Abstract
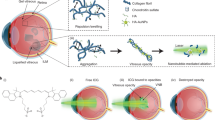
Internal-tamponade agents are crucial surgical adjuncts in vitreoretinal surgery. Clinically used endotamponade agents act through buoyancy forces, yet can result in prolonged post-operative positioning, temporary loss of vision, raised intra-ocular pressure, cataract formation or the need for additional removal surgery. Here, we describe a thermogelling polymer that provides an internal tamponade effect through surface tension and swelling counter-forces. We tested the long-term biocompatibility of the polymer endotamponade in rabbit vitrectomy models, and its surgical efficacy and biocompatibility in a non-human primate retinal-detachment model. We also show that, while the thermogel biodegrades during the three months following surgery, it promotes the reformation of a vitreous-like body that mimics the biophysical properties of the natural vitreous. The thermogelling endotamponade might serve as a long-term vitreous substitute.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The authors declare that all data supporting the results in this study are available within the paper and its Supplementary Information. The MS proteomics data have been deposited to the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org/) via the PRIDE partner repository, with the dataset identifier PXD009525.
References
Sebag, J. & Balazs, E. A. Morphology and ultrastructure of human vitreous fibers. Invest. Ophthalmol. Vis. Sci. 30, 1867–1871 (1989).
Foster, W. J. Vitreous substitutes. Expert Rev. Ophthalmol. 3, 211–218 (2008).
Lee, B., Litt, M. & Buchsbaum, G. Rheology of the vitreous body. Part I: Viscoelasticity of human vitreous. Biorheology 29, 521–533 (1992).
Los, L. I., van der Worp, R. J., van Luyn, M. J. & Hooymans, J. M. Age-related liquefaction of the human vitreous body: LM and TEM evaluation of the role of proteoglycans and collagen. Invest. Ophthalmol. Vis. Sci. 44, 2828–2833 (2003).
Mitry, D., Charteris, D. G., Fleck, B. W., Campbell, H. & Singh, J. The epidemiology of rhegmatogenous retinal detachment: geographical variation and clinical associations. Br. J. Ophthalmol. 94, 678–684 (2010).
Park, S. J., Choi, N. K., Park, K. H. & Woo, S. J. Five year nationwide incidence of rhegmatogenous retinal detachment requiring surgery in Korea. PLoS One 8, e80174 (2013).
Kleinberg, T. T., Tzekov, R. T., Stein, L., Ravi, N. & Kaushal, S. Vitreous substitutes: a comprehensive review. Surv. Ophthalmol. 56, 300–323 (2011).
Newsom, R. S. et al. Sudden visual loss after removal of silicone oil. Retina 24, 871–877 (2004).
Su, X. et al. Recent progress in using biomaterials as vitreous substitutes. Biomacromolecules 16, 3093–3102 (2015).
Kopecek, J. Polymer chemistry: swell gels. Nature 417, 388–391 (2002).
Nakagawa, M., Tanaka, M. & Miyata, T. Evaluation of collagen gel and hyaluronic acid as vitreous substitutes. Ophthalmic. Res. 29, 409–420 (1997).
Hong, Y. et al. Crosslinked poly(1-vinyl-2-pyrrolidinone) as a vitreous substitute. J. Biomed. Mater. Res. 30, 441–448 (1996).
Mueller-Jensen, K. Polyacrylamide as an alloplastic vitreous implant. Albrecht Von Graefes Arch. Klin. Exp. Ophthalmol. 189, 147–158 (1973).
Barth, H., Crafoord, S., Andreasson, S. & Ghosh, F. A cross-linked hyaluronic acid hydrogel (Healaflow(®)) as a novel vitreous substitute. Graefes Arch. Clin. Exp. Ophthalmol. 254, 697–703 (2016).
Pritchard, C. D. et al. Evaluation of viscoelastic poly(ethylene glycol) sols as vitreous substitutes in an experimental vitrectomy model in rabbits. Acta Biomater. 7, 936–943 (2011).
Leone, G. et al. PVA/STMP based hydrogels as potential substitutes of human vitreous. J. Mater. Sci. Mater. Med. 21, 2491–2500 (2010).
Maruoka, S. et al. Biocompatibility of polyvinylalcohol gel as a vitreous substitute. Curr. Eye Res. 31, 599–606 (2006).
Hayashi, K. et al. Fast-forming hydrogel with ultralow polymeric content as an artificial vitreous body. Nat. Biomed. Eng. 1, 0044 (2017).
Uesugi, K. et al. A self-assembling peptide gel as a vitreous substitute: a rabbit study. Invest. Ophthalmol. Vis. Sci. 58, 4068–4075 (2017).
Swindle, K. E., Hamilton, P. D. & Ravi, N. In situ formation of hydrogels as vitreous substitutes: Viscoelastic comparison to porcine vitreous. J. Biomed. Mater. Res. A 87, 656–665 (2008).
Katagiri, Y. et al. Application of thermo-setting gel as artificial vitreous. Jpn. J. Ophthalmol. 49, 491–496 (2005).
Lorget, F. et al. Characterization of the pH and temperature in the rabbit, pig, and monkey eye: key parameters for the development of long-acting delivery ocular strategies. Mol. Pharm. 13, 2891–2896 (2016).
Loh, X. J., Yee, B. J. & Chia, F. S. Sustained delivery of paclitaxel using thermogelling poly(PEG/PPG/PCL urethane)s for enhanced toxicity against cancer cells. J. Biomed. Mater. Res. A 100, 2686–2694 (2012).
Li, Z., Zhang, Z., Liu, K. L., Ni, X. & Li, J. Biodegradable hyperbranched amphiphilic polyurethane multiblock copolymers consisting of poly(propylene glycol), poly(ethylene glycol), and polycaprolactone as in situ thermogels. Biomacromolecules 13, 3977–3989 (2012).
Loh, X. J., Goh, S. H. & Li, J. New biodegradable thermogelling copolymers having very low gelation concentrations. Biomacromolecules 8, 585–593 (2007).
Loh, X. J. et al. Biodegradable thermogelling poly(ester urethane)s consisting of poly(lactic acid)–thermodynamics of micellization and hydrolytic degradation. Biomaterials 29, 2164–2172 (2008).
Liow, S. S. et al. Thermogels: In situ gelling biomaterial. ACS Biomater. Sci. Eng. 2, 295–316 (2016).
Stanzel, B. V. et al. Subretinal delivery of ultrathin rigid-elastic cell carriers using a metallic shooter instrument and biodegradable hydrogel encapsulation. Invest. Ophthalmol. Vis. Sci. 53, 490–500 (2012).
Liu, Z., Yu, N., Holz, F. G., Yang, F. & Stanzel, B. V. Enhancement of retinal pigment epithelial culture characteristics and subretinal space tolerance of scaffolds with 200 nm fiber topography. Biomaterials 35, 2837–2850 (2014).
Akash, M. S. & Rehman, K. Recent progress in biomedical applications of Pluronic (PF127): Pharmaceutical perspectives. J. Control Release 209, 120–138 (2015).
Loh, X. J., Goh, S. H. & Li, J. Hydrolytic degradation and protein release studies of thermogelling polyurethane copolymers consisting of poly[(R)-3-hydroxybutyrate], poly(ethylene glycol), and poly(propylene glycol). Biomaterials 28, 4113–4123 (2007).
Loh, X. J., Guerin, W. & Guillaume, S. M. Sustained delivery of doxorubicin from thermogelling poly(PEG/PPG/PTMC urethane)s for effective eradication of cancer cells. J. Mater. Chem. 22, 21249–21256 (2012).
Bishop, P. The biochemical structure of mammalian vitreous. Eye 10, 664–670 (1996).
Bishop, P. N. Structural macromolecules and supramolecular organisation of the vitreous gel. Prog. Retin. Eye Res. 19, 323–344 (2000).
Fraser, J. R., Laurent, T. C. & Laurent, U. B. Hyaluronan: its nature, distribution, functions and turnover. J. Intern. Med. 242, 27–33 (1997).
Day, A. J. & Prestwich, G. D. Hyaluronan-binding proteins: tying up the giant. J. Biol. Chem. 277, 4585–4588 (2002).
Itakura, H., Kishi, S., Kotajima, N. & Murakami, M. Vitreous collagen metabolism before and after vitrectomy. Graefes Arch. Clin. Exp. Ophthalmol. 243, 994–998 (2005).
Qiao, H. et al. The characterisation of hyalocytes: the origin, phenotype, and turnover. Br. J. Ophthalmol. 89, 513–517 (2005).
Bloom, G. D. & Balazs, E. A. An electron microscopic study of hyalocytes. Exp. Eye Res. 4, 249–255 (1965).
Freeman, H. M. The lens and vitreous. Arch. Ophthalmol. 80, 132–144 (1968).
Theocharis, D. A. et al. Hyaluronan and chondroitin sulfate proteoglycans in the supramolecular organization of the mammalian vitreous body. Connect. Tissue Res. 49, 124–128 (2008).
Moore, A. N. et al. Nanofibrous peptide hydrogel elicits angiogenesis and neurogenesis without drugs, proteins, or cells. Biomaterials 161, 154–163 (2018).
Engler, A. J., Sen, S., Sweeney, H. L. & Discher, D. E. Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 (2006).
Saha, K. et al. Substrate modulus directs neural stem cell behavior. Biophys. J. 95, 4426–4438 (2008).
Banerjee, A. et al. The influence of hydrogel modulus on the proliferation and differentiation of encapsulated neural stem cells. Biomaterials 30, 4695–4699 (2009).
Discher, D. E. et al. Matrix mechanosensing: from scaling concepts in ‘omics data to mechanisms in the nucleus, regeneration, and cancer. Annu. Rev. Biophys. 46, 295–315 (2017).
Hytonen, V. P. & Wehrle-Haller, B. Mechanosensing in cell-matrix adhesions - Converting tension into chemical signals. Exp. Cell Res. 343, 35–41 (2016).
Stoppel, W. L. et al. Terminal sterilization of alginate hydrogels: efficacy and impact on mechanical properties. J. Biomed. Mater. Res. B 102, 877–884 (2014).
Bernhardt, A. et al. Improved sterilization of sensitive biomaterials with supercritical carbon dioxide at low temperature. PLoS ONE 10, e0129205 (2015).
Chan, B. et al. Poly(carbonate urethane)-based thermogels with enhanced drug release efficacy for chemotherapeutic applications. Polymers 10, 89 (2018).
Liow, S. S. et al. Long-term real-time in vivo drug release monitoring with AIE thermogelling polymer. Small 13, 1603404 (2017).
Al-Nawaiseh, S. et al. A step by step protocol for subretinal surgery in rabbits. J. Vis. Exp. 115, 53927 (2016).
Ryan, S. J. in Retina Vol. 3, 5th edn (eds Wong, I. Y. & Wong, D.) Ch. 104 (Saunders, 2012).
Ryan, S. J. in Retina Vol. 3, 5th edn (ed. Charles, S.) Ch. 101 (Saunders, 2012).
Struble, C., Howard, S. & Relph, J. Comparison of ocular tissue weights (volumes) and tissue collection techniques in commonly used preclinical animal species. Acta Ophthalmol. 92, S005 (2014).
Finger, P. T., Moshfeghi, D. M., Smith, P. D. & Perry, H. D. Microwave cyclodestruction for glaucoma in a rabbit model. Arch. Ophthalmol. 109, 1001–1004 (1991).
Seidehamel, R. J. & Dungan, K. W. Characteristics and pharmacologic utility of an intraocular pressure (IOP) model in unanesthetized rabbits. Invest. Ophthalmol. 13, 319–322 (1974).
Gherezghiher, T., March, W. F., Nordquist, R. E. & Koss, M. C. Laser-induced glaucoma in rabbits. Exp. Eye Res. 43, 885–894 (1986).
Patrick, J. How to handle cataract surgery post-operative complications. Newgradoptometry.com https://newgradoptometry.com/handle-cataract-surgery-post-operative-complications (2016).
Cioboata, M. et al. Benefits of anterior chamber paracentesis in the management of glaucomatous emergencies. J. Med. Life 7, 5–6 (2014).
Kitnarong, N., Boonyaleepun, S., Sakiyalak, D., Ruangvaravate, N. & Metheetrairut, A. Immediate anterior chamber paracentesis with a 30-gauge needle for acute primary angle closure. J. Clin. Exp. Ophthalmol. 8, 657 (2017).
McCulloch, D. L. et al. ISCEV Standard for full-field clinical electroretinography (2015 update). Doc. Ophthalmol. 130, 1–12 (2015).
Bach, M. et al. ISCEV standard for clinical pattern electroretinography (PERG): 2012 update. Doc. Ophthalmol. 126, 1–7 (2013).
Acknowledgements
This study was supported by a Biomedical Engineering Programme (BEP) grant, A*STAR, Singapore (2014 POC/1521480032), the NUS Start-up grant NUHSRO/2016/100/SU/01 and IAF-PP (HMBS Domain) H17/01/a0/013 (OrBID): OculaR BIomaterials and Device. We would like to acknowledge the veterinary team at the Translational Pre-Clinical Model Platform (Singapore Eye Research Institute, Singapore) for providing support in NHP surgery preparation and animal follow-up.
Author information
Authors and Affiliations
Contributions
X.S., X.J.L., G.L. and Z. Liu designed experiments and prepared the manuscript. G.L., P.Z. and X.S. performed the in vivo animal surgeries. Z. Liu performed the in vivo assessment of animals post-surgery. V.A.B. provided assistance in large-animal surgeries. S.S.L., Z. Li and M.J.T. synthesized thermogels and performed rheological assessments. R.L. provided assistance in rheological assessments of the thermogels. S.L.L. and J.G. performed MS analysis and A.A.-S. performed the vitreous proteome analysis. G.E.H. analysed the ERG data and contributed to manuscript preparation. B.H.P. performed immunohistochemistry experiments. S.K. performed the H&E histology experiments. W.H. provided assistance in ex vivo analysis of retina and manuscript preparation. C.W.T.T., C.K.C. and P.Z. provided clinical inputs on clinical application of thermogel, and preparation of the manuscript. All authors reviewed and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary figures, and video and table captions.
Supplementary Dataset
List of proteins identified by MS-based LFQ proteomics analysis.
Supplementary Video 1
Gel injection in the rabbit eye.
Supplementary Video 2a
Dissection of the EPC-filled eye, 3 months post-operation.
Supplementary Video 2b
Dissection of the native vitreous.
Supplementary Video 2c
Dissection of the operated control (BSS-filled eye), 3 months post-operation.
Supplementary Video 3a
SD-OCT volume scan of the retinotomy site of the non-human primate, 12 months post-operation.
Supplementary Video 3b
SD-OCT volume scan of the macula of the non-human primate, 2 months post-operation.
Rights and permissions
About this article
Cite this article
Liu, Z., Liow, S.S., Lai, S.L. et al. Retinal-detachment repair and vitreous-like-body reformation via a thermogelling polymer endotamponade. Nat Biomed Eng 3, 598–610 (2019). https://doi.org/10.1038/s41551-019-0382-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-019-0382-7
This article is cited by
-
In vivo evaluation of a Nano-enabled therapeutic vitreous substitute for the precise delivery of triamcinolone to the posterior segment of the eye
Drug Delivery and Translational Research (2024)
-
Injectable PTHF-based thermogelling polyurethane implants for long-term intraocular application
Biomaterials Research (2022)
-
A bio-functional polymer that prevents retinal scarring through modulation of NRF2 signalling pathway
Nature Communications (2022)
-
Submacular integration of hESC-RPE monolayer xenografts in a surgical non-human primate model
Stem Cell Research & Therapy (2021)
-
Use of biomaterials for sustained delivery of anti-VEGF to treat retinal diseases
Eye (2020)