Abstract
Preclinical models of aortic stenosis can induce left ventricular pressure overload and coarsely control the severity of aortic constriction. However, they do not recapitulate the haemodynamics and flow patterns associated with the disease. Here we report the development of a customizable soft robotic aortic sleeve that can mimic the haemodynamics and biomechanics of aortic stenosis. By allowing for the adjustment of actuation patterns and blood-flow dynamics, the robotic sleeve recapitulates clinically relevant haemodynamics in a porcine model of aortic stenosis, as we show via in vivo echocardiography and catheterization studies, and a combination of in vitro and computational analyses. Using in vivo and in vitro magnetic resonance imaging, we also quantified the four-dimensional blood-flow velocity profiles associated with the disease and with bicommissural and unicommissural defects re-created by the robotic sleeve. The design of the sleeve, which can be adjusted on the basis of computed tomography data, allows for the design of patient-specific devices that may guide clinical decisions and improve the management and treatment of patients with aortic stenosis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the article and its Supplementary Information. The raw and analysed data generated during the study are available from the corresponding authors on reasonable request. Source data are provided with this paper.
References
Maglio, S., Park, C., Tognarelli, S., Menciassi, A. & Roche, E. T. High-fidelity physical organ simulators: from artificial to bio-hybrid solutions. IEEE Trans. Med. Robot. Bionics 3, 349–361 (2021).
Park, C. et al. An organosynthetic dynamic heart model with enhanced biomimicry guided by cardiac diffusion tensor imaging. Sci. Robot. 5, eaay9106 (2020).
Bhattacharya, D., Ali, S. J. V., Cheng, L. K. & Xu, W. RoSE: a robotic soft esophagus for endoprosthetic stent testing. Soft Robot. 8, 397–415 (2021).
Dang, Y. et al. SoGut: a soft robotic gastric simulator. Soft Robot. 8, 273–283 (2021).
Ranunkel, O., Güder, F. & Arora, H. Soft robotic surrogate lung. ACS Appl. Bio Mater. 2, 1490–1497 (2019).
Horvath, M. A. et al. An organosynthetic soft robotic respiratory simulator. APL Bioeng. 4, 026108 (2020).
Lu, X., Xu, W. & Li, X. A soft robotic tongue—mechatronic design and surface reconstruction. IEEE ASME Trans. Mechatron. 22, 2102–2110 (2017).
Horvath, M. A. et al. Design and fabrication of a biomimetic circulatory simulator with overlaid flow and respiration mechanism for single ventricle physiology. In 2020 8th IEEE RAS/EMBS International Conference for Biomedical Robotics and Biomechatronics (BioRob) 74–79 (IEEE, 2020).
Nkomo, V. T. et al. Burden of valvular heart diseases: a population-based study. Lancet 368, 1005–1011 (2006).
Mrsic, Z., Hopkins, S. P., Antevil, J. L. & Mullenix, P. S. Valvular heart disease. Prim. Care Clin. Pract. 45, 81–94 (2018).
Carabello, B. A. & Paulus, W. J. Aortic stenosis. Lancet 373, 956–966 (2009).
Bonow, R. O. & Greenland, P. Population-wide trends in aortic stenosis incidence and outcomes. Circulation 131, 969–971 (2015).
Coffey, S. et al. Global epidemiology of valvular heart disease. Nat. Rev. Cardiol. 18, 853–864 (2021).
Grossman, W., Jones, D. & McLaurin, L. P. Wall stress and patterns of hypertrophy in the human left ventricle. J. Clin. Invest. 56, 56–64 (1975).
Borlaug, B. A. & Paulus, W. J. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur. Heart J. 32, 670–679 (2011).
Borlaug, B. A. Evaluation and management of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 17, 559–573 (2020).
Taniguchi, T. et al. Sudden death in patients with severe aortic stenosis: observations from the CURRENT AS registry. J. Am. Heart Assoc. 7, e008397 (2018).
Otto, C. M. et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 143, e72–e227 (2021).
Brennan, J. M. et al. Long-term survival after aortic valve replacement among high-risk elderly patients in the United States. Circulation 126, 1621–1629 (2012).
Jones, J. M. et al. Repeat heart valve surgery: risk factors for operative mortality. J. Thorac. Cardiovasc. Surg. 122, 913–918 (2001).
Yarbrough, W. M. et al. Progressive induction of left ventricular pressure overload in a large animal model elicits myocardial remodeling and a unique matrix signature. J. Thorac. Cardiovasc. Surg. 143, 215–223 (2012).
Olver, T. D. et al. Western diet-fed, aortic-banded ossabaw swine. JACC Basic Transl. Sci. 4, 404–421 (2019).
Torres, W. M. et al. Changes in myocardial microstructure and mechanics with progressive left ventricular pressure overload. JACC Basic Transl. Sci. 5, 463–480 (2020).
Singh, G. K. Congenital aortic valve stenosis. Children 6, 69 (2019).
Roberts, W. C. & Ko, J. M. Frequency by decades of unicuspid, bicuspid, and tricuspid aortic valves in adults having isolated aortic valve replacement for aortic stenosis, with or without associated aortic regurgitation. Circulation 111, 920–925 (2005).
OʼBrien, K. D. Epidemiology and genetics of calcific aortic valve disease. J. Investig. Med. 55, 284–291 (2007).
Brantley, H. P., Nekkanti, R., Anderson, C. A. & Kypson, A. P. Three-dimensional echocardiographic features of unicuspid aortic valve stenosis correlate with surgical findings. Echocardiography 29, E204–E207 (2012).
Moller, J. H., Nakib, A., Eliot, R. S. & Edwards, J. E. Symptomatic congenital aortic stenosis in the first year of life. J. Pediatr. 69, 728–734 (1966).
Singh, S. et al. Unicuspid unicommissural aortic valve: an extremely rare congenital anomaly. Tex. Heart Inst. J. 42, 273–276 (2015).
Baillargeon, B., Rebelo, N., Fox, D. D., Taylor, R. L. & Kuhl, E. The Living Heart Project: a robust and integrative simulator for human heart function. Eur. J. Mech. A 48, 38–47 (2014).
Saikrishnan, N., Kumar, G., Sawaya, F. J., Lerakis, S. & Yoganathan, A. P. Accurate assessment of aortic stenosis. Circulation 129, 244–253 (2014).
Rajani, R., Hancock, J. & Chambers, J. B. The art of assessing aortic stenosis. Heart 98, iv14–iv22 (2012).
Borlaug, B. A. The pathophysiology of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 11, 507–515 (2014).
Rosalia, L., Ozturk, C., Van Story, D., Horvath, M. A. & Roche, E. T. Object-oriented lumped-parameter modeling of the cardiovascular system for physiological and pathophysiological conditions. Adv. Theory Simul. 4, 2000216 (2021).
Rosalia, L., Ozturk, C. & Roche, E. T. Lumped-parameter and finite element modeling of heart failure with preserved ejection fraction. J. Vis. Exp. 2021, e62167 (2021).
Baumgartner, H. et al. Recommendations on the echocardiographic assessment of aortic valve stenosis: a focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur. Heart J. Cardiovasc. Imaging 18, 254–275 (2017).
Bahlmann, E. et al. Prognostic value of energy loss index in asymptomatic aortic stenosis. Circulation 127, 1149–1156 (2013).
Hachicha, Z., Dumesnil, J. G. & Pibarot, P. Usefulness of the valvuloarterial impedance to predict adverse outcome in asymptomatic aortic stenosis. J. Am. Coll. Cardiol. 54, 1003–1011 (2009).
Dyverfeldt, P., Hope, M. D., Tseng, E. E. & Saloner, D. Magnetic resonance measurement of turbulent kinetic energy for the estimation of irreversible pressure loss in aortic stenosis. JACC Cardiovasc. Imaging 6, 64–71 (2013).
Binter, C. et al. Turbulent kinetic energy assessed by multipoint 4-dimensional flow magnetic resonance imaging provides additional information relative to echocardiography for the determination of aortic stenosis severity. Circ. Cardiovasc. Imaging 10, e005486 (2017).
Dice, L. R. Measures of the amount of ecologic association between species. Ecology 26, 297–302 (1945).
Iung, B. et al. A prospective survey of patients with valvular heart disease in Europe: the Euro Heart Survey on Valvular Heart Disease. Eur. Heart J. 24, 1231–1243 (2003).
Mishra, S. & Kass, D. A. Cellular and molecular pathobiology of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 18, 400–423 (2021).
Rosalia, L. et al. Device-based solutions to improve cardiac physiology and hemodynamics in heart failure with preserved ejection fraction. JACC Basic Transl. Sci. 6, 772–795 (2021).
Rosalia, L., Saeed, Y. M. & Roche, E. T. in Advances in Cardiovascular Technology (eds Karimov, J. H. et al.) 625–640 (Elsevier, 2022). https://doi.org/10.1016/B978-0-12-816861-5.00015-0
Pibarot, P. et al. Moderate aortic stenosis and heart failure with reduced ejection fraction. JACC Cardiovasc. Imaging 12, 172–184 (2019).
Azevedo, C. F. et al. Prognostic significance of myocardial fibrosis quantification by histopathology and magnetic resonance imaging in patients with severe aortic valve disease. J. Am. Coll. Cardiol. 56, 278–287 (2010).
Goldsmith, E. C., Bradshaw, A. D. & Spinale, F. G. Cellular mechanisms of tissue fibrosis. 2. Contributory pathways leading to myocardial fibrosis: moving beyond collagen expression. Am. J. Physiol. Physiol. 304, C393–C402 (2013).
Zhu, Y. et al. Novel bicuspid aortic valve model with aortic regurgitation for hemodynamic status analysis using an ex vivo simulator. J. Thorac. Cardiovasc. Surg. 163, e161–e171 (2022).
Gao, F., Guo, Z., Sakamoto, M. & Matsuzawa, T. Fluid-structure interaction within a layered aortic arch model. J. Biol. Phys. 32, 435–454 (2007).
Shirakawa, T. et al. Towards a clinical implementation of measuring the elastic modulus of the aorta from cardiac computed tomography images. IEEE Trans. Biomed. Eng. 68, 3543–3553 (2021).
Nader, E. et al. Blood rheology: key parameters, impact on blood flow, role in sickle cell disease and effects of exercise. Front. Physiol. 10, 1329 (2019).
Price, R. R. et al. Quality assurance methods and phantoms for magnetic resonance imaging: report of AAPM nuclear magnetic resonance Task Group No. 1. Med. Phys. 17, 287–295 (1990).
van Ooij, P. et al. Wall shear stress estimated with phase contrast MRI in an in vitro and in vivo intracranial aneurysm. J. Magn. Reson. Imaging 38, 876–884 (2013).
Guccione, J. M. & McCulloch, A. D. Mechanics of active contraction in cardiac muscle: Part I—constitutive relations for fiber stress that describe deactivation. J. Biomech. Eng. 115, 72–81 (1993).
Genet, M., Lee, L. C., Baillargeon, B., Guccione, J. M. & Kuhl, E. Modeling pathologies of diastolic and systolic heart failure. Ann. Biomed. Eng. 44, 112–127 (2016).
Mitchell, C. et al. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 32, 1–64 (2019).
Stypmann, J. et al. Echocardiographic assessment of global left ventricular function in mice. Lab. Anim. 43, 127–137 (2009).
Pibarot, P., Garcia, D. & Dumesnil, J. G. Energy loss index in aortic stenosis. Circulation 127, 1101–1104 (2013).
Nuis, R.-J. et al. Impact of valvulo-arterial impedance on long-term quality of life and exercise performance after transcatheter aortic valve replacement. Circ. Cardiovasc. Interv. 13, e008372 (2020).
Itoh, T. et al. Body surface area measurement in juvenile miniature pigs using a computed tomography scanner. Exp. Anim. 66, 229–233 (2017).
Acknowledgements
We acknowledge funding from the Harvard-Massachusetts Institute of Technology Health Sciences and Technology programme, the SITA Foundation Award from the Institute for Medical Engineering and Science, the MathWorks Engineering Fellowship Fund, the Fulbright-Turkey Fellowship, the Hassenfeld Research Scholarship, the Massachusetts General Hospital SPARK Award, and grants R01HL151704, R01HL135242 and R01HL159010 from the National Institutes of Health (NIH) National Heart Lung and Blood Institute (NHLBI). We thank N. Jin for providing the Siemens WIP for the 4D flow MRI sequence used in this study, and BioHues Digital for creating the illustrations in Figs. 1a and 3a.
Author information
Authors and Affiliations
Contributions
L.R., C.O., M.P., E.T.R. and C.T.N. conceived the hypothesis and designed the experiments. L.R., C.O., J.C.-F., Y.F., Y.N., M.S., D.G., A.M., S.C., R.A.E., E.M.G., J.H.K., S.Y., B.P.B., A.N.F., R.A.L., E.R.E. and J.L.G performed the experiments. L.R., C.O., E.T.R. and C.T.N. analysed the results and wrote the manuscript. L.R. and C.O. contributed equally to this work. E.T.R. and C.T.N. equally supervised this research.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Lyes Kadem, Amanda Randles and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
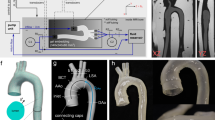
Extended Data Fig. 1 Sleeve tensioning and representative hemodynamic data in vitro and in silico under quasi-static and dynamic conditions.
a, Illustration of an adequately and loosely implanted sleeve, showing the sleeve-to-aorta diameter ratio for classification. Ratios smaller than 1 would result in over-tensioning, and thus in aortic pre-constriction. Ratios greater than 1.8 would cause under-tensioning. b, Representative in vivo peak aortic flow velocity on MRI at baseline (BL), intermediate (Int: 3 mL), and full (4 mL) constrictions for the loose, intermediate, and adequately-pretensioned sleeve. c, Representative in vitro LVP and AoP under quasi-static actuation conditions. d, Representative LVP tracing in vivo measured under quasi-static actuation conditions. e, Representative LVP and AoP tracings in vitro with dynamic actuation. f, Representative LVP tracing in vivo with dynamic actuation. The ON and OFF marks indicate the intervals during which the sleeve was inflated or deflated respectively, following the start of the triggering.
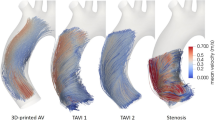
Extended Data Fig. 2 In vivo MRI aortic flow streamlines and turbulence kinetic energy (TKE).
a, Aortic flow streamlines for the stenosis (AS), bicommissural (Bi), and unicommissural (Uni) constriction profiles. b, TKE map of the aorta of a longitudinal cross-section of the aorta and for the same constriction profiles. c, Distribution of elements across ranges of TKE within the aortic domain. d, TKE line plots along an aortic diameter at the sleeve plane.
Extended Data Fig. 3 CFD hemodynamics and TKE.
a, Aortic flow streamlines for the stenosis, bicommissural, and unicommissural constriction profiles, with details of transverse planes at the ascending aorta (P1), aortic arch (P2), and descending aorta (P3). b, 2D velocity vector maps of a longitudinal cross-section of the aorta for the three constriction profiles. c, TKE map of the same longitudinal cross-section for the three constriction profiles. d, Distribution of elements across ranges of TKE within the aortic CFD domain. e, TKE line plots along an aortic diameter at the sleeve plane.
Extended Data Fig. 4 In vivo hemodynamic studies under dynamic actuation.
a–f, Clinical metrics of AS obtained via echocardiography and LV catheterization. These include the (a) iEOA, (b) ΔPmax, (c) ΔPmean, (d) vmax, (e) ELI, (f) ZVA. Error bars, s.d., n = 5 for each data point, with 5 consecutive measurements taken for 1 animal. g, 2D velocity vector maps of the aorta with corresponding flow cross-sectional planes at the ascending aorta (P1), aortic arch (P2), and descending aorta (P3). h, Corresponding TKE map of the aorta.
Extended Data Fig. 5 Global and local hemodynamics in the presence or absence of a healthy valve in series with the aortic sleeve in vitro.
(a) EOA, (b) ΔPmax, (c) ΔPmean, (d) LVPmax calculated at baseline (valve only; BL) and at 8 psi under dynamic actuation with (valve + sleeve) and without (sleeve only) a valve proximal to the sleeve. Error bars, s.d., n = 15 actuation cycles for each data point. e, LV PV loops at BL and 10 psi for the same groups. f, LV PV loops at BL, and for quasi-static and dynamic actuation. g-h, Longitudinal and cross-sectional 2D velocity vectors (g) before and (h) during actuation of the soft robotic aortic sleeve (sleeve only) and during actuation with a porcine valve inserted proximally to the sleeve (valve + sleeve). Results illustrate the cross-sectional geometry of the mock aortic vessel at the sleeve plane both prior to and during actuation for the two groups (sleeve only and valve + sleeve). Arrows indicate the direction of flow. Scale bar, 1.0 cm.
Supplementary information
Supplementary Information
Supplementary notes, figures, tables and references.
Supplementary video 1
Quasi-static and dynamic actuation of the biomimetic soft robotic sleeve in a mock circulatory loop.
Supplementary video 2
Magnetic resonance imaging of the biomimetic soft robotic sleeve under dynamic actuation for various actuation profiles in a mock circulatory loop.
Supplementary video 3
Echocardiography and magnetic resonance imaging of the biomimetic soft robotic sleeve under quasi-static and dynamic actuation in vivo.
Supplementary video 4
Computational fluid-dynamics modelling for the prediction of aortic flow owing to dynamic actuation of the biomimetic soft robotic sleeve.
Supplementary data
Source data for Supplementary Figs. 3b–d.
Source data
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rosalia, L., Ozturk, C., Coll-Font, J. et al. A soft robotic sleeve mimicking the haemodynamics and biomechanics of left ventricular pressure overload and aortic stenosis. Nat. Biomed. Eng 6, 1134–1147 (2022). https://doi.org/10.1038/s41551-022-00937-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-022-00937-8
This article is cited by
-
Acausal Modelling of Advanced-Stage Heart Failure and the Istanbul Heart Ventricular Assist Device Support with Patient Data
Cardiovascular Engineering and Technology (2023)
-
A soft robot that mimics aortic stenosis
Nature Reviews Materials (2022)