Abstract
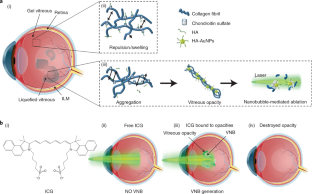
In myopia, diabetes and ageing, fibrous vitreous liquefaction and degeneration is associated with the formation of opacities inside the vitreous body that cast shadows on the retina, appearing as ‘floaters’ to the patient. Vitreous opacities degrade contrast sensitivity function and can cause notable impairment in vision-related quality of life. Here we introduce ‘nanobubble ablation’ for safe destruction of vitreous opacities. Following intravitreal injection, hyaluronic acid-coated gold nanoparticles and indocyanine green, which is widely used as a dye in vitreoretinal surgery, spontaneously accumulate on collagenous vitreous opacities in the eyes of rabbits. Applying nanosecond laser pulses generates vapour nanobubbles that mechanically destroy the opacities in rabbit eyes and in patient specimens. Nanobubble ablation might offer a safe and efficient treatment to millions of patients suffering from debilitating vitreous opacities and paves the way for a highly safe use of pulsed lasers in the posterior segment of the eye.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Source data are provided with this paper. All data supporting the findings of this study are available within the paper and its Supplementary Information. Any further related information can be provided by the corresponding authors on reasonable request.
References
Chiti, F. & Dobson, C. M. Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 75, 333–366 (2006).
Sebag, J. Vitreous and vision degrading myodesopsia. Prog. Retin. Eye Res. https://doi.org/10.1016/j.preteyeres.2020.100847 (2020).
Asakura, A. Histochemistry of hyaluronic acid of the bovine vitreous body by electronmicroscopy. Nippon Ganka Gakkai Zasshi 89, 179–191 (1985).
Sebag, J. & Balazs, E. A. Morphology and ultrastructure of human vitreous fibers. Invest. Ophthalmol. Vis. Sci. 30, 1867–1871 (1989).
Sebag, J. Floaters and the quality of life. Am. J. Ophthalmol. 152, 3–4.e1 (2011).
Webb, B. F., Webb, J. R., Schroeder, M. C. & North, C. S. Prevalence of vitreous floaters in a community sample of smartphone users. Int. J. Ophthalmol. 6, 402–405 (2013).
Zou, H., Liu, H., Xu, X. & Zhang, X. The impact of persistent visually disabling vitreous floaters on health status utility values. Qual. Life Res. 22, 1507–1514 (2013).
Wagle, A. M., Lim, W.-Y., Yap, T.-P., Neelam, K. & Au Eong, K.-G. Utility values associated with vitreous floaters. Am. J. Ophthalmol. 152, 60–65.e1 (2011).
Kim, Y.-K. et al. Psychological distress in patients with symptomatic vitreous floaters. J. Ophthalm. https://doi.org/10.1155/2017/3191576 (2017).
Milston, R., Madigan, M. C. & Sebag, J. Vitreous floaters: etiology, diagnostics, and management. Surv. Ophthalmol. 61, 211–227 (2016).
Sebag, J., Yee, K. M. P., Wa, C. A., Huang, L. C. & Sadun, A. A. Vitrectomy for floaters: prospective efficacy analyses and retrospective safety profile. Retina 34, 1062–1068 (2014).
Macherner, R. The development of pars plana vitrectomy: a personal account. Graefes Arch. Clin. Exp. Ophthalmol. 233, 453–468 (1995).
Holekamp, N. M., Shui, Y.-B. & Beebe, D. C. Vitrectomy surgery increases oxygen exposure to the lens: a possible mechanism for nuclear cataract formation. Am. J. Ophthalmol. 139, 302–310 (2005).
Kunimoto, D. Y. & Kaiser, R. S. Incidence of endophthalmitis after 20- and 25-gauge vitrectomy. Ophthalmology 114, 2133–2137 (2007).
Tsai, W. F., Chen, Y. C. & Su, C. Y. Treatment of vitreous floaters with neodymium YAG laser. Br. J. Ophthalmol. 77, 485–488 (1993).
Procedure guide: vitreous opacities. Ellex Medical Pty https://www.ellex.com/uploads/Ellex-Laser-Procedure-Guide-Vitreolysis-Opacities-VB0002H-A4-ELECTRONIC.pdf (2022).
Rockwell, B. A., Thomas, R. J. & Vogel, A. Ultrashort laser pulse retinal damage mechanisms and their impact on thresholds. Med. Laser Appl. 25, 84–92 (2010).
Delaney, Y. M., Oyinloye, A. & Benjamin, L. Nd:YAG vitreolysis and pars plana vitrectomy: surgical treatment for vitreous floaters. Eye 16, 21–26 (2002).
Koo, E. H., Haddock, L. J., Bhardwaj, N. & Fortun, J. A. Cataracts induced by neodymium–yttrium-aluminium-garnet laser lysis of vitreous floaters. Br. J. Ophthalmol. 101, 709–711 (2017).
Xiong, R., Xu, R. X., Huang, C., Smedt, S. D. & Braeckmans, K. Stimuli-responsive nanobubbles for biomedical applications. Chem. Soc. Rev. https://doi.org/10.1039/C9CS00839J (2021).
Xiong, R. et al. Comparison of gold nanoparticle mediated photoporation: vapor nanobubbles outperform direct heating for delivering macromolecules in live cells. ACS Nano 8, 6288–6296 (2014).
Liu, J. et al. Repeated photoporation with graphene quantum dots enables homogeneous labeling of live cells with extrinsic markers for fluorescence microscopy. Light Sci. Appl. 7, 47 (2018).
Harizaj, A. et al. Photoporation with biodegradable polydopamine nanosensitizers enables safe and efficient delivery of mRNA in human T cells. Adv. Funct. Mater. 31, 2102472 (2021).
Barras, A. et al. Carbon quantum dots as a dual platform for the inhibition and light-based destruction of collagen fibers: implications for the treatment of eye floaters. Nanoscale Horiz. https://doi.org/10.1039/D1NH00157D (2021).
Hua, D. et al. Bubble forming films for spatial selective cell killing. Adv. Mater. https://doi.org/10.1002/adma.202008379 (2021).
Ueno, N., Sebag, J., Hirokawa, H. & Chakrabarti, B. Effects of visible-light irradiation on vitreous structure in the presence of a photosensitizer. Exp. Eye Res. 44, 863–870 (1987).
Filas, B. A., Zhang, Q., Okamoto, R. J., Shui, Y.-B. & Beebe, D. C. Enzymatic degradation identifies components responsible for the structural properties of the vitreous body. Invest. Ophthalmol. Vis. Sci. 55, 55–63 (2014).
Sauvage, F. et al. Photoablation of human vitreous opacities by light-induced vapor nanobubbles. ACS Nano 13, 8401–8416 (2019).
Ziefuss, A. R., Reich, S., Reichenberger, S., Levantino, M. & Plech, A. In situ structural kinetics of picosecond laser-induced heating and fragmentation of colloidal gold spheres. Phys. Chem. Chem. Phys. 22, 4993–5001 (2020).
Pan, Y. et al. Size-dependent cytotoxicity of gold nanoparticles. Small 3, 1941–1949 (2007).
Desmettre, T., Devoisselle, J. M. & Mordon, S. Fluorescence properties and metabolic features of indocyanine green (ICG) as related to angiography. Surv. Ophthalmol. 45, 15–27 (2000).
Burk, S. E., Da Mata, A. P., Snyder, M. E., Rosa, R. H. & Foster, R. E. Indocyanine green-assisted peeling of the retinal internal limiting membrane. Ophthalmology 107, 2010–2014 (2000).
Seitz, B. & Langenbucher, A. Lasers in ophthalmology. Lancet 356, S26 (2000).
Masse, F., Ouellette, M., Lamoureux, G. & Boisselier, E. Gold nanoparticles in ophthalmology. Med. Res. Rev. 39, 302–327 (2019).
Pereira, D. V. et al. Effects of gold nanoparticles on endotoxin-induced uveitis in rats. Invest. Ophthalmol. Vis. Sci. 53, 8036–8041 (2012).
Chen, F., Si, P., Zerda, Adela, V. Jokerst, J. & Myung, D. Gold nanoparticles to enhance ophthalmic imaging. Biomater. Sci. 9, 367–390 (2021).
Hayashi, A., Naseri, A., Pennesi, M. E. & de Juan, E. Subretinal delivery of immunoglobulin G with gold nanoparticles in the rabbit eye. Jpn. J. Ophthalmol. 53, 249–256 (2009).
Rodrigues, E. B., Meyer, C. H., Mennel, S. & Farah, M. E. Mechanisms of intravitreal toxicity of indocyanine green dye: implications for chromovitrectomy. Retina 27, 958–970 (2007).
Kwok, A. K. H., Lai, T. Y. Y., Yew, D. T. W. & Li, W. W. Y. Internal limiting membrane staining with various concentrations of indocyanine green dye under air in macular surgeries. Am. J. Ophthalmol. 136, 223–230 (2003).
Wels, M., Roels, D., Raemdonck, K., De Smedt, S. C. & Sauvage, F. Challenges and strategies for the delivery of biologics to the cornea. J. Control. Release 333, 560–578 (2021).
Del Amo, E. M. et al. Pharmacokinetic aspects of retinal drug delivery. Prog. Retin. Eye Res. 57, 134–185 (2017).
Käsdorf, B. T., Arends, F. & Lieleg, O. Diffusion regulation in the vitreous humor. Biophys. J. 109, 2171–2181 (2015).
Lapotko, D. Optical excitation and detection of vapor bubbles around plasmonic nanoparticles. Opt. Express 17, 2538–2556 (2009).
Xiong, R. et al. Laser-assisted photoporation: fundamentals, technological advances and applications. Adv. Phys. X 1, 596–620 (2016).
Lukianova-Hleb, E. et al. Plasmonic nanobubbles as transient vapor nanobubbles generated around plasmonic nanoparticles. ACS Nano 4, 2109–2123 (2010).
Lukianova-Hleb, E. Y. et al. Hemozoin-generated vapor nanobubbles for transdermal reagent- and needle-free detection of malaria. Proc. Natl Acad. Sci. USA 111, 900–905 (2014).
Palanker, D. Femtosecond lasers for ophthalmic surgery enabled by chirped-pulse amplification. N. Engl. J. Med. 379, 2267–2269 (2018).
American National Standard for Safe Use of Lasers ANSI Z136.1-2014 (Laser Institute of America, 2015); https://www.lia.org/store/product/ansi-z1361-2014-safe-use-lasers-electronic-version
Acknowledgements
This research was supported by the Research Foundation Flanders (FWO, 12X3222N, F.S.).
Author information
Authors and Affiliations
Contributions
F.S., K.B. and S.C.D.S. conceived the concept of nanobubble ablation of vitreous opacities. F.S., V.V.H., R.X. and A.H. performed and analysed the in vitro/ex vivo experiments. R.X. and K.B. designed the optical set-up. J.S. contributed to the writing of the manuscript and performed vitrectomies. J.C.F. synthesized and characterized the AuNPs. V.P.N. and Y.L. performed the experiments in rabbits. F.S., V.P.N. and Y.L. performed the analysis of the experiments in rabbits (OCT, PAM, histology and ERG). F.S., S.C.D.S., V.P.N. and Y.M.P. designed the in vivo experiments. K.R., D.R., M.-J.T., K.P., K.B., J.S., Y.M.P., A.H. and S.C.D.S. advised and provided guidance on experiments and data analysis. All authors discussed the experimental results and jointly wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Lingam Gopal, James McLaughlan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–6.
Supplementary Video 1
ICG (0.5 mg ml−1) mixed with exogenous collagen opacities (0.02 mg ml−1) locally generate VNBs leading to their mechanical destruction.
Supplementary Video 2
ICG (0.5 mg ml−1) mixed with opacities obtained in patients during vitrectomy locally generate VNBs leading to their mechanical destruction.
Source data
Source Data Fig. 3
Number of pulses needed for the destruction of collagen fibres as a function of the fluence and type of photosensitizer.
Source Data Fig. 5
Source data of electroretinograms (b-wave amplitude and implicit time).
Rights and permissions
About this article
Cite this article
Sauvage, F., Nguyen, V.P., Li, Y. et al. Laser-induced nanobubbles safely ablate vitreous opacities in vivo. Nat. Nanotechnol. 17, 552–559 (2022). https://doi.org/10.1038/s41565-022-01086-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-022-01086-4
This article is cited by
-
No side effects on rabbit retina or vitreous microenvironment by nd:YAG laser vitreolysis
BMC Ophthalmology (2024)
-
3D printing by stereolithography using thermal initiators
Nature Communications (2024)
-
Accurate prediction of the optical properties of nanoalloys with both plasmonic and magnetic elements
Nature Communications (2024)
-
Multimodal photoacoustic microscopy, optical coherence tomography, and fluorescence imaging of USH2A knockout rabbits
Scientific Reports (2023)