Abstract
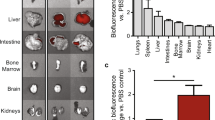
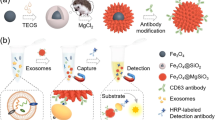
The systemic biodistribution of endogenous extracellular vesicles is central to the maintenance of tissue homeostasis. Here, we show that angiogenesis and heart function in infarcted heart tissue can be ameliorated by the local accumulation of exosomes collected from circulation using magnetic nanoparticles. The nanoparticles consist of a Fe3O4 core and a silica shell that is decorated with poly (ethylene glycol) conjugated through hydrazone bonds to two types of antibody, which bind either to CD63 antigens on the surface of extracellular vesicles or to myosin-light-chain surface markers on injured cardiomyocytes. On application of a local magnetic field, accumulation of the nanoparticles and cleavage of the hydrazone bonds under the acidic pH of injured cardiac tissue lead to the local release of the captured exosomes. In rabbit and rat models of myocardial infarction, the magnetic-guided accumulation of captured CD63-expressing exosomes in infarcted tissue led to reductions in infarct size as well as improved left-ventricle ejection fraction and angiogenesis. The approach could be used to manipulate endogenous exosome biodistribution for the treatment of other diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The main data supporting the results of this study are available within the paper and its Supplementary Information. The data used to make the graphs are provided as Supplementary Information. The raw and analysed datasets generated during the study are too large to be publicly shared, yet they are available for research purposes from the corresponding authors on reasonable request.
References
Thery, C., Zitvogel, L. & Amigorena, S. Exosomes: composition, biogenesis and function. Nat. Rev. Immunol. 2, 569–579 (2002).
Valadi, H. et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9, 654–659 (2007).
Steinbichler, T. B., Dudas, J., Riechelmann, H. & Skvortsova, I. I. The role of exosomes in cancer metastasis. Semin. Cancer Biol. 44, 170–181 (2017).
Skog, J. et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 10, 1470–1476 (2008).
Aznar, E. et al. Gated materials for on-command release of guest molecules. Chem. Rev. 116, 561–718 (2016).
Huo, M., Wang, L., Chen, Y. & Shi, J. Tumor-selective catalytic nanomedicine by nanocatalyst delivery. Nat. Commun. 8, 357 (2017).
Xing, P. & Zhao, Y. Multifunctional nanoparticles self-assembled from small organic building blocks for biomedicine. Adv. Mater. 28, 7304–7339 (2016).
Chen, X. et al. Light-Induced hydrogel based on tumor-targeting mesoporous silica nanoparticles as a theranostic platform for sustained cancer treatment. ACS Appl. Mater. Inter. 8, 15857–15863 (2016).
Chen, X. et al. Dual bioresponsive mesoporous silica nanocarrier as an “AND” logic gate for targeted drug delivery cancer cells. Adv. Funct. Mater. 24, 6999–7006 (2014).
Nahrendorf, M., Pittet, M. J. & Swirski, F. K. Monocytes: protagonists of infarct inflammation and repair after myocardial infarction. Circulation 121, 2437–2445 (2010).
Vicencio, J. M. et al. Plasma exosomes protect the myocardium from ischemia-reperfusion injury. J. Am. Coll. Cardiol. 65, 1525–1536 (2015).
Sahoo, S. & Losordo, D. W. Exosomes and cardiac repair after myocardial infarction. Circ. Res. 114, 333–344 (2014).
Giricz, Z. et al. Cardioprotection by remote ischemic preconditioning of the rat heart is mediated by extracellular vesicles. J. Mol. Cell. Cardiol. 68, 75–78 (2014).
Armstrong, J. P., Holme, M. N. & Stevens, M. M. Re-engineering extracellular vesicles as smart nanoscale therapeutics. ACS Nano 11, 69–83 (2017).
Bian, S. et al. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J. Mol. Med. 92, 387–397 (2014).
Gallet, R. et al. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur. Heart J. 38, 201–211 (2017).
Bei, Y. et al. Exercise-induced circulating extracellular vesicles protect against cardiac ischemia-reperfusion injury. Basic Res. Cardiol. 112, 38 (2017).
Cheng, M. et al. Circulating myocardial microRNAs from infarcted hearts are carried in exosomes and mobilise bone marrow progenitor cells. Nat. Commun. 10, 959 (2019).
Li, H. et al. Coronary serum exosomes derived from patients with myocardial ischemia regulate angiogenesis through the miR-939-mediated nitric oxide signaling pathway. Theranostics 8, 2079–2093 (2018).
Zile, M. R. et al. Relationship between the temporal profile of plasma microRNA and left ventricular remodeling in patients after myocardial infarction. Circ. Cardiovasc. Genet. 4, 614–619 (2011).
Luther, K. M. et al. Exosomal miR-21a-5p mediates cardioprotection by mesenchymal stem cells. J. Mol. Cell. Cardiol. 119, 125–137 (2018).
Dong, S. et al. MicroRNA expression signature and the role of microRNA-21 in the early phase of acute myocardial infarction. J. Biol. Chem. 284, 29514–29525 (2009).
Mathieu, M., Martin-Jaular, L., Lavieu, G. & Thery, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 21, 9–17 (2019).
Kamerkar, S. et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 546, 498–503 (2017).
Thery, C., Amigorena, S., Raposo, G. & Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 30, 3.22.1–3.22.29 (2006).
Vandergriff, A. C. et al. Magnetic targeting of cardiosphere-derived stem cells with ferumoxytol nanoparticles for treating rats with myocardial infarction. Biomaterials 35, 8528–8539 (2014).
Garbern, J. C., Minami, E., Stayton, P. S. & Murry, C. E. Delivery of basic fibroblast growth factor with a pH-responsive, injectable hydrogel to improve angiogenesis in infarcted myocardium. Biomaterials 32, 2407–2416 (2011).
Lott, J. A. Serum enzyme determinations in the diagnosis of acute myocardial infarction: an update. Hum. Pathol. 15, 706–716 (1984).
Wolf, R. E. et al. Evaluation of serum creatine kinase and lactate dehydrogenase in experimental myocardial infarction, atriotomies, and thoracotomies. Ann. Thorac. Surg. 41, 378–386 (1986).
Xu, R., Greening, D. W., Zhu, H. J., Takahashi, N. & Simpson, R. J. Extracellular vesicle isolation and characterization: toward clinical application. J. Clin. Invest. 126, 1152–1162 (2016).
Farooqi, A. A. et al. Exosome biogenesis, bioactivities and functions as new delivery systems of natural compounds. Biotechnol. Adv. 36, 328–334 (2018).
Peinado, H. et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 18, 883–891 (2012).
Becker, A. et al. Extracellular vesicles in cancer: cell-to-cell mediators of metastasis. Cancer Cell 30, 836–848 (2016).
Tran, T. H., Mattheolabakis, G., Aldawsari, H. & Amiji, M. Exosomes as nanocarriers for immunotherapy of cancer and inflammatory diseases. Clin. Immunol. 160, 46–58 (2015).
Im, H. et al. Label-free detection and molecular profiling of exosomes with a nano-plasmonic sensor. Nat. Biotechnol. 32, 490–495 (2014).
Shao, H. et al. Chip-based analysis of exosomal mRNA mediating drug resistance in glioblastoma. Nat. Commun. 6, 6999 (2015).
Tavallaie, R. et al. Nucleic acid hybridization on an electrically reconfigurable network of gold-coated magnetic nanoparticles enables microRNA detection in blood. Nat. Nanotechnol. 13, 1066–1071 (2018).
Melo, S. A. et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 523, 177–182 (2015).
Colao, I. L., Corteling, R., Bracewell, D. & Wall, I. Manufacturing exosomes: a promising therapeutic platform. Trends Mol. Med. 24, 242–256 (2018).
Alvarez-Erviti, L. et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 29, 341–345 (2011).
Koppers-Lalic, D., Hogenboom, M. M., Middeldorp, J. M. & Pegtel, D. M. Virus-modified exosomes for targeted RNA delivery; a new approach in nanomedicine. Adv. Drug Deliv. Rev. 65, 348–356 (2013).
Ostrowski, M. et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat. Cell Biol. 12, 19–30 (2010).
Headland, S. E. et al. Neutrophil-derived microvesicles enter cartilage and protect the joint in inflammatory arthritis. Sci. Transl. Med. 7, 315ra190 (2015).
Zhang, B. et al. HucMSC-exosome mediated-Wnt4 signaling is required for cutaneous wound healing. Stem Cells 33, 2158–2168 (2015).
Hoshino, A. et al. Tumour exosome integrins determine organotropic metastasis. Nature 527, 329–335 (2015).
Gutierrez-Vazquez, C., Villarroya-Beltri, C., Mittelbrunn, M. & Sanchez-Madrid, F. Transfer of extracellular vesicles during immune cell-cell interactions. Immunol. Rev. 251, 125–142 (2013).
Fleshner, M. & Crane, C. R. Exosomes, DAMPs and miRNA: features of stress physiology and immune homeostasis. Trends Immunol. 38, 768–776 (2017).
Kowal, J. et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl Acad. Sci. USA 113, E968–E977 (2016).
Cheng, K. et al. Magnetic antibody-linked nanomatchmakers for therapeutic cell targeting. Nat. Commun. 5, 4880 (2014).
Zhang, H. et al. Ultrasmall ferrite nanoparticles synthesized via dynamic simultaneous thermal decomposition for high-performance and multifunctional T1 magnetic resonance imaging contrast agent. ACS Nano 11, 3614–3631 (2017).
Liu, Q. et al. Genetic targeting of sprouting angiogenesis using Apln-CreER. Nat. Commun. 6, 6020 (2015).
Kapnisi, M. et al. Auxetic cardiac patches with tunable mechanical and conductive properties toward treating myocardial infarction. Adv. Funct. Mater. 28, 1800618 (2018).
Ferreira, J. C. B. et al. A selective inhibitor of mitofusin 1-βIIPKC association improves heart failure outcome in rats. Nat. Commun. 10, 329 (2019).
Yu, W. et al. GATA4 regulates Fgf16 to promote heart repair after injury. Development 143, 936–949 (2016).
Ottersbach, A. et al. Improved heart repair upon myocardial infarction: combination of magnetic nanoparticles and tailored magnets strongly increases engraftment of myocytes. Biomaterials 155, 176–190 (2018).
Qi, H. et al. Blood exosomes endowed with magnetic and targeting properties for cancer therapy. ACS Nano 10, 3323–3333 (2016).
Mahaffey, K. W., Raya, T. E., Pennock, G. D., Morkin, E. & Goldman, S. Left ventricular performance and remodeling in rabbits after myocardial infarction. Effects of a thyroid hormone analogue. Circulation 91, 794–801 (1995).
Makkar, R. R. et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet 379, 895–904 (2012).
Acknowledgements
This work was supported by the National Key Research and Development Program of China (no. 2016YFC1101400 to Y.J.), the National Natural Science Foundation of China (no. 81601606 to X.C. and no. 31670995 to S.L.), the Young Elite Scientist Sponsorship Program by CAST (no. 2017QNRC001 to S.L.), the Fundamental Research Funds for the Central Universities (no. 2016qngz02 to X.C.), the National Natural Science Foundation of Shaanxi Province (no. 2017JM5023 to X.C.), the open fund of the State Key Laboratory of Military Stomatology (no. 2017KA02 to X.C.) and the ARC Centre of Excellence in Convergent Bio-Nano Science and Technology (no. CE140100036 to J.J.G.), and the ARC Laureate Fellowship (no. FL150100060 to J.J.G.).
Author information
Authors and Affiliations
Contributions
S.L., X.C., L.B. and T.L. contributed to the study design, data acquisition and interpretation. P.Y. and Y.B. characterized the properties of magnetic nanoparticles. X.Y. and X.Q. contributed to data analysis and interpretation. J.X. and F.P. performed the animal experiments. J.J.G. refined the manuscript. S.L., X.C. and Y.J. developed the concept and supervised experiments. All of the authors contributed to writing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary figures.
Supplementary Dataset 1
The data used to generate the figures.
Rights and permissions
About this article
Cite this article
Liu, S., Chen, X., Bao, L. et al. Treatment of infarcted heart tissue via the capture and local delivery of circulating exosomes through antibody-conjugated magnetic nanoparticles. Nat Biomed Eng 4, 1063–1075 (2020). https://doi.org/10.1038/s41551-020-00637-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-020-00637-1
This article is cited by
-
Enhancing aortic valve drug delivery with PAR2-targeting magnetic nano-cargoes for calcification alleviation
Nature Communications (2024)
-
Advances in the study of exosomes derived from mesenchymal stem cells and cardiac cells for the treatment of myocardial infarction
Cell Communication and Signaling (2023)
-
Therapeutic angiogenesis and tissue revascularization in ischemic vascular disease
Journal of Biological Engineering (2023)
-
Preparation and PET/CT imaging of implant directed 68Ga-labeled magnetic nanoporous silica nanoparticles
Journal of Nanobiotechnology (2023)
-
The current status of stimuli-responsive nanotechnologies on orthopedic titanium implant surfaces
Journal of Nanobiotechnology (2023)