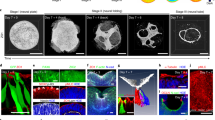
Abstract
Organoids with region-specific architecture could facilitate the repair of injuries of the central nervous system. Here we show that human astrocytes can be directly reprogrammed into early neuroectodermal cells via the overexpression of OCT4, the suppression of p53 and the provision of the small molecules CHIR99021, SB431542, RepSox and Y27632. We also report that the activation of signalling mediated by fibroblast growth factor, sonic hedgehog and bone morphogenetic protein 4 in the reprogrammed cells induces them to form spinal-cord organoids with functional neurons specific to the dorsal and ventral domains. In mice with complete spinal-cord injury, organoids transplanted into the lesion differentiated into spinal-cord neurons, which migrated and formed synapses with host neurons. The direct reprogramming of human astrocytes into neurons may pave the way for in vivo neural organogenesis from endogenous astrocytes for the repair of injuries to the central nervous system.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The data supporting the results in this study are available within the Article and its Supplementary Information. The RNA sequencing data are available from the Gene Expression Omnibus database via the accession number GSE168635. The raw single-cell RNA sequencing data are available from the Genome Sequence Archive database via the accession number https://ngdc.cncb.ac.cn/gsa-human/browse/HRA002541. Source data are provided with this paper.
References
Anderson, M. A. et al. Required growth facilitators propel axon regeneration across complete spinal cord injury. Nature 561, 396–400 (2018).
Courtine, G. & Sofroniew, M. V. Spinal cord repair: advances in biology and technology. Nat. Med. 25, 898–908 (2019).
Sofroniew, M. V. Dissecting spinal cord regeneration. Nature 557, 343–350 (2018).
Li, K. et al. Human iPS cell-derived astrocyte transplants preserve respiratory function after spinal cord injury. Exp. Neurol. 271, 479–492 (2015).
Andersen, J. et al. Generation of functional human 3D cortico-motor assembloids. Cell 183, 1913–1929 (2020).
Lu, P. et al. Prolonged human neural stem cell maturation supports recovery in injured rodent CNS. J. Clin. Invest. 127, 3293–3305 (2017).
Kawai, M. et al. Long-term selective stimulation of transplanted neural stem/progenitor cells for spinal cord injury improves locomotor function. Cell Rep. 37, 110019 (2021).
Fischer, I., Dulin, J. N. & Lane, M. A. Transplanting neural progenitor cells to restore connectivity after spinal cord injury. Nat. Rev. Neurosci. 21, 366–383 (2020).
Kumamaru, H. et al. Generation and post-injury integration of human spinal cord neural stem cells. Nat. Methods 15, 723–731 (2018).
Lu, P. et al. Long-distance axonal growth from human induced pluripotent stem cells after spinal cord injury. Neuron 83, 789–796 (2014).
Nori, S. et al. Grafted human-induced pluripotent stem-cell-derived neurospheres promote motor functional recovery after spinal cord injury in mice. Proc. Natl Acad. Sci. USA 108, 16825–16830 (2011).
Assinck, P., Duncan, G. J., Hilton, B. J., Plemel, J. R. & Tetzlaff, W. Cell transplantation therapy for spinal cord injury. Nat. Neurosci. 20, 637–647 (2017).
Marchini, A. et al. Multifunctionalized hydrogels foster hNSC maturation in 3D cultures and neural regeneration in spinal cord injuries. Proc. Natl Acad. Sci. USA 116, 7483–7492 (2019).
Lai, B. Q. et al. A modular assembly of spinal cord-like tissue allows targeted tissue repair in the transected spinal cord. Adv. Sci. 5, 1800261 (2018).
Ogura, T., Sakaguchi, H., Miyamoto, S. & Takahashi, J. Three-dimensional induction of dorsal, intermediate and ventral spinal cord tissues from human pluripotent stem cells. Development 145, dev162214 (2018).
Koffler, J. et al. Biomimetic 3D-printed scaffolds for spinal cord injury repair. Nat. Med. 25, 263–269 (2019).
Di Lullo, E. & Kriegstein, A. R. The use of brain organoids to investigate neural development and disease. Nat. Rev. Neurosci. 18, 573–584 (2017).
Pasca, S. P. The rise of three-dimensional human brain cultures. Nature 553, 437–445 (2018).
Lancaster, M. A. et al. Cerebral organoids model human brain development and microcephaly. Nature 501, 373–379 (2013).
Park, S. E., Georgescu, A. & Huh, D. Organoids-on-a-chip. Science 364, 960–965 (2019).
Qian, X. Y. et al. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell 165, 1238–1254 (2016).
Cakir, B. et al. Engineering of human brain organoids with a functional vascular-like system. Nat. Methods 16, 1169–1175 (2019).
Pasca, A. M. et al. Human 3D cellular model of hypoxic brain injury of prematurity. Nat. Med. 25, 784–791 (2019).
Trujillo, C. A. et al. Complex oscillatory waves emerging from cortical organoids model early human brain network development. Cell Stem Cell 25, 558–569 (2019).
Li, H. D. & Chen, G. In vivo reprogramming for CNS repair: regenerating neurons from endogenous glial cells. Neuron 91, 728–738 (2016).
Niu, W. Z. et al. In vivo reprogramming of astrocytes to neuroblasts in the adult brain. Nat. Cell Biol. 15, 1164–1194 (2013).
Srivastava, D. & DeWitt, N. In vivo cellular reprogramming: the next generation. Cell 166, 1386–1396 (2016).
Zheng, Y. Y. et al. In vivo reprogramming of reactive astrocytes into functional neurons after cervical spinal cord injury. J. Neurotrauma 35, 180–181 (2018).
Cervo, P. R. D. et al. Induction of functional dopamine neurons from human astrocytes in vitro and mouse astrocytes in a Parkinson’s disease model. Nat. Biotechnol. 35, 444–452 (2017).
Gao, L. F. et al. Direct generation of human neuronal cells from adult astrocytes by small molecules. Stem Cell Rep. 8, 538–547 (2017).
Guo, Z. Y. et al. In vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer’s disease model. Cell Stem Cell 14, 188–202 (2014).
Heinrich, C. et al. Directing astroglia from the cerebral cortex into subtype specific functional neurons. PLoS Biol. 8, e1000373 (2010).
Masserdotti, G. et al. Transcriptional mechanisms of proneural factors and REST in regulating neuronal reprogramming of astrocytes. Cell Stem Cell 17, 74–88 (2015).
Zhang, L. et al. Small molecules efficiently reprogram human astroglial cells into functional neurons. Cell Stem Cell 17, 735–747 (2015).
Heins, N. et al. Glial cells generate neurons: the role of the transcription factor Pax6. Nat. Neurosci. 5, 308–315 (2002).
Qian, H. et al. Reversing a model of Parkinson’s disease with in situ converted nigral neurons. Nature 582, 550–556 (2020).
Zhou, H. et al. Glia-to-neuron conversion by CRISPR-CasRx alleviates symptoms of neurological disease in mice. Cell 181, 590–603 (2020).
Mertens, J., Marchetto, M. C., Bardy, C. & Gage, F. H. Evaluating cell reprogramming, differentiation and conversion technologies in neuroscience. Nat. Rev. Neurosci. 17, 424–437 (2016).
Huangfu, D. W. et al. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat. Biotechnol. 26, 1269–1275 (2008).
Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007).
Banito, A. et al. Senescence impairs successful reprogramming to pluripotent stem cells. Gene Dev. 23, 2134–2139 (2009).
Beausejour, C. M. et al. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J. 22, 4212–4222 (2003).
Hafner, A., Bulyk, M. L., Jambhekar, A. & Lahav, G. The multiple mechanisms that regulate p53 activity and cell fate. Nat. Rev. Mol. Cell Biol. 20, 199–210 (2019).
Lancaster, M. A. & Knoblich, J. A. Generation of cerebral organoids from human pluripotent stem cells. Nat. Protoc. 9, 2329–2340 (2014).
Bayraktar, O. A. et al. Astrocyte layers in the mammalian cerebral cortex revealed by a single-cell in situ transcriptomic map. Nat. Neurosci. 23, 500–509 (2020).
Delile, J. et al. Single cell transcriptomics reveals spatial and temporal dynamics of gene expression in the developing mouse spinal cord. Development 146, dev173807 (2019).
Yin, J. C. et al. Chemical conversion of human fetal astrocytes into neurons through modulation of multiple signaling pathways. Stem Cell Rep. 12, 488–501 (2019).
Gascon, S., Masserdotti, G., Russo, G. L. & Gotz, M. Direct neuronal reprogramming: achievements, hurdles, and new roads to success. Cell Stem Cell 21, 18–34 (2017).
Barkers, R. A., Gotz, M. & Parmar, M. New approaches for brain repair—from rescue to reprogramming. Nature 557, 329–334 (2018).
Yang, H. et al. Sonic hedgehog effectively improves Oct4-mediated reprogramming of astrocytes into neural stem cells. Mol. Ther. 27, 1467–1482 (2019).
Zhu, S. et al. Small molecules enable OCT4-mediated direct reprogramming into expandable human neural stem cells. Cell Res. 24, 126–129 (2014).
Rosenzweig, E. S. et al. Restorative effects of human neural stem cell grafts on the primate spinal cord. Nat. Med. 24, 484–490 (2018).
Acknowledgements
This study was supported by the National Key Research and Development Program of China (grant no. 2021ZD0202500), the National Natural Science Foundation of China (grant no. 32070956 to Z.S., grant nos. 32130044 and 31630029 to Y.S. and grant no. 32000680 to S.D.), the Fundamental Research Funds for Central Universities from Xiamen University (grant no. 20720180040 to Z.S.), the Natural Science Foundation of Shanghai (grant no. 20ZR1405200 to Z.S.), the MOE Frontiers Center for Brain Science Fund and the Starting Fund from Fudan University.
Author information
Authors and Affiliations
Contributions
Z.S., J.X. and S.F. designed the experiments. J.X., S.F., Y.H. and Z.S. performed the astrocyte reprogramming, organoid analysis and transplantation. S.D. and S.F. conducted the electrophysiological studies. H.L. performed the single-cell RNA sequencing analysis. X.L., J.X. and S.F. isolated astrocytes from the patients. Z.S., J.X. and S.F. conducted the RNA sequencing analysis. Z.S., J.X., S.F. and S.D. wrote the manuscript and data interpretation. S.C. and Y.S. reviewed the data interpretation and manuscript content. Z.S. supported this study financially.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Qixin Chen, Woong Sun and Seungkwon You for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Characterization of hAD-Organs.
a, Phase images of Op53-CSBRY induced rosette-like clusters and the expanded red box (left) showing zoom-in images (right) of rosette-like clusters at 14 days. Scale bars, left: 400 μm, right: 100 μm. b, Phase image of hAD-Organs in suspension culture for over 15 weeks. c - d, Representative images of sections from hAD-Organs showing neuroepithelium-like structures at week 7. Showing neural progenitor marker SOX2 and PAX6, and neuronal marker MAP2. Scale bar, 250 μm. e and f, Immunostaining of PAX6+, CTIP2+ and TBR1+ and Reelin+ cells located in different cortical layers in 10-week-old hAD-Organs. Scale bar, 50 μm. g, Representive image of hGFAP::GFP and brightfield in 3-week-old cortical organoid (n = 3). Scale bar, 500 μm. h - k, Showing expression of MAP2, S100ꞵ, SOX2, and FOXG1 in 3-week-old cortical organoid. Scale bars, h and i: 500 μm, j and k: 50 μm. (Representative images from three sections of organoids with similar results (c-f, h-k).
Extended Data Fig. 2 Characterization of human astrocytes.
a, Characterizing human primary astrocytes (HA1800) by immunostaining for GFAP, S100ꞵ, SOX2, PAX6 and MAP2. Scale bar, 100 μm. b, Quantitative analysis of the population of human primary astrocytes at several randomaly chosen fields per coverslip (n = 3 independent experiments). Data are presented as mean ± SEM. c, Neurosphere assay of human primary astrocytes comparing with Op53-CSBRY induced astrocytes. Scale bar, 200 μm. d, Immunostaining of primary human astrocytes with GFAP and MAP2 treated with either ISX-9 (10 μM) (left), or retinoic acid (RA, 100 nM) for 7 days respectively. Scale bars, 50 μm. e, Immunostaining for MAP2 after astrocytes induced by Op53-CSBRY and infected with hGFAP::GFP virus at day 7 (n = 3 independent experiments). Scale bars, 50 μm. f, Immunostaining for MAP2 in Op53-CSBRY induced astrocytes infected with hGFAP::GFP virus at day 26 (n = 3 independent experiments). Scale bar, 25 μm. g, Live cell tracking of the conversion of human astrocytes during Op53-CSBRY reprogramming. Scale bars, 25 μm.
Extended Data Fig. 3 Characterization of human astrocytes by single-cell RNA-sequencing.
a, Graph showing cell percentage of expression of selected pluripotecy (ESC), neuron layer (NL), white matter (WM), upper layer astrocyte, upper-layer-biased pan-astrocyte/gray matter astrocyte and deep layer astrocyte related genes in brain cortical astrocytes. b - f, tSNE plots showing gene expression map of representative pluripotecy marker gene LIN28A, NANOG and POU5F1 (b), neuron gene RBFOX3 and DCX (c), upper layer astrocyte gene ADIPOR2, EGOT and SPRY1 (d), upper-layer-biased pan-astrocyte/gray matter astrocyte gene ITM2B, BSG and IGFBP2 (e), and deep layer astrocyte gene EFHD2, DKK3 and ID3 (f).
Extended Data Fig. 4 Characterization of hADSC-Organs.
a and b, Immunostaining images of dorsal and ventral pMN progenitor markers Nkx6.1 and Olig3 labeled with neuron markers. Scale bar, 25 μm. c, The horizontal plane image of sections from hADSC-Organs (representative images from three sections of spinal-cord organoids with similar results) stained for neural markers TUJ1 and MAP2. Scale bar, 200 μm. d, Image of spinal cord motor neuron (ChAT+/MAP2+) in 10-week-old hADSC-Organs (representative images from three sections of spinal-cord organoids with similar results). Scale bar, 100 μm. e, Quantitative analysis of neuronal subtypes of hADSC-Organs at week 10 stained for neuronal markers ChAT, HB9, GAD67 and VGLUT (n = 3 sections of organoids). f and g, Immunostaining of GFAP+, TUJ1+ and MAP2+ cells in dissociated 10-week-old hADSC-Organs at several randomaly chosen fields per coverslip (n = 3 independent experiments). Scale bar, 50 μm. h, Quantitative the percentage of cells in dissociated 10-week-old hADSC-Organs for MAP2, TUJ1 and GFAP positive cells at several randomaly chosen fields per coverslip (n = 3 independent experiments). i, Representative images of ChAT+ spinal motor neurons co-localized with MAP2+ cells in dissociated 10-week-old hADSC-Organs at several randomaly chosen fields per coverslip (n = 3 independent experiments). Scale bar, 50 μm. j and k, Cluster analysis of differentially expressed genes show that the gene expression profile of hADSC-Organs was significantly different from that of hAD-Organs. The colour bar represents scaled gene expression value (j). Spinal cord specific markers MNX1, ChAT, ISL1, LHX3, Nkx6.1, NKX2.2 and HOX family were significantly high expression in hADSC-Organs. (n = 3 independent experiments). The colour bar represents FPKM value (k). l, KEGG Pathway enrichment assay demonstrating up-regulated genes comparing hADSC-Organs with hAD-Organs. Data are presented as mean ± SEM.
Extended Data Fig. 5 Gene-expression profiles of astrocytes reprogrammed via Op53-CSBRY.
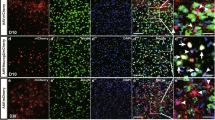
a, Sample distance matrix showing three independent biological replicates treated by CSBRY. b,Volcano plots showing the results of RNA-seq after CSBRY/DMSO control treatment of Op53. Each dot represents a single gene, including upregulated, downregulated, and filtered genes. FDR ≤ 0.05 and log2FC > 1. c, Showing significantly different expression (DE) genes were regulated by CSBRY/DMSO control treatment of Op53. FDR ≤ 0.05 and log2FC > 1. d, CSBRY regulated the cancer genes TP53, KLF4 and MYC (n = 3 independent experiments). Multiple unpaired Student’s t-test was used for comparing each groups. e - f, Gray scale image and quantification of Nestin protein expression in human primary astrocytes, OP53 control group at day 1, 7 and 14, and OP53-CSBRY treat group at day 7 and 14 (n = 3 independent experiments). Data are presented as mean ± SEM. Ordinary one-way ANOVA with Dunnett’s multiple comparison test was used for multiple comparisons. g, Immunostaining of pluripotent markers of NANOG, SSEA4 and TRA-1-60 in human primary astrocyte (top row) and OP53-CSBRY treated cells (bottom row) at several randomaly chosen fields per coverslip (n = 3 independent experiments). Scale bar, 50 μm. Data are presented as mean ± SEM.
Extended Data Fig. 6 Functional characterization of induced neurons from human astrocytes.
a, Example trace showing spontaneous firing in the same cell shown in Fig. 7b. b, Group data comparing the RMP at different culture stages. For group 80-100 days: Without AP, n = 18 vs. With AP, n = 14; For 100-140 days: Without AP, n = 88 vs. With AP, n = 47. Data are presented as mean ± SEM. Two-sample Student’s t-test was used for comparing with AP and without AP in each group. c, Group data comparing the RMP in cells with single AP and repetitive AP. Single AP: n = 65 vs. Repetitive AP: n = 23. Data are presented as mean ± SEM. Two-sample Student’s t-test was used for comparing. d, Group data comparing the peak amplitudes of Na+ currents. Single AP: n = 46 vs. Repetitive APs: n = 23. Data are presented as mean ± SEM. Mann-Whitney test was used for comparing between single and repetitive APs. e, An example current trace showing two spontaneously occurring putative postsynaptic currents (PSC). The second PSC event (arrow) was expanded for clarity (bottom). f, An example synaptic ultrastructure (red arrow) in 10-week-old hAD-Organs (n = 3 independent experiments). Scale bar, 100 nm. g, Left, merged image of IR-DIC and HB9::RFP positive of an example recorded cells isolated from 9-week-old hADSC-Organs (n = 3 independent experiments). Scale bar, 10 μm. Right, membrane potential responses (top) to intracellular injections of step currents (bottom). The blue traces indicate the responses to +50/−10 pA current pulses. h, The percentage of cells without AP (white), with single AP (blue) and repetitive AP (red) (top). The number of recorded cells in each group was provided.
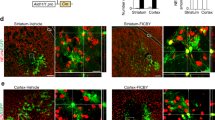
Extended Data Fig. 7 Transplantation of hADSC-Organs into a complete spinal cord injury in NOD-SCID mice.
a, Representative images of 7-week-old hADSC-Organs expressed high GFP after transduction with LV-Ubi-GFP virus infection (n = 25 organoids). Scale bar, 500 μm. b, The procedure of hADSC-Organs transplantation showing the site of the spinal cord amputation. c, The posterior soles of mice with complete spinal cord injury showed hindlimb paralysis with the valgus phenomenon. d, Representative images survival of GFP+ cells in grafted hADSC-Organs in mice (n = 3). Scale bar, 50 μm. e, Representative images survival of GFP+ cells in the grafted field of hADSC-Organs were human nuclei positive of hNUMA (n = 3 independent experiments). Scale bar, 50 μm. f, Open field score of grafted hADSC-Organs mice with 5 different treatments. The number of experiments (n) are summarized in Supplementary Table 1. Each treatment for 10–25 mice and in total 5 groups for 70 mice. Data are presented as mean ± SEM. g and h, Representative images of the spinal cord from g, the Matrigel only group and h, hADSC-Organs +MBG group and an expanded image of the outlined region (white box) where the hADSC-Organs were implanted (n = 3 mice). Scale bars, g: 250 μm, h: left, 250 μm, right, 50 μm.
Supplementary information
Supplementary Information
Supplementary Figs. 1–4, Tables 1 and 2 and captions for Supplementary Videos 1–3.
Supplementary Video 1
Live imaging of the conversion of human astrocytes into neurons.
Supplementary Video 2
Vehicle control in mice with complete SCI.
Supplementary Video 3
Grafted hADSC-Organs in mice with complete SCI.
Supplementary Table 1
BBB score of mice transplanted with hADSC-Organs.
Source data
Source Data Fig. 5e
Unprocessed western blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, J., Fang, S., Deng, S. et al. Generation of neural organoids for spinal-cord regeneration via the direct reprogramming of human astrocytes. Nat. Biomed. Eng 7, 253–269 (2023). https://doi.org/10.1038/s41551-022-00963-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-022-00963-6
This article is cited by
-
Tissue engineering RPE sheet derived from hiPSC-RPE cell spheroids supplemented with Y-27632 and RepSox
Journal of Biological Engineering (2024)