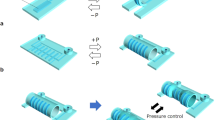
Abstract
Improvements in ingestible electronics with the capacity to sense physiological and pathophysiological states have transformed the standard of care for patients. Yet, despite advances in device development, significant risks associated with solid, non-flexible gastrointestinal transiting systems remain. Here, we report the design and use of an ingestible, flexible piezoelectric device that senses mechanical deformation within the gastric cavity. We demonstrate the capabilities of the sensor in both in vitro and ex vivo simulated gastric models, quantify its key behaviours in the gastrointestinal tract using computational modelling and validate its functionality in awake and ambulating swine. Our proof-of-concept device may lead to the development of ingestible piezoelectric devices that might safely sense mechanical variations and harvest mechanical energy inside the gastrointestinal tract for the diagnosis and treatment of motility disorders, as well as for monitoring ingestion in bariatric applications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Jacobson, B. & Mackay, R. S. A pH-endoradiosonde. Lancet 272, 1224 (1957).
Cassilly, D. et al. Gastric emptying of a non-digestible solid: assessment with simultaneous SmartPill pH and pressure capsule, antroduodenal manometry, gastric emptying scintigraphy. Neurogastroenterol Motil. 20, 311–319 (2008).
Belknap, R. et al. Feasibility of an ingestible sensor-based system for monitoring adherence to tuberculosis therapy. PLoS ONE 8, e53373 (2013).
Traverso, G. et al. Physiologic status monitoring via the gastrointestinal tract. PLoS ONE 10, e0141666 (2015).
Iddan, G., Meron, G., Glukhovsky, A. & Swain, P. Wireless capsule endoscopy. Nature 405, 417 (2000).
Au-Yeung, K. Y. et al. Early clinical experience with networked system for promoting patient self-management. Am. J. Manag. Care 17, e277–e287 (2011).
Liao, Z., Gao, R., Xu, C. & Li, Z. S. Indications and detection, completion, and retention rates of small-bowel capsule endoscopy: a systematic review. Gastrointest. Endosc. 71, 280–286 (2010).
Dagdeviren, C. et al. Conformal piezoelectric energy harvesting and storage from motions of the heart, lung, and diaphragm. Proc. Natl Acad. Sci. USA 111,1927–1932 (2014).
Dagdeviren, C. et al. Recent progress in flexible and stretchable piezoelectric devices for mechanical energy harvesting, sensing and actuation. Extreme Mech. Lett. 9, 269–281 (2016).
Dagdeviren, C. The future of bionic dynamos. Science 354, 1109 (2016).
Mostafalu, P. & Sonkusale, S. Flexible and transparent gastric battery: energy harvesting from gastric acid for endoscopy application. Biosens. Bioelectron. 54,292–296 (2014).
Kim, J. Y. et al. Self-deployable current sources fabricated from edible materials. J. Mater. Chem. B 1, 3781–3788 (2013).
Persano, L. et al. High performance, flexible piezoelectric devices based on aligned arrays of nanofibers of poly(vinylidenefluoride-co-trifluoroethylene). Nat. Commun. 4, 1633 (2013).
Persano, L., Dagdeviren, C., Marrucio, C., De Lorenzis, L. & Pisignano, D. Cooperativity in the enhanced piezoelectric response of polymer nanowires. Adv. Mater. 26, 7574–7580 (2014).
Dagdeviren, C. et al. Transient, biocompatible electronics and energy harvesters based on ZnO. Small 9, 3398–3404 (2013).
Sirohi, J. & Chopra, I. Fundamental understanding of piezoelectric strain sensors. J. Intell. Mater. Syst. Struct. 11, 246–257 (2000).
Yong, Y. K., Fleming, A. J. & Moheimani, S. O. A novel piezoelectric strain sensor for simultaneous damping and tracking control of a high-speed nanopositioner. IEEE/ASME Trans. Mechatron. 18, 1113–1121 (2013).
Shi, Y., & Dagdeviren, C., Rogers, J. A., Gao, C. F. & Huang, Y. An analytic model for skin modulus measurement via conformal piezoelectric systems. J. Appl. Mech. T. ASME 82, 091007 (2015).
Su, Y., Li, S., Li, R. & Dagdeviren, C. Splitting of neutral mechanical plane of conformal, multilayer piezoelectric mechanical energy harvester. Appl. Phys. Lett. 107, 041905 (2015).
Dagdeviren, C. et al. Conformal piezoelectric systems for clinical and experimental characterization of soft tissue biomechanics. Nat. Mater. 14, 728–736 (2015).
Dagdeviren, C. et al. Conformable amplified lead zirconate titanate sensors with enhanced piezoelectric response for cutaneous pressure monitoring. Nat. Commun. 5, 4496 (2014).
Famm, K., Litt, B., Tracey, K. J., Boyden, E. S. & Slaoui, M. Drug discovery: a jump-start for electroceuticals. Nature 496, 159–161 (2013).
Yang, S. et al. Thermally resistant UV-curable epoxy–siloxane hybrid materials for light emitting diode (LED) encapsulation. J. Mater. Chem. 22, 8874–8880 (2012).
Yagnamurthy, S. N. Electromechanical Behavior of PZT Thin Film Composites for RF-MEMS. Masters Thesis, Univ. Illinois at Urbana Champaign (2009).
Su, Y. J., Quian, C. F., Zhao, M. H. & Zhang, T. Y. Microbridge testing of silicon oxide/silicon nitride bilayer films deposited on silicon wafers. Acta Materialia 48, 4901–4915 (2000).
Sharpe, W. et al. Strain measurements of silicon dioxide microspecimens by digital imaging processing. Exp. Mech. 47, 649–658 (2007).
Bellinger, A. M. et al. Oral, ultra-long-lasting drug delivery: application toward malaria elimination goals. Sci. Transl. Med. 8, 365 (2016).
Poeggel, S. et al. Optical fibre pressure sensors in medical applications. Sensors 15, 17115–17148 (2015).
Merritt, J. S. & Weinhaus, F. The pressure curve for a rubber balloon. Am. J. Phys. 46, 976–977 (1978).
Yu, L., Kim, B. J. & Meng, E. Chronically implanted pressure sensors: challenges and state of the field. Sensors 14, 20620–20644 (2014).
Bures, J. et al. Small intestinal bacterial overgrowth syndrome. World J. Gastroenterol. 16, 2978–2990 (2010).
Beyerlein, L. et al. Correlation between symptoms developed after the oral ingestion of 50 g lactose and results of hydrogen breath testing for lactose intolerance. Aliment. Pharmacol. Ther. 27, 659–665 (2008).
Nadeau, P. et al. Prolonged energy harvesting for ingestible devices.Nat. Biomed. Eng. 1, 0022 (2017).
Yeo, W.-H. et al. Multifunctional epidermal electronics printed directly onto the skin. Adv. Mater. 25, 2773–2778 (2013).
Holzapfel, G. A. Nonlinear Solid Mechanics (John Wiley & Sons, Chichester, 2000).
Overvelde, J. T. B., Kloek, T., D'haen, J. J. A. & Bertoldi, K. Amplifying the response of soft actuators by harnessing snap-through instabilities. Proc. Natl Acad. Sci. USA 112, 10863–10868 (2015).
Jones, R. M. Mechanics of Composite Materials (Taylor & Francis Group, 1998).
Acknowledgements
We thank J. Haupt and M. Jamiel for help with the in vivo swine work. We thank Y-A. Lee for assistance with the SEM. We thank the Hope Babette Tang Histology Facility at the Koch Institute at MIT for the histology work and consultation. We also thank the MIT Microsystems Technology Laboratories and MIT Microscopy Core Facility. C.D. thanks the late G. Caliskanoglu for useful suggestions on the device design. This work was funded in part by a postdoctoral fellowship from the Swiss National Foundation (to T.v.E.), National Institutes of Health grant EB-000244, the Max Planck Research Award (Award Ltr Dtd. 2/11/08), the Alexander von Humboldt-Stiftung Foundation (to R.L.) and the Division of Gastroenterology, Brigham and Women’s Hospital (to G.T.).
Author information
Authors and Affiliations
Contributions
C.D. designed and fabricated the PZT GI-S. C.D. and G.T. designed the in vitro, ex vivo and in vivo experiments. T.v.E. performed the cell culture study and studied the biocompatibility of the PZT GI-S. C.D., P.J., T.B. and Z.W. designed an in vitro setup to simulate stomach behaviour and conducted in vitro trials of PZT GI-S. F.J. performed ABAQUS/Standard for finite element modelling. C.D., S.S., C.C. and L.B. designed and conducted the ex vivo studies. C.D., P.J., S.M., J.C., A.H. and G.T. performed in vivo evaluations of the GI-S in Yorkshire swine models. All authors discussed and interpreted the results, and wrote and edited the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Information
Supplementary figures.
Rights and permissions
About this article
Cite this article
Dagdeviren, C., Javid, F., Joe, P. et al. Flexible piezoelectric devices for gastrointestinal motility sensing. Nat Biomed Eng 1, 807–817 (2017). https://doi.org/10.1038/s41551-017-0140-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-017-0140-7
This article is cited by
-
End-to-end design of ingestible electronics
Nature Electronics (2024)
-
A conformable phased-array ultrasound patch for bladder volume monitoring
Nature Electronics (2023)
-
Location-aware ingestible microdevices for wireless monitoring of gastrointestinal dynamics
Nature Electronics (2023)
-
Bio-impedance method to monitor colon motility response to direct distal colon stimulation in anesthetized pigs
Scientific Reports (2022)
-
Wearable, wireless, multi-sensor device for monitoring tissue circulation after free-tissue transplantation: a multicentre clinical trial
Scientific Reports (2022)