Abstract
The development of gene therapies for the treatment of diseases of the central nervous system has been hindered by the limited availability of adeno-associated viruses (AAVs) that efficiently traverse the blood–brain barrier (BBB). Here, we report the rational design of AAV9 variants displaying cell-penetrating peptides on the viral capsid and the identification of two variants, AAV.CPP.16 and AAV.CPP.21, with improved transduction efficiencies of cells of the central nervous system on systemic delivery (6- to 249-fold across 4 mouse strains and 5-fold in cynomolgus macaques, with respect to the AAV9 parent vector). We also show that the neurotropism of AAV.CPP.16 is retained in young and adult macaques, that this variant displays enhanced transcytosis at the BBB as well as increased efficiency of cellular transduction relative to AAV9, and that it can be used to deliver antitumour payloads in a mouse model of glioblastoma. AAV capsids that can efficiently penetrate the BBB will facilitate the clinical translation of gene therapies aimed at the central nervous system.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. All raw and analysed datasets generated during the study are too large to be publicly shared, yet they are available for research purposes from the corresponding author on reasonable request. Source data are provided with this paper.
References
Hudry, E. & Vandenberghe, L. H. Therapeutic AAV gene transfer to the nervous system: a clinical reality. Neuron 101, 839–862 (2019).
Deverman, B. E., Ravina, B. M., Bankiewicz, K. S., Paul, S. M. & Sah, D. W. Y. Gene therapy for neurological disorders: progress and prospects. Nat. Rev. Drug. Discov. 17, 641–659 (2018).
Gray, S. J. The evolution of adeno-associated virus capsids for CNS gene therapy. Cell Gene Ther. Insights 5, 1361–1368 (2019).
Murlidharan, G., Samulski, R. J. & Asokan, A. Biology of adeno-associated viral vectors in the central nervous system. Front. Mol. Neurosci. 7, 76 (2014).
Pardridge, W. M. The blood–brain barrier: bottleneck in brain drug development. NeuroRx 2, 3–14 (2005).
Foust, K. D. et al. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat. Biotechnol. 27, 59–65 (2009).
Gao, G. et al. Clades of adeno-associated viruses are widely disseminated in human tissues. J. Virol. 78, 6381–6388 (2004).
Gray, S. J. et al. Preclinical differences of intravascular AAV9 delivery to neurons and glia: a comparative study of adult mice and nonhuman primates. Mol. Ther. 19, 1058–1069 (2011).
Zhang, H. et al. Several rAAV vectors efficiently cross the blood–brain barrier and transduce neurons and astrocytes in the neonatal mouse central nervous system. Mol. Ther. 19, 1440–1448 (2011).
Ellsworth, J. L. et al. Clade F AAVHSCs cross the blood brain barrier and transduce the central nervous system in addition to peripheral tissues following intravenous administration in nonhuman primates. PLoS ONE 14, e0225582 (2019).
Mendell, J. R. et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N. Engl. J. Med. 377, 1713–1722 (2017).
Choudhury, S. R. et al. Widespread central nervous system gene transfer and silencing after systemic delivery of novel AAV-AS vector. Mol. Ther. 24, 726–735 (2016).
Chan, K. Y. et al. Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat. Neurosci. 20, 1172–1179 (2017).
Deverman, B. E. et al. Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nat. Biotechnol. 34, 204–209 (2016).
Hanlon, K. S. et al. Selection of an efficient AAV vector for robust CNS transgene expression. Mol. Ther. Methods Clin. Dev. 15, 320–332 (2019).
Hudry, E. et al. Efficient gene transfer to the central nervous system by single-stranded Anc80L65. Mol. Ther. Methods Clin. Dev. 10, 197–209 (2018).
Nonnenmacher, M. et al. Rapid evolution of blood–brain-barrier-penetrating AAV capsids by RNA-driven biopanning. Mol. Ther. Methods Clin. Dev. 20, 366–378 (2021).
Deo, A. K., Theil, F. P. & Nicolas, J. M. Confounding parameters in preclinical assessment of blood–brain barrier permeation: an overview with emphasis on species differences and effect of disease states. Mol. Pharm. 10, 1581–1595 (2013).
Song, H. W. et al. Transcriptomic comparison of human and mouse brain microvessels. Sci. Rep. 10, 12358 (2020).
O’Brown, N. M., Pfau, S. J. & Gu, C. Bridging barriers: a comparative look at the blood–brain barrier across organisms. Genes Dev. 32, 466–478 (2018).
Zhang, Y. et al. Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences with mouse. Neuron 89, 37–53 (2016).
Hordeaux, J. et al. The neurotropic properties of AAV-PHP.B are limited to C57BL/6J mice. Mol. Ther. 26, 664–668 (2018).
Hordeaux, J. et al. The GPI-linked protein LY6A drives AAV-PHP.B transport across the blood–brain barrier. Mol. Ther. 27, 912–921 (2019).
Matsuzaki, Y. et al. Intravenous administration of the adeno-associated virus-PHP.B capsid fails to upregulate transduction efficiency in the marmoset brain. Neurosci. Lett. 665, 182–188 (2018).
Huang, Q. et al. Delivering genes across the blood–brain barrier: LY6A, a novel cellular receptor for AAV-PHP.B capsids. PLoS ONE 14, e0225206 (2019).
Batista, A. R. et al. Ly6a differential expression in blood–brain barrier is responsible for strain specific central nervous system transduction profile of AAV-PHP.B. Hum. Gene Ther. 31, 90–102 (2020).
Goertsen, D. et al. AAV capsid variants with brain-wide transgene expression and decreased liver targeting after intravenous delivery in mouse and marmoset. Nat. Neurosci. 25, 106–115 (2021).
Kardani, K., Milani, A., S, H. S. & Bolhassani, A. Cell penetrating peptides: the potent multi-cargo intracellular carriers. Expert Opin. Drug Deliv. 16, 1227–1258 (2019).
Böhmová, E. et al. Cell-penetrating peptides: a useful tool for the delivery of various cargoes into cells. Physiol. Res. 67, s267–s279 (2018).
Oller-Salvia, B., Sánchez-Navarro, M., Giralt, E. & Teixidó, M. Blood–brain barrier shuttle peptides: an emerging paradigm for brain delivery. Chem. Soc. Rev. 45, 4690–4707 (2016).
Stalmans, S. et al. Cell-penetrating peptides selectively cross the blood–brain barrier in vivo. PLoS ONE 10, e0139652 (2015).
Cho, C. F. et al. Blood–brain-barrier spheroids as an in vitro screening platform for brain-penetrating agents. Nat. Commun. 8, 15623 (2017).
Meng, Y. et al. Cell-penetrating peptides enhance the transduction of adeno-associated virus serotype 9 in the central nervous system. Mol. Ther. Methods Clin. Dev. 21, 28–41 (2021).
Zhang, X., He, T., Chai, Z., Samulski, R. J. & Li, C. Blood–brain barrier shuttle peptides enhance AAV transduction in the brain after systemic administration. Biomaterials 176, 71–83 (2018).
Wang, D., Tai, P. W. L. & Gao, G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 18, 358–378 (2019).
Büning, H. & Srivastava, A. Capsid modifications for targeting and improving the efficacy of AAV vectors. Mol. Ther. Methods Clin. Dev. 12, 248–265 (2019).
Lee, E. J., Guenther, C. M. & Suh, J. Adeno-associated virus (AAV) vectors: rational design strategies for capsid engineering. Curr. Opin. Biomed. Eng. 7, 58–63 (2018).
Muller, O. J. et al. Random peptide libraries displayed on adeno-associated virus to select for targeted gene therapy vectors. Nat. Biotechnol. 21, 1040–1046 (2003).
Münch, R. C. et al. Off-target-free gene delivery by affinity-purified receptor-targeted viral vectors. Nat. Commun. 6, 6246 (2015).
Girod, A. et al. Genetic capsid modifications allow efficient re-targeting of adeno-associated virus type 2. Nat. Med. 5, 1052–1056 (1999).
Yoshida, T. et al. Bax-inhibiting peptide derived from mouse and rat Ku70. Biochem. Biophys. Res. Commun. 321, 961–966 (2004).
Gomez, J. A. et al. Cell-penetrating penta-peptides (CPP5s): measurement of cell entry and protein-transduction activity. Pharmaceuticals 3, 3594–3613 (2010).
Samaranch, L. et al. AAV9-mediated expression of a non-self protein in nonhuman primate central nervous system triggers widespread neuroinflammation driven by antigen-presenting cell transduction. Mol. Ther. 22, 329–337 (2014).
Hordeaux, J. et al. Adeno-associated virus-induced dorsal root ganglion pathology. Hum. Gene Ther. 31, 808–818 (2020).
Kotchey, N. M. et al. A potential role of distinctively delayed blood clearance of recombinant adeno-associated virus serotype 9 in robust cardiac transduction. Mol. Ther. 19, 1079–1089 (2011).
Di Pasquale, G. et al. Bovine AAV transcytosis inhibition by tannic acid results in functional expression of CFTR in vitro and altered biodistribution in vivo. Gene Ther. 19, 576–581 (2012).
Tugizov, S. M., Herrera, R. & Palefsky, J. M. Epstein–Barr virus transcytosis through polarized oral epithelial cells. J. Virol. 87, 8179–8194 (2013).
Wen, P. Y. & Kesari, S. Malignant gliomas in adults. N. Engl. J. Med. 359, 492–507 (2008).
Lim, M., Xia, Y., Bettegowda, C. & Weller, M. Current state of immunotherapy for glioblastoma. Nat. Rev. Clin. Oncol. 15, 422–442 (2018).
Sarkaria, J. N. et al. Is the blood–brain barrier really disrupted in all glioblastomas? A critical assessment of existing clinical data. Neuro. Oncol. 20, 184–191 (2018).
Freeman, G. J. et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 192, 1027–1034 (2000).
Okazaki, T. & Honjo, T. PD-1 and PD-1 ligands: from discovery to clinical application. Int. Immunol. 19, 813–824 (2007).
Nduom, E. K. et al. PD-L1 expression and prognostic impact in glioblastoma. Neuro. Oncol. 18, 195–205 (2016).
Cloughesy, T. F. et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat. Med. 25, 477–486 (2019).
Engeland, C. E. et al. CTLA-4 and PD-L1 checkpoint blockade enhances oncolytic measles virus therapy. Mol. Ther. 22, 1949–1959 (2014).
Jun, H. J. et al. Acquired MET expression confers resistance to EGFR inhibition in a mouse model of glioblastoma multiforme. Oncogene 31, 3039–3050 (2012).
Karjoo, Z., Chen, X. & Hatefi, A. Progress and problems with the use of suicide genes for targeted cancer therapy. Adv. Drug Deliv. Rev. 99, 113–128 (2016).
Sawada, M., Hayes, P. & Matsuyama, S. Cytoprotective membrane-permeable peptides designed from the Bax-binding domain of Ku70. Nat. Cell Biol. 5, 352–357 (2003).
Fell, V. L. & Schild-Poulter, C. The Ku heterodimer: function in DNA repair and beyond. Mutat. Res. Rev. Mutat. Res. 763, 15–29 (2015).
Merkel, S. F. et al. Trafficking of adeno-associated virus vectors across a model of the blood–brain barrier; a comparative study of transcytosis and transduction using primary human brain endothelial cells. J. Neurochem. 140, 216–230 (2017).
Simonato, M. et al. Progress in gene therapy for neurological disorders. Nat. Rev. Neurol. 9, 277–291 (2013).
Hocquemiller, M., Giersch, L., Audrain, M., Parker, S. & Cartier, N. Adeno-associated virus-based gene therapy for CNS diseases. Hum. Gene Ther. 27, 478–496 (2016).
Parker Kerrigan, B. C., Shimizu, Y., Andreeff, M. & Lang, F. F. Mesenchymal stromal cells for the delivery of oncolytic viruses in gliomas. Cytotherapy 19, 445–457 (2017).
Jain, R. K. et al. Angiogenesis in brain tumours. Nat. Rev. Neurosci. 8, 610–622 (2007).
Bhaskaran, V., Yao, Y., Bei, F. & Peruzzi, P. Engineering, delivery, and biological validation of artificial microRNA clusters for gene therapy applications. Nat. Protoc. 14, 3538–3553 (2019).
Bhere, D. et al. Simultaneous downregulation of miR-21 and upregulation of miR-7 has anti-tumor efficacy. Sci. Rep. 10, 1779 (2020).
Reardon, D. A. et al. Glioblastoma eradication following immune checkpoint blockade in an orthotopic, immunocompetent model. Cancer Immunol. Res. 4, 124–135 (2016).
Reardon, D. A. et al. Phase II study to evaluate safety and efficacy of MEDI4736 (durvalumab) + radiotherapy in patients with newly diagnosed unmethylated MGMT glioblastoma (new unmeth GBM). J. Clin. Oncol. 37, 2032–2032 (2019).
Ene, C. I. et al. Anti-PD-L1 antibody direct activation of macrophages contributes to a radiation-induced abscopal response in glioblastoma. Neuro. Oncol. 22, 639–651 (2020).
Hambardzumyan, D., Gutmann, D. H. & Kettenmann, H. The role of microglia and macrophages in glioma maintenance and progression. Nat. Neurosci. 19, 20–27 (2016).
D’Costa, S. et al. Practical utilization of recombinant AAV vector reference standards: focus on vector genomes titration by free ITR qPCR. Mol. Ther. Methods Clin. Dev. 5, 16019 (2016).
Meliani, A. et al. Determination of anti-adeno-associated virus vector neutralizing antibody titer with an in vitro reporter system. Hum. Gene Ther. Methods 26, 45–53 (2015).
Acknowledgements
We thank K. J. Smith and H. Hirai for providing feedback on the manuscript, G. Freeman for providing the PD-L1 antibody, A. Charest and R. Piranlioglu for providing the GBM1694 cells, H. Jin for providing AAV reagents and D. Feng, Y. Ni and B. J. Scott for technical assistance. This study was supported by a Brigham and Women’s Hospital sundry fund (to F.B.).
Author information
Authors and Affiliations
Contributions
F.B. conceived and supervised the study, with input from E.A.C.; Y.Y., J.Wang, Y.L. and Y.Q. performed experiments and collected data, with inputs from K.W., Y.Z., Y.C., Z.Y., J.Wan and J.L.; H.N., S.E.L. and C.-F.C. contributed to methodology; F.B., Y.Y., J.W., Y.L. and Y.Q. analysed data. F.B. and Y.Y. wrote the manuscript with input from all the authors.
Corresponding author
Ethics declarations
Competing interests
F.B. and E.A.C. receive royalties from patents generated by this study (Patent applications: PCT/US19/41386, PCT/US21/12746 and PCT/US22/73051). F.B. is a co-founder and scientific advisor of Brave Bio Inc., an AAV gene therapy start-up. The other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
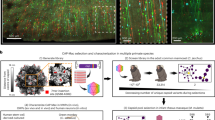
Extended Data Fig. 1 Initial round of in vivo selection revealing potential candidates for further optimization.
a, Fluorescence images of sagittal brain sections in mice 21 days after intravenous injection of 1 × 1010 vg of single-stranded AAV-EF1α-H2B-RFP. White dots indicate RFP-positive cells. Scale bars: 1000 μm for whole brain section and 30 μm for insets. b, Fluorescence images of sagittal brain sections in mice 21 days after intravenous injection of 1 × 1011 vg of selected AAVs-EF1α-H2B-RFP. Scale bars: 1,000 μm for whole brain section and 300 μm for insets. c, Percentage of transduced brain cells and relative fold change of AAV transduction as quantified by counting transduced RFP-positive cells using ImageJ. n = 3 mice per group, mean ± s.e.m., one-way ANOVA with Tukey’s post-test.
Extended Data Fig. 2 Sequence optimization of Bip inserts yields additional AAV variants with further enhanced brain tropism.
a, Sequences of standard 5-mer Bips, as well as derivatives during optimization. b, Fluorescence images of sagittal brain sections in mice 21 days after intravenous injection of 1 × 1011 vg of AAVs-EF1α-H2B-RFP. Scale bars: 1000 μm for whole brain section and 300 μm for insets. c, Percentage of transduced brain cells and relative fold change of AAV transduction in the brain as quantified by counting RFP-positive cells using ImageJ. d, Percentage of transduced liver cells by AAVs relative to DAPI counter-staining. White dots indicate RFP-positive cells. n = 3 mice per group, mean ± s.e.m., one-way ANOVA with Tukey’s post-test.
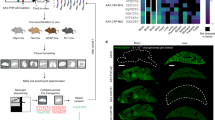
Extended Data Fig. 3 Brain transductions by AAV.CPP.16 and AAV.CPP.21 in four common mouse strains.
a-l, Representative fluorescence images of brain regions in C57BL/6J, BALB/cJ, FVB/NJ and 129S1/SvlmJ mice 21 days after intravenous injection of 1 × 1011 vg of AAVs-EF1α-H2B-RFP (AAV9, a-d; AAV.CPP.16, e-h; AAV.CPP.21, i-l). White dots indicate RFP-positive cells. Scale bars: 500 μm. m, quantification of transduced brain cells in C57BL/6J at a high dose of AAVs (1 × 1012 vg, IV). n = 3 mice per group, mean ± s.e.m., one-way ANOVA with Tukey’s post-test.
Extended Data Fig. 4 Viral genome distribution of AAV.CPP.16 vs. AAV9 in BALB/cJ.
5 × 1011 vg AAVs-CAG-GFP-WPRE were intravenously injected to BALB/cJ mice and AAV genomic DNA was isolated 21 days later. AAV viral genomes in selected CNS regions (a) and peripheral organs (b) were measured by qPCR using primers targeting WPRE and normalized to mouse genome (targeting glucagon). n = 4 mice per group, mean ± s.e.m., Student’s t-test.
Extended Data Fig. 5 Transduction of oligodendrocytes, motor neurons and peripheral tissues by AAV.CPP.16 and AAV9 in BALB/cJ.
a-e, Transduced oligodendrocytes (Olig2+) in the brain and quantification on percentage. Scale bar for a, b: 25 μm. Scale bar for c, d: 10 μm. n = 3 mice per group, mean ± s.e.m. f, Immunostaining of spinal cord sections. Motor neurons were visualized using ChAT antibody. Scale bars: 250 μm for the left two and 50 μm for the right two. g, Transduction of peripheral tissues and quantification on relative fold change. BALB/cJ mice were examined 3 weeks after intravenous injection of 1× 1012 vg AAV.CPP.16-CAG-H2B-GFP or AAV9-CAG-H2B-GFP. Mean ± s.e.m., n = 4-8, Student’s t-test.
Extended Data Fig. 6 Imaging, western blot and histological analysis for AAV.CPP.16-aPD-L1-treated GBM-bearing mice.
a, Timeline for procedures in a mouse glioblastoma (GBM) model. b, Example of bioluminescence imaging for verification of tumor formation at day 7 post tumor implantation. c, aPD-L1-HA expression in the liver and muscle as measured by western blot. d-f, Representative images of hematoxylin-eosin-stained brain sections in a long-term survivor mouse treated with AAV.CPP.16-aPD-L1. Little residual tumor tissue was detectable suggesting eradication of GBM. g, Immunostaining showing AAV.CPP.16-mediated expression of aPD-L1-HA and astrogliosis in one long-term-surviving animal. Scale bar in g: 25 μm. n = 3-5 mice per group, mean ± s.e.m., one-way ANOVA with Tukey’s post-test.
Extended Data Fig. 7 Flow cytometry analysis of tumor-infiltrating lymphocytes (TILs).
a, Gating strategies for all panels. Lymphocyte populations were selected based on SSC-H and FSC-H parameters, followed by FSC-H and FSC-A gating to eliminate doublets. CD45 marks for all immune cells. b, Representative flow cytometry plots and quantification of T cells (CD3+/CD45+) and their subtypes (CD4+/CD3+ T cells, CD8+/CD3+ T cells, IFNg+/CD3+ T cells, Granzyme B+/CD8+ T cells, CD25+/Foxp3 + /CD4+ Tregs). n = 3-11 mice per group, mean ± s.e.m., one-way ANOVA with Tukey’s post-test.
Extended Data Fig. 8 Pro-survival effect by monoclonal antibodies against PD-L1 in GL261 mouse GBM model.
a, Experimental timeline for studying the effect of PD-L1 mAB in the GL261 GBM model. A large dose of PD-L1 mAB (339.6A2, mouse IgG1, κ; 2.25 mg in total) was administered. b, Kaplan–Meier survival curves showing survival outcome of PD-L1 mAB or control in the GL261 GBM model. n = 6 each group, Log-rank test used to define statistical significance.
Extended Data Fig. 9 Pro-survival effect of systematic therapy of AAV.CPP.16-aPD-L1 in a genetic GBM tumor model.
a, Experimental timeline. GBM1694 tumor cells with human wild-type EGFR over-expression and Ink4a/Arf-PTEN co-deletion were implanted into BALB/c mice to model GBM. 1 × 1012 vg of AAV.CPP.16-CAG-aPD-L1-HA were administered at day 5 post tumor implantation. b, Kaplan–Meier survival curves showing survival times of AAV.CPP.16-aPD-L1 or PBS control. n = 6 each group, Log-rank test used to define statistical significance.
Supplementary information
Supplementary Information
Supplementary figures and list of supplementary tables.
Supplementary Tables
Supplementary Tables 1–4.
Supplementary Tables
Source data for the supplementary figures.
Source data
Source Data Fig. 5
Unprocessed western blots.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yao, Y., Wang, J., Liu, Y. et al. Variants of the adeno-associated virus serotype 9 with enhanced penetration of the blood–brain barrier in rodents and primates. Nat. Biomed. Eng 6, 1257–1271 (2022). https://doi.org/10.1038/s41551-022-00938-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-022-00938-7
This article is cited by
-
Adeno-associated virus as a delivery vector for gene therapy of human diseases
Signal Transduction and Targeted Therapy (2024)
-
The blood–brain barrier: structure, regulation, and drug delivery
Signal Transduction and Targeted Therapy (2023)
-
AAV-based in vivo gene therapy for neurological disorders
Nature Reviews Drug Discovery (2023)
-
Therapeutic strategies for autism: targeting three levels of the central dogma of molecular biology
Translational Psychiatry (2023)
-
Genetic Approaches for Neural Circuits Dissection in Non-human Primates
Neuroscience Bulletin (2023)