Rodríguez, R. M. et al. SARS-CoV-2 RapidPlex: a graphene-based multiplexed telemedicine platform for rapid and low-cost COVID-19 diagnosis and monitoring. Matter 3, 1981–1998 (2020).
Zhuang, G., Katakura, Y., Omasa, T., Kishimoto, M. & Suga, K.-I. Measurement of association rate constant of antibody-antigen interaction in solution based on enzyme-linked immunosorbent assay. J. Biosci. Bioeng. 92, 330–336 (2001).
Acknowledgements
This project was supported by the American Heart Association grant 19TPA34850157, National Institutes of Health (NIH) grants R01HL155815 and R21DK13266, National Science Foundation grant 2145802, Office of Naval Research grants N00014-21-1-2483 and N00014-21-1-2845, High Impact Pilot Research Award T31IP1666 from the Tobacco-Related Disease Research Program, Sloan Research Fellowship and the Technology Ventures Internal Project Fund at Cedars-Sinai. J.T. was supported by the National Science Scholarship from the Agency of Science Technology and Research (A*STAR) Singapore. E.D. was supported by NIH grant T32EB027629. We gratefully acknowledge critical support and infrastructure provided for this work by the Kavli Nanoscience Institute at Caltech. We acknowledge support from the Beckman Institute of Caltech to the Molecular Materials Research Center and Jake Evans for help with XPS. The Proteome Exploration Laboratory is supported by the Beckman Institute and NIH grant 1S10OD02001301. We thank G. R. Rossman for assistance in Raman spectroscopy. We also thank E. Bayoumi, E. Pascual and P.-E. Chen at Cedars-Sinai Medical Center for their assistance in participant recruitment. We thank R. M. Torrente-Rodríguez for constructive feedback on manuscript preparation.
Author information
Authors and Affiliations
Contributions
W.G. and J.T. initiated the concept and designed the overall studies. W.G. supervised the work. J.T., J. Min and Y.S. led the experiments and collected the overall data. C.X., J.L., T.-Y.W., E.D. and T.-F.C. contributed to sensor characterization and validation. J. Moore, J.H., E.H., T.P., P.C., J.J.H. and H.B.R. contributed to the design of the human trials and to the system’s evaluation in the participants. All authors contributed to data analysis and provided feedback on the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Nae-Eung Lee and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary notes, figures, tables and references.
Supplementary Video 1
Wearable nanobiosensor for automatic, non-invasive and wireless inflammation monitoring.
Supplementary Video 2
Laboratory flow test illustrating the automatic microfluidic immunosensing.
Supplementary Video 3
On-body flow test showing the delivery and refreshment of black dye in the detection reservoir after 5 min of iontophoretic sweat induction.
Source data
Source Data Figs. 3 and 5
Source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tu, J., Min, J., Song, Y. et al. A wireless patch for the monitoring of C-reactive protein in sweat. Nat. Biomed. Eng 7, 1293–1306 (2023). https://doi.org/10.1038/s41551-023-01059-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-023-01059-5
This article is cited by
-
Interindividual- and blood-correlated sweat phenylalanine multimodal analytical biochips for tracking exercise metabolism
Nature Communications (2024)
-
A physicochemical-sensing electronic skin for stress response monitoring
Nature Electronics (2024)
-
Interstitial fluid-based wearable biosensors for minimally invasive healthcare and biomedical applications
Communications Materials (2024)
-
Miniaturized implantable temperature sensors for the long-term monitoring of chronic intestinal inflammation
Nature Biomedical Engineering (2024)
-
The potential of wearable sweat sensors in heart failure management
Nature Electronics (2024)