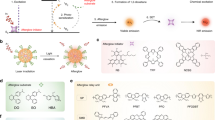
Abstract
Molecular imaging via afterglow luminescence minimizes tissue autofluorescence and increases the signal-to-noise ratio. However, the induction of afterglow requires the prior irradiation of light, which is attenuated by scattering and absorption in tissue. Here we report the development of organic nanoparticles producing ultrasound-induced afterglow, and their proof-of-concept application in cancer immunotheranostics. The ‘sonoafterglow’ nanoparticles comprise a sonosensitizer acting as an initiator to produce singlet oxygen and subsequently activate a substrate for the emission of afterglow luminescence, which is brighter and detectable at larger tissue depths (4 cm) than previously reported light-induced afterglow. We formulated sonoafterglow nanoparticles containing a singlet-oxygen-cleavable prodrug for the immune-response modifier imiquimod that specifically turn on in the presence of the inflammation biomarker peroxynitrite, which is overproduced by tumour-associated M1-like macrophages. Systemic delivery of the nanoparticles allowed for sonoafterglow-guided treatment of mice bearing subcutaneous breast cancer tumours. The high sensitivity and depth of molecular sonoafterglow imaging may offer advantages for the real-time in vivo monitoring of physiopathological processes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. The raw and analyzed datasets generated during the study are too large to be publicly shared, yet they are available for research purposes from the corresponding author on reasonable request. Source data are provided with this paper.
References
Michalet, X. et al. Quantum dots for live cells, in vivo imaging, and diagnostics. Science 307, 538–544 (2005).
Gao, X., Cui, Y., Levenson, R. M., Chung, L. W. & Nie, S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat. Biotechnol. 22, 969–976 (2004).
Hong, G., Antaris, A. L. & Dai, H. Near-infrared fluorophores for biomedical imaging. Nat. Biomed. Eng. 1, 0010 (2017).
Chang, B. et al. A phosphorescent probe for in vivo imaging in the second near-infrared window. Nat. Biomed. Eng. 6, 629–639 (2022).
Miao, Q. et al. Molecular afterglow imaging with bright, biodegradable polymer nanoparticles. Nat. Biotechnol. 35, 1102–1110 (2017).
De Chermont, Q. L. M. et al. Nanoprobes with near-infrared persistent luminescence for in vivo imaging. Proc. Natl Acad. Sci. USA 104, 9266–9271 (2007).
Maldiney, T. et al. The in vivo activation of persistent nanophosphors for optical imaging of vascularization, tumours and grafted cells. Nat. Mater. 13, 418–426 (2014).
Li, Z. et al. Direct aqueous-phase synthesis of sub-10 nm “luminous pearls” with enhanced in vivo renewable near-infrared persistent luminescence. J. Am. Chem. Soc. 137, 5304–5307 (2015).
Jiang, Y. et al. A generic approach towards afterglow luminescent nanoparticles for ultrasensitive in vivo imaging. Nat. Commun. 10, 2064 (2019).
So, M.-K., Xu, C., Loening, A. M., Gambhir, S. S. & Rao, J. Self-illuminating quantum dot conjugates for in vivo imaging. Nat. Biotechnol. 24, 339–343 (2006).
Shaffer, T. M., Pratt, E. C. & Grimm, J. Utilizing the power of Cerenkov light with nanotechnology. Nat. Nanotechnol. 12, 106–117 (2017).
Xu, Y., Liu, H. & Cheng, Z. Harnessing the power of radionuclides for optical imaging: Cerenkov luminescence imaging. J. Nucl. Med. 52, 2009–2018 (2011).
Lu, L. et al. NIR-II bioluminescence for in vivo high contrast imaging and in situ ATP-mediated metastases tracing. Nat. Commun. 11, 4192 (2020).
Xie, C., Zhen, X., Miao, Q., Lyu, Y. & Pu, K. Self-assembled semiconducting polymer nanoparticles for ultrasensitive near-infrared afterglow imaging of metastatic tumours. Adv. Mater. 30, 1801331 (2018).
Wu, L. et al. H2S-activatable near-infrared afterglow luminescent probes for sensitive molecular imaging in vivo. Nat. Commun. 11, 446 (2020).
He, S., Xie, C., Jiang, Y. & Pu, K. An organic afterglow protheranostic nanoassembly. Adv. Mater. 31, 1902672 (2019).
Jiang, Y. & Pu, K. Molecular probes for autofluorescence-free optical imaging. Chem. Rev. 121, 13086–13131 (2021).
Chen, H. et al. LiGa5O8: Cr-based theranostic nanoparticles for imaging-guided X-ray induced photodynamic therapy of deep-seated tumours. Mater. Horiz. 4, 1092–1101 (2017).
Pei, P. et al. X-ray-activated persistent luminescence nanomaterials for NIR-II imaging. Nat. Nanotechnol. 16, 1011–1018 (2021).
Chen, Y. et al. Ultra-high-frequency radio-frequency acoustic molecular imaging with saline nanodroplets in living subjects. Nat. Nanotechnol. 16, 717–724 (2021).
Flannigan, D. J. & Suslick, K. S. Plasma formation and temperature measurement during single-bubble cavitation. Nature 434, 52–55 (2005).
Ostrovskii, I., Korotchenkov, O., Goto, T. & Grimmeiss, H. Sonoluminescence and acoustically driven optical phenomena in solids and solid–gas interfaces. Phys. Rep. 311, 1–46 (1999).
Wu, X. et al. Sono-optogenetics facilitated by a circulation-delivered rechargeable light source for minimally invasive optogenetics. Proc. Natl Acad. Sci. USA 116, 26332–26342 (2019).
Fridman, W. H., Pages, F., Sautes-Fridman, C. & Galon, J. The immune contexture in human tumours: impact on clinical outcome. Nat. Rev. Cancer 12, 298–306 (2012).
Van Elssen, C. H. et al. Noninvasive imaging of human immune responses in a human xenograft model of graft-versus-host disease. J. Nucl. Med. 58, 1003–1008 (2017).
Grivennikov, S. I., Greten, F. R. & Karin, M. Immunity, inflammation, and cancer. Cell 140, 883–899 (2010).
Szabó, C., Ischiropoulos, H. & Radi, R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat. Rev. Drug Discov. 6, 662–680 (2007).
Wynn, T. A., Chawla, A. & Pollard, J. W. Macrophage biology in development, homeostasis and disease. Nature 496, 445–455 (2013).
Huang, J., Jiang, Y., Li, J., Huang, J. & Pu, K. Molecular chemiluminescent probes with a very long near-infrared emission wavelength for in vivo imaging. Angew. Chem. Int. Ed. 60, 3999–4003 (2021).
Su, X. et al. Interferon-γ regulates cellular metabolism and mRNA translation to potentiate macrophage activation. Nat. Immunol. 16, 838–849 (2015).
Rodell, C. B. et al. TLR7/8-agonist-loaded nanoparticles promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy. Nat. Biomed. Eng. 2, 578–588 (2018).
Lester, S. N. & Li, K. Toll-like receptors in antiviral innate immunity. J. Mol. Biol. 426, 1246–1264 (2014).
Friedman, A. A., Letai, A., Fisher, D. E. & Flaherty, K. T. Precision medicine for cancer with next-generation functional diagnostics. Nat. Rev. Cancer 15, 747–756 (2015).
Chen, Q. et al. In situ sprayed bioresponsive immunotherapeutic gel for post-surgical cancer treatment. Nat. Nanotechnol. 14, 89–97 (2019).
Smyth, M. J., Ngiow, S. F., Ribas, A. & Teng, M. W. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat. Rev. Clin. Oncol. 13, 143–158 (2016).
Sindrilaru, A. et al. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J. Clin. Invest. 121, 985–997 (2011).
Klebanoff, C. A., Gattinoni, L. & Restifo, N. P. CD8+ T-cell memory in tumor immunology and immunotherapy. Immunol. Rev. 211, 214–224 (2006).
Van Manen, L. et al. A practical guide for the use of indocyanine green and methylene blue in fluorescence-guided abdominal surgery. J. Surg. Oncol. 118, 283–300 (2018).
Huang, J., Huang, J., Cheng, P., Jiang, Y. & Pu, K. Near-infrared chemiluminescent reporters for in vivo imaging of reactive oxygen and nitrogen species in kidneys. Adv. Funct. Mater. 30, 2003628 (2020).
Hanna, E., Abadi, R. & Abbas, O. Imiquimod in dermatology: an overview. Int J. Dermatol. 55, 831–844 (2016).
Dumortier, G., Grossiord, J. L., Agnely, F. & Chaumeil, J. C. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm. Res. 23, 2709–2728 (2006).
Ouyang, J. et al. Ultrasound mediated therapy: recent progress and challenges in nanoscience. Nano Today 35, 100949 (2020).
Canavese, G. et al. Nanoparticle-assisted ultrasound: a special focus on sonodynamic therapy against cancer. Chem. Eng. J. 340, 155–172 (2018).
Rajamma, D. B., Anandan, S., Yusof, N. S. M., Pollet, B. G. & Ashokkumar, M. Sonochemical dosimetry: a comparative study of Weissler, Fricke and terephthalic acid methods. Ultrason Sonochem. 72, 105413 (2021).
Zhao, P. et al. Aggregation-enhanced sonodynamic activity of phthalocyanine-artesunate conjugates. Angew. Chem. Int. Ed. 61, e202113506 (2022).
Chao, Y. et al. Combined local immunostimulatory radioisotope therapy and systemic immune checkpoint blockade imparts potent antitumour responses. Nat. Biomed. Eng. 2, 611–621 (2018).
van der Meel, R. et al. Smart cancer nanomedicine. Nat. Nanotechnol. 14, 1007–1017 (2019).
Wang, C. et al. In situ activation of platelets with checkpoint inhibitors for post-surgical cancer immunotherapy. Nat. Biomed. Eng. 1, 0011 (2017).
Xu, J. et al. A general strategy towards personalized nanovaccines based on fluoropolymers for post-surgical cancer immunotherapy. Nat. Nanotechnol. 15, 1043–1052 (2020).
Ubil, E. et al. Tumor-secreted Pros1 inhibits macrophage M1 polarization to reduce antitumor immune response. J. Clin. Invest. 128, 2356–2369 (2018).
Michaelis, K. A. et al. The TLR7/8 agonist R848 remodels tumor and host responses to promote survival in pancreatic cancer. Nat. Commun. 10, 4682 (2019).
Schaap, A. P., Chen, T.-S., Handley, R. S., DeSilva, R. & Giri, B. P. Chemical and enzymatic triggering of 1,2-dioxetanes. 2: fluoride-induced chemiluminescence from tert-butyldimethylsilyloxy-substituted dioxetanes. Tetrahedron Lett. 28, 1155–1158 (1987).
Acknowledgements
K.P. acknowledges financial support from the Singapore National Research Foundation (NRF) (NRF-NRFI07-2021-0005), and the Singapore Ministry of Education, Academic Research Fund Tier 1 (2019-T1-002-045, RG125/19, RT05/20) and Academic Research Fund Tier 2 (MOE-T2EP30220-0010).
Author information
Authors and Affiliations
Contributions
K.P. conceived and designed the study. C.X. and Y.J. performed nanoparticle construction. C.X. performed in vivo experiments. J.H. performed the chemical synthesis. C.X., S.H. and C.Z. performed in vitro characterization and cell experiments. K.P. and C.X. analyzed the data and drafted the manuscript. All authors contributed to the writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks the anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary figures, tables, schematics and NMR spectra.
Source data
Source Data for Fig. 1
Source data.
Source Data for Fig. 2
Source data.
Source Data for Fig. 3
Source data.
Source Data for Fig. 4
Source data.
Source Data for Fig. 5
Source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, C., Huang, J., Jiang, Y. et al. Nanoparticles with ultrasound-induced afterglow luminescence for tumour-specific theranostics. Nat. Biomed. Eng 7, 298–312 (2023). https://doi.org/10.1038/s41551-022-00978-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-022-00978-z
This article is cited by
-
Photooxidation triggered ultralong afterglow in carbon nanodots
Nature Communications (2024)
-
In vivo ultrasound-induced luminescence molecular imaging
Nature Photonics (2024)
-
Acidity-activatable upconversion afterglow luminescence cocktail nanoparticles for ultrasensitive in vivo imaging
Nature Communications (2024)
-
Preparation of AIEgen-based near-infrared afterglow luminescence nanoprobes for tumor imaging and image-guided tumor resection
Nature Protocols (2024)
-
Illuminating cancer with sonoafterglow
Nature Photonics (2024)