Abstract
Carbamazepine (CBZ) is a frequently-detected aqueous pharmaceutical due to its extensive use and persistence in the environment. CBZ could not be efficiently removed by biological processes which led to its disposal in natural water bodies. This study coupled the Fenton process as pretreatment with the activated sludge process for aqueous CBZ removal. Fenton degradation studies showed that the application of excessive reagents might cause a decrease in CBZ removal. Apparent CBZ degradation at neutral pH supported the use of the Fenton process as pretreatment for CBZ removal. Treated with the hybrid combination system, CBZ, COD, and TOC removal were enhanced. The EEM analysis evidenced the biodegradable intermediates formed during the hybrid process. CBZ degradation pathways were explored using LC-MS analytical results and literature studies. Given the low biodegradability of CBZ, using the Fenton process as a pretreatment before sequencing batch reactor-activated sludge (SBR-AS) is an alternative to improve the aqueous CBZ treatment.
Similar content being viewed by others
Introduction
The occurrence of contaminants of emerging concern (CEC) in aquatic systems such as wastewater, groundwater, and drinking water has drawn great attention during the past few decades. Drug residues, also recognized as pharmaceutically active compounds, are one of the influential categories of CEC due to their possible detrimental impacts on aquatic life and human health1,2. Drug residues entered the hydrological circulation in a variety of routes, including bodily excretion, improper disposal of unwanted/expired drugs, discharge of untreated or ineffectively treated wastewater, etc3,4. Since over 3000 pharmaceutical ingredients are used nowadays, these substances that are released into the environment can pose serious threats to the ecosystem by causing negative effects on organisms and bioaccumulate at trace levels5. Moreover, pharmaceutically active compounds may enter human food chains through source water abstraction and drinking water distribution systems if the substances were not removed by treatment plants6.
Carbamazepine (CBZ), a popular anticonvulsant and mood-stabilizing drug used to treat epilepsy and bipolar disorder, has been one of the most frequently detected pharmaceutical compounds in the environment7. CBZ is oftentimes discharged from households and hospitals and reaches municipal wastewater treatment plants (WWTPs)4,6,8,9. Due to its molecular structure and chemical properties, CBZ showed persistence in conventional treatment processes4. Although CBZ removals by several well-known advanced technologies were reported, few of them were demonstrated to be effective for CBZ removal in WWTPs10. CBZ has been proposed as an anthropogenic marker of sewage contamination to assess water treatment efficiency11,12. The frequent release of CBZ residuals leads to its widespread occurrence in natural water bodies. The CBZ distribution may be further facilitated due to strengthened atmospheric and hydrological circulations caused by the global warming effect10.
Recently, different physical, chemical, and biological treatment processes have been employed to remove CBZ from wastewater. The activated carbon separation and nanofiltration were the main physical technologies that showed an excellent treatment performance13,14. However, the issues of membrane/adsorbent regeneration, efficiency maintenance, and high operation cost need to be considered when applied to the real wastewater treatment15. Advanced oxidation processes (AOPs) have been reported to be efficient to remove aqueous CBZ in laboratory studies5,16 and the Fenton oxidation possesses several advantages for in-situ application, such as effective performance, simplicity in design, and low cost of operation. The Fenton process decomposes CBZ by generating hydroxyl radicals from hydrogen peroxide catalyzed by ferrous ions, and the hydroxyl radical act as the principal component in the Fenton-related degradation systems17,18. Nevertheless, the practical applications of the Fenton process are constrained by the acidic reacting environment required for the best performance, or the possible formation of detrimental degradation by-products due to incomplete mineralization19. Since the pharmaceutical compounds could be toxic to microorganisms, the CBZ removal using conventional biological processes such as activated sludge was not favored10. De la Cruz et al.20 assessed the degradation of several micropollutants at a given municipal WWTP with the traditional activated sludge process. No CBZ degradation was observed, implying that the biological treatment was ineffective and an additional process was needed if CBZ removal was expected.
The classic Fenton process generates the hydroxyl radical which has a strong oxidizing ability without selectivity. It can decompose the organic compounds easily, forming oxidation intermediates or achieving mineralization with a sufficient amount of oxidants. Although the contaminants can be fully degraded by the Fenton process, the mineralization rate of pharmaceutical compounds remains relatively low12,21. The metabolites formed during ozonation and sonolysis of CBZ have been reported to be readily biodegradable22,23,24. In other words, AOPs may not mineralize CBZ completely, but they may reduce toxicity and increase its biodegradability, suggesting that the AOP may act as a pretreatment process before a biological treatment25.
In light of the above statements, this study aimed to explore the possibility of using the Fenton pretreatment to treat the manufacturing wastewater containing CBZ before merging with wastewater streams from other sectors (i.e., offices, dormitories, restaurants) in an industrial/pharmaceutical manufacturing facility. Usually, wastewater in an industrial factory consists of water streams from various sectors, including business & operation offices, restaurants, dormitories, and manufacturing processes, the wastewater from which usually contains organic loadings resulting from domestic activities such as washing, showing, cooking, and excretion. The wastewater from manufacturing processes mostly contains the residuals of the raw materials. All these water streams from various sectors were merged and introduced into a conventional wastewater treatment plant (WWTP, usually containing an activated sludge process) before being discharged into the environment.
Thus, the Fenton pretreatment was expected to degrade/destroy the CBZ to form more biodegradable molecular fractions before mixing with other wastewater streams. The combined wastewater was subjected to the activated sludge treatment in the wastewater treatment system. The CBZ removal efficiencies by the Fenton process at various reagent dosages were evaluated, and the experimental condition with the optimal removal performance was used for subsequent AOP-SBR combination treatment. The degradation products were examined and the possible CBZ degradation pathways were investigated. Also, the overall removing performance of the combined system was evaluated, the future application of such a combination system was discussed, and possible implications were addressed.
Results and discussion
Degradation and mineralization of CBZ by Fenton process
For CBZ degradation by Fenton process, the initial solution pH was around 3 and the initial concentration of CBZ was 50 μM. The Fenton reagents concentrations were at the fixed [H2O2]: [Fe2+] ratio of 1:1 (starting from 100 μM to 10000 μM following the stoichiometry of the hydroxyl radical generation reaction in the Fenton system, Eq. (1)) and the results are shown in Fig. 1A–D. The CBZ demonstrated a degradation efficiency of 87.08% when H2O2 and Fe2+ were both at the concentrations of 100 μM. CBZ was completely decomposed in the first 30 s as the reagent dosage was increased above 500 μM. In contrast to the degradation efficiencies, the highest mineralization efficiency was 67.60% when H2O2 and Fe2+ were both at the concentrations of 3000 μM. Adding more reagents (both the oxidant and catalyst) to the system did not necessarily increase the mineralization because the excessive reagents might compete with the target contaminant for hydroxyl radicals.
Hydrogen peroxide plays an important role in the Fenton process, which acts as the precursor to generate hydroxyl radicals. Thus, experiments were conducted at a constant ferrous ion (i.e., the catalyst) concentration of 100 μM with various hydrogen peroxide (i.e., the oxidant) concentrations, and the results are shown in Fig. 1E, F. CBZ was degraded almost completely in 30 min after the reaction took place as the H2O2 concentrations were raised to 500 μM or more. In the presence of Fe2+, the higher H2O2 concentration resulted in a more efficient CBZ removal because of increased hydroxyl radical generation. The mineralization efficiency was around 5% at the 100 μM H2O2 concentration and remained relatively low (<15%) even when the H2O2 concentration increased to 2000 μM. The removal ratio became even lower when the H2O2 concentration further increased to 5000 μM. Figure 1 shows the incomplete mineralization of CBZ by the classic Fenton process. Hermosilla et al.26 also reported a similar result of poor TOC removal (i.e., around 20–30%) in phenol degradation by the classic Fenton process with 30,000 μM H2O2 and 800 μM Fe2+. A similar result was shown by Ye et al.27 that the pentachlorophenol mineralization at 2000 μM H2O2 and 5 ppm Fe2+ was less than 10%.
Equations (1)–(6) are the major reacting mechanism of the Fenton process19, in which an increase in H2O2 can enhance the generation of ·OH with the continuous supply of Fe2+ according to Eqs. (1), (5), (6),
However, the excessive H2O2 and Fe2+ are detrimental to the Fenton chain reaction because of the following reasons. First, excessive H2O2 may act as the hydroxyl radical scavenger. In Eq. (2), H2O2 can react with the generated ·OH reducing the available amount of ·OH for target compound degradation, and the hydroperoxyl radical (·HO2) is generated as well to compete with the target compound for hydroxyl radicals (Eq. (3))28,29. Second, the overdose of H2O2 accelerates its self-decomposition and decreases the available amount of H2O2 for ·OH generation (Eq. (4))27. Third, excessive Fe2+ promotes partial ·OH scavenging as indicated in Eq. (5)30. In short, the more Fenton reagents within the proportional range are in the system, the higher degradation and mineralization efficiencies might be observed. Overdosing H2O2 or Fe2+ is not conducive to the facilitation of the Fenton process.
The rate constants of Eqs. (1), (2), (5), (6) were 6331, 2.7 × 107 32, 3.2 × 108 33, and 0.0134 M−1 S−1, respectively. In the study by Xiao et al.35, the rate constant of CBZ reacting with ·OH was 4.63 × 109 M−1 S−1, which was higher than those of H2O2 and Fe2+interacting with ·OH. After the Fenton reaction was initiated, CBZ was degraded by the generated ·OH following Eq. (1). The excessive Fe2+ consumed the ·OH (Eq. (5)) so the overall removal and mineralization may be reduced. Also, the excessive H2O2 scavenged the ·OH (Eq. (2)) in a fashion faster than the reduction of Fe3+ to Fe2+ by H2O2 (Eq. (6)), resulting in the limited CBZ removal performance. Cui et al.36 proposed a conceptual threshold of H2O2 concentration. If under critical concentration, more H2O2 leads to a higher ·OH generation and promotes the decomposition of target contaminants. On the contrary, the degradation rate may decrease with an increase in oxidant dose if the critical concentration is reached. Similar results were observed in the present study. Meanwhile, the high degradation efficiency with a low mineralization rate implied that the ·OH could only destroy the CBZ structure into intermediates rather than mineralize CBZ.
Kinetics study of CBZ removal by Fenton process
In Fig. 1, the CBZ demonstrated a fast decomposition at the beginning and subsequent slow removal. A similar phenomenon was reported in the study by Chan and Chu37, in which a two-stage model including a rapid phase I degradation followed by a retarded phase II degradation to describe the Fenton kinetics was proposed. In rapid phase I, the Fenton reaction was characterized as a process with a rapid initial reaction due to the fast radical generation by a relatively high ferrous ion concentration. After the initial production and consumption of oxidative reagents, the Fenton process moved to the stagnant stage because of the short-lived property of hydroxyl radicals and the radical generation limitation caused by the retardation of the iron ions redox cycle. In the present study, the observed degradation of the target contaminant CBZ appeared to be a two-stage degradation. By using the linear regression method with the least square analysis, the second-order kinetic was found best fit with the data both in Phases I and II. The two kinetic rate constants were obtained by Eqs. (7) and (8).
where [CBZ]0 is the initial concentration of CBZ (i.e., 0.05 mM). The breakpoint time, tb (s), was assessed by calculating the intersection of the two regression lines of Phase I and II. Table 1 shows the calculated rate constants (k1 and k2) and breakpoint times (tb) at different experimental conditions.
The regression curves of CBZ at different Fenton reagent ratios are shown in Fig. 2. With the increasing [H2O2] at a given [Fe2+] (i.e., 50 μM), the degradation in Phase I was accelerated, increasing the k1 and reducing the breakpoint time. However, k1 reached its maximum at an H2O2 concentration of 2000 μM, instead of 5000 μM, indicating that the excessive H2O2 in Phase I decreased the CBZ removal. This observation showed that the excessive H2O2 increased the hydroxyl radical generation, but the resulting hydroxyl radicals may not necessarily react with the target compound and its organic fractions.
Effect of initial pH on the Fenton process
The Fenton process has been reported to be less efficient in contaminant removal in neutral conditions as compared to the operation in an acidic environment (i.e., pH = 3) because Fe2+ may be oxidized into Fe3+ at neutral pH, forming Fe(OH)3 precipitate by reacting with hydroxyl ions. Under such a circumstance, the reduction of Fe3+ to Fe2+ by H2O2 (Eq. (6)) becomes unfavorable, resulting in less available Fe2+ and a decrease in Fenton removal efficiency38. While H2O2 may auto-decompose in the neutral environment, its decrease in ·OH generation due to H2O2 self-depletion is less significant compared to that resulting from the decreased amount in ferrous ions39.
In the field practice, the wastewater usually has a pH value ranging between 6 and 940. If the Fenton process is applied in a neutral condition, maintaining a sufficient amount of Fe2+ for ·OH generation is important. To assure the success of the real application of the Fenton process, the CBZ degradation by the Fenton process was conducted in neutral conditions and the results are presented in Fig. 3. The CBZ degradation in the acidic condition is presented for comparison. Also, the wastewater stream from a manufacturing process usually contains little buffering capacity, and most of the dissolved substances are from the residuals of the raw materials.
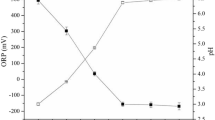
With the stoichiometry of 100 μM:100 μM for [H2O2]:[Fe2+], the degradation and mineralization efficiencies were 87.08% and 6.04% in acidic conditions and decreased to 76.05% and 4.41% in the neutral condition, respectively. A similar CBZ degradation result reported by Xiang et al.41 in nearly neutral circumstances (i.e., pH = 6) by FeS/PDS advanced oxidation process has shown a 71.2% removal in 60 min, indicating that the Fe2+ may remain soluble with an appreciable amount for ·OH production. CBZ contains an amide (azepine) group which was a strong electron-withdrawing functional group (EWG)42. The EWGs display a relatively stable anion property43 that may lead to the chelation between CBZ and ferrous ion to prevent Fe(OH)3 formation at neutral pH. More information regarding the CBZ-Fe3+ formation was included in the supplementary material. While Fe2+ remained in the solution, the Fenton reaction can proceed continuously, causing the observed CBZ removal and pH drop. It is worth noting that the pH value has dropped from 7 to 3.8 because of the emergence of intermediate acids during the degradation process. Reduction in pH shifted the system to an acidic condition and facilitated the degradation of CBZ.
Carbamazepine removal by combining the Fenton process with activated sludge
The CBZ degradation was conducted by combining the Fenton process with an activated sludge system, and the results are shown in Table 2. The low removal efficiency in the biological process only revealed that the activated sludge treatment process was not effective in removing CBZ from wastewater. Since CBZ is moderately hydrophobic, its removal via sorption onto activated sludge has been reported to range between 5 and 20%10,44. Hai et al.4 summarized the CBZ removal rates in various activated sludge systems and reported that the removal is almost negligible. Several studies also reported that CBZ exhibited very poor biodegradability and low sorption capacity in an activated sludge system45,46. Moreover, Urase and Kikuta47 have proposed that the maximum adsorption ratio of CBZ is less than 30%. As stated earlier, the electron-withdrawing function group of amide in the CBZ molecular structure makes it resistant to biodegradation48. In this study, the CBZ removal by the activated sludge system might be attributed to (1) biological degradation, (2) sludge sorption, and (3) complexation and sedimentation with coexisting compounds in the activated sludge system. However, CBZ removal by sorption seemed limited in a biological treatment system.
Since the COD removal ratio was calculated based on the COD removing performance after the pretreated solution was introduced into the SBR (i.e., 0 h of activated sludge treatment), the COD removal ratio would be affected by the COD base value. In neutral pretreatment, the degradation efficiency was less than that in the acidic environment, so the concentrations of CBZ and its intermediates in the influent were higher, resulting in a higher calculated COD removal ratio.
Given the apparent CBZ removal in the Fenton process at neutral conditions, a combination of the Fenton process and activated sludge treatment was employed to explore the CBZ removal in this hybrid process. The Fenton process was operated as a pretreatment to decompose CBZ before biodegradation, and the results are presented in Table 2 and Fig. 4. In Fig. 4, the degradation, mineralization, and COD removal showed that the activated sludge was able to degrade the oxidation by-products of CBZ after the Fenton pretreatment. The pretreatment with 200 μM H2O2 and 100 μM Fe2+ in acidic conditions showed the highest relative degradation and mineralization of 97.90% and 28.87%, respectively, implying that the oxidation by-products of CBZ were more readily biodegradable than the parent compound of CBZ.
The Zahn-Wellens test of CBZ and its oxidation (by-)products for evaluating their inherent biodegradability was performed by Monsalvo et al.5. Their results showed that CBZ inhibited microbial activity. The biodegradability of CBZ oxidation (by-)products by the Fenton process was enhanced and their toxicity was reduced. A similar result was reported by Nguyen et al.49 that the CBZ removal rate by the anoxic biological process in groundwater after Fenton-like pretreatment (i.e., PMS/Fe2+ system) has been enhanced. In this hybrid combination process, the Fenton process was used to decompose the non-biodegradable organic structures into fragments that would be readily degraded by microorganisms. The results showed that the utilization of the Fenton process as a pretreatment before activated sludge could be an alternative to improve aqueous CBZ removal.
EEMs for CBZ degradation by the hybrid system
The excitation-emission matrix (EEM) by fluorescence spectrometry has been employed to characterize the dissolved organic matter in water. The EEMs of different pretreatment conditions are shown in Fig. 5. The general EEM features and the positions of fluorophores reflecting organic molecular structures are presented in Fig. 5A. Area A (yellow empty box) reflects fluvic acid-like (FA-like) structures and emerges in the λex/λem range of 220–270 nm/380–550 nm. Area C (red empty box) implies the emergence of humic acid-like (HA-like) substances and occurs in the λex/λem range of 300–370 nm/ 400–500 nm. Area B (white empty box) suggests the occurrence of Tyrosine-like materials and takes place in the range of λex/λem around 225–237 nm/ 309–321 nm. Area T (pink empty box) represents Tryptophan-like molecules and is found in the range of λex/λem around 225–237 nm/ 340–381 nm50.
In Fig. 5D, G, J, apparent signals were observed in Area C after the Fenton oxidation in comparison to Fig. 5A, indicating the formation of oxidation products containing HA-like structures after CBZ degradation by ·OH attack. The pretreated solution was introduced into the aerated activated sludge reactor for biological treatment, and the signal intensities of Area C in Fig. 5E, H, K decreased because of the dilution effect. The peak intensities of Areas A, B, and T in Fig. 5B were considered as the reference background of activated sludge. Low biodegradation was observed without the Fenton pretreatment, as given in Table 2, and a similar situation was also observed in the EEMs spectrum shown in Fig. 5B, C. With the Fenton pretreatment, the biological removal of CBZ shifted the fluorescence signals towards the regions with shorter wavelengths (i.e., blue-shift, as shown in Fig. 5F, I, L). To be more specific, the peaks in Areas A and C were blue-shifted along the excitation/emission axes. The blue-shifting phenomenon of EEM signals may be associated with the following reasons: (i) the decomposition of aromatic moieties, (ii) the breakage of large molecules into smaller fragments, (iii) the decrease in the number of aromatic rings, (iv) the decrement of conjugated bonds in a chain structure, (v) the conversion of a linear system to a nonlinear system, and (vi) the elimination of particular functional groups including carbonyl, hydroxyl, and amine groups51. The blue-shifted results of the observed EEM spectrum indicated the decomposition of the aromatic structure of CBZ and the formation of molecular fractions through the activated sludge treatment. Tyrosine- and tryptophan-like (i.e., signals in Areas B and T) compounds are small organic matter predominant in wastewater and suitable for microbial degradation52. The occurrence of peaks in Area T represented the formation of organics under microbial oxidation after biological treatment, confirming the enhancement of the overall CBZ biodegradability.
Mass spectrometry for CBZ degradation by the hybrid system
The oxidation products of CBZ during the hybrid combination process were characterized using liquid chromatography-mass spectrometry (LC-MS). The chromatographic illustration of extracted mass spectrometry is presented in supplementary data (Supplementary Figs. 1–4). Table 3 shows the oxidation products that were also reported by the previous studies5,53,54. Hydroxycarbamazapine (m/z = 275.0791) has isomers (e.g., Carbamazepine-10,11-epoxide) due to the position variation of additional hydroxyl groups, leading to the peaks of different retention times, which was similar to hydroxyacridine (m/z = 196.0757). Most of the oxidation products have lower intensities at the [H2O2]:[Fe2+] ratio of 2:1 than that of 1:1, indicating that the oxidation products might be further oxidized with additional hydroxyl radicals generated by extra H2O2. In contrast, Acridine-9-carboxylic acid and C15H12N2O3 have higher intensities at [H2O2]: [Fe2+] ratio of 2:1 than 1:1, inferring that they may be formed as secondary oxidation products.
In a biological system, bacteria play a key role in contaminant removal. The organic contaminant was consumed and various intermediates might be formed, depending on the microbial community involved in the degradation55,56. The purpose of the present study is to evaluate the removal performance using the Fenton process as a pretreatment for an activated system treating pharmaceutical wastewater and the microbial composition of the biological system was not further determined. In a biological system, the degradation pathway may vary significantly depending on the participating bacterial genera. The reacting mechanism is not like those in chemical degradation, in which an oxidant may attack certain sites or atoms to form intermediates. This point can be reflected by the fact that very few studies regarding the degradation pathway of a biological wastewater treatment system were reported. In the present study, the LC-MS analysis showed that the degradation products by the Fenton oxidation (i.e., the Fenton pretreatment) were also observed in the sample collected after the combined system. These intermediates were not shown again in Fig. 6 for clarity. For the combined system, the signal intensities before and after the activated sludge system varied due to the biological reactions that might degrade the parament contaminant (while a portion of CBZ was not degraded in the pretreatment) and its intermediates. In the meantime, the biological reactions may combine two molecular fractions to form byproducts with higher molecular weight as well.
Five unidentified compounds (P1~P5) listed in Table 4 were detected in the single batch activated sludge treatment experiments of CBZ. P1, P2, and P3 were the oxidation products that were also found in the Fenton oxidation, implying that the activated sludge and Fenton treatment might have a few common oxidation pathways that generated similar products. P4 and P5 were the CBZ biodegradation products that did not appear in the Fenton oxidation. The intensities of these five products increased after a 4-h biological reaction, evidencing themselves as the biodegradation products.
Six different m/z ratios, which were P1 to P5, and hydroxycarbamazepine, were further discussed to evaluate the degradation mechanism of the combined treatment system. The degradation products occurring at the m/z= 275.0791 showed a decreasing trend of their intensities in the activated sludge treatment, indicating that these compounds could be further biodegraded after the Fenton oxidation. In the hybrid system, P1 was removed at the beginning of the biodegradation. The concentration of P2 and P3 increased as the biodegradation continued, implying that the formation of P2 and P3 might occur through the decomposition of the resulting intermediates from the Fenton process. The observed intensities of P4 were enhanced after 3 h, suggesting that P4 is a later degradation product. The MS signal of P5 increased during the activated sludge treatment and the double peaks showed the possibility of isomer existence. The exact compound formulas and chemical structures of unknown CBZ degradation products were not observed in this study.
Degradation pathway of CBZ by the hybrid system
Based on the products identified by LC-MS, the CBZ degradation pathway was proposed (Fig. 6). The reactions in the Fenton process were mostly hydroxylation due to the involvement of ·OH, and the hydroxylation preferentially happened at the sites with higher frontier electron density (FED)54. The generated ·OH directly attacked the unsaturated double bond on the benzene ring and/or the central heterocycle of CBZ, forming hydroxylated products (i.e., hydroxycarbamazepine) through the addition of ·OH, and the resulting hydroxycarbamazepine could be further transformed to C15H12N2O357. The potential energy surface profiles for ·OH reactions with CBZ were studied by Xiao et al.35. For ·OH reactions, all reactions were thermodynamically favorable except for the single electron transfer pathway and two additional carbon sites (i.e., C4 and C5 of CBZ). Thus, radical addition on the unsaturated carbon bonds of the heterocyclic and bilateral aromatic rings was possibly the dominant oxidation pathway due to its low energy barrier, forming the hydroxylated products of hydroxycarbamazepine.
Hydroxycarbamazepine underwent further hydroxylation at the olefinic double bond on heterocycle and carbamazepine-10,11-epoxide was formed through an elimination step58. Dihydroxycarbazepine and carbamazepine-o-quinone formation occurred through continuous ·OH attacks of carbamazepine-10,11-epoxide. With hydrogen rearrangement, the ring size of the central heterocycle was reduced and acridine-9-carbaldehyde was formed. Subsequently, the contraction process could happen on the ring of dihydroxycarbazepine and convert the formed molecule into acridine59. Acridine-9-carbaldehyde is an important intermediate for the ring-cleavage process and might be hydroxylated to other oxidized compounds by ·OH (i.e., acridone, acridine-9-carboxylic acid, acridine, hydroxyacridine). Degradation products might tend to break down via the ring cleavage process and generate low molecular weight compounds such as single aromatic ring acids and simple organic acids53. The unknown degradation products P1–P5 are also shown in Fig. 6. P1, P2, and P3 were found both after the Fenton oxidation and biodegradation; P4 and P5 were found only after the activated sludge treatment. For the hybrid system, the molecular structures in Tables 3 and 4 were observed in the effluent with different concentration levels as compared to the effluent from the Fenton pretreatment.
In summary, Carbamazepine removal by the Fenton process was investigated at various H2O2 and Fe2+ concentrations. At the fixed [H2O2]: [Fe2+] ratio of 1:1, CBZ removal reached almost 100% when [H2O2] was 500 μM or above. The best mineralization (67.60%) occurred at [H2O2]: [Fe2+] ratio of 3000:3000, showing that the optimum reagent dosage for best CBZ removal was not necessarily having the highest applied doses. With a given Fe2+ concentration, increasing H2O2 concentration may not necessarily promote mineralization. Excess in H2O2, and Fe2+ could be the hydroxyl radical scavengers in the Fenton chain reactions, causing the reduction in CBZ mineralization. The CBZ removal by the Fenton process was assessed using a two-stage kinetic model and the breakpoint time was found shorter as the Fenton reagent concentrations increased. The second-order kinetic model was found best fitted with the CBZ degradation in both stages.
In the activated sludge process, the CBZ removal ratio was 10.03%, implying that the physicochemical properties of CBZ showed resistance to the degradation by the activated sludge. In the combined treatment system, the CBZ treatment efficiency of 80.70% was achieved by the Fenton pretreatment under neutral conditions, thus suggesting that it is promising to combine the Fenton pretreatment and biological treatment for CBZ removal in neutral pH conditions, which could solve the practical problem of pH adjustment when applying the classic Fenton process to treat the real wastewater. In this study, the hybrid combination system, in which CBZ was pretreated by the Fenton process and the resulting (by-)products were introduced into a biological process, was effective in removing CBZ.
The degradation products generated from the Fenton process and activated sludge were analyzed by EEM and LC-MS. The blue-shifted signal inferred the decomposition of aromatic rings and the formation of smaller fragments. Tyrosine- and tryptophan-like signals that represent the organics subjected to microbial oxidation appeared after the biological treatment, supporting the enhancement of the overall CBZ biodegradability using the hybrid combination process. This study showed the possibility of using the Fenton process as a pretreatment before the conventional biological treatment when treating aqueous emerging contaminants that contain electron-withdrawing functional groups in the structure.
Implications for field practices
The present study demonstrated the feasibility of using a hybrid combination of the Fenton and activated sludge processes to treat CBZ in industrial wastewater. As reported in the literature, the Fenton process is effective when operating in acidic conditions. However, our investigation showed that CBZ degradation by the Fenton process at neutral conditions was >75% due to its electron-withdrawing molecular structure, implying the possibility of using the Fenton process as a pretreatment to degrade CBZ without further pH adjustment in the field application.
With the Fenton pretreatment, the CBZ molecules were degraded into smaller organic fractions before biological treatment (i.e., activated sludge in the present study). By inspecting the treatment efficiencies for the inflow, flow after the Fenton pretreatment, and outflow from the activated sludge, the COD removal was increased as the wastewater traveled along with the treatment process, showing the potential to include the Fenton pretreatment in a biological process. However, the improvement of treatment efficiency for TOC was not as significant as for COD. For effluent control, BOD/ COD (instead of TOC) was often regulated since the main function of a biological system is to decrease oxygen demand rather than to mineralize the target compound. The hybrid combination system is especially promising in treating pharmaceutical wastewater containing target compounds that have functional groups with strong electron-withdrawing ability.
In a pharmaceutical factory, wastewater streams from different departments (e.g., offices, restaurants, dormitories, manufacturing processes, etc.) were collected and introduced into a wastewater treatment system to meet the required regulation. The wastewater from manufacturing processes mostly contains raw materials (e.g., CBZ in this study), in which the Fenton pretreatment could be applied before its mixing with other wastewater streams to avoid the scavenging effects by co-dissolved organic matters. On the other hand, wastewater may contain trace inorganic elements (e.g., Fe2+ and Fe3+)60 which can trigger the Fenton reaction and reduce the addition of required catalyst.
Methods
Chemicals and reagents
The reagent grade CBZ with >99% purity was purchased from Sigma-Aldrich. For the Fenton process, reagent-grade chemicals H2O2, FeSO4, and Na2S2O3 were obtained from Sigma-Aldrich, Avantor, and Alfa Aesar, respectively. The deionized water was used for solution preparation. For pH adjustment, H2SO4 and NaOH were used to adjust the solution pH to 3 before Fenton experimentation and to 7 before the solution was introduced into the biological process. For the HPLC and LC-MS mobile phase, the reagent grade methanol was purchased from Honeywell.
Fenton treatment process
The initial concentration of CBZ in Fenton experiments was 50 μM, and the acidic FeSO4 stock solution was added to make a 1000 mL carbamazepine solution under given experimental conditions. Sulfuric acid was used to adjust pH to 3 to ensure the Fenton process was operated in an optimal environment. The degradation experiment started with the addition of a given concentration of hydrogen peroxide into the solution, and the pH was measured. Samples were collected at the beginning, 30, 60, 120, 300, 600, 1800 s after the reaction took place. Sodium thiosulfate solution was used to quench the reaction immediately after samples were collected. Samples were filtered through the 0.22 μm filter and analyzed using HPLC, TOC, EEM, and MS.
Similar to those experiments at pH 3, the Fenton degradation was also conducted at pH 7 using the Fenton reagent doses that had the optimal treatment efficiency at pH 3. The pH value was adjusted by adding H2SO4 and/or NaOH before the Fenton reaction started. However, the buffer addition is not considered in the present study due to the interference between ferrous ions and buffer solution, causing a reduction in Fenton degradation under neutral conditions61,62.
Sequencing batch reactor operation
For the activated sludge treatment process, a sequencing batch reactor (SBR) was designed to be the bioreactor. The SBR reactor has a working volume of 4 liters and was equipped with an aquarium-type air pump and aeration disc diffuser at the bottom of the reactor for appropriate aeration (maintaining the dissolved concentration above 3.0 mg/L). SBR was operated sequentially with a cycle time of 4 h including filling (15 min), aeration (210 min), settling (10 min), and discharge (5 min). The inoculum of activated sludge was taken from the wastewater treatment plant in Tucheng Industrial Park in New Taipei City, Taiwan, and operated in the laboratory with synthetic wastewater for one month as the adaptation period. The synthetic wastewater contained a fixed ratio of COD, nitrogen, and phosphorus at 100:5:1 for maintaining the bacteria growth in the SBR. The main carbon, nitrogen, and phosphorus sources were CH3COONa 3H2O (300 mg/L COD), NH4Cl (15 mg/L), and KH2PO4 (3 mg/L), respectively. CaCl2·2H2O (9.2 mg/L), MgSO4·7H2O (25.6 mg/L), and nutrient solution (25 μL/L) were also used to prepare the synthetic wastewater. The nutrient solution consisted of the following compounds per liter: FeCl3 (9 g), H3BO3 (1.5 g), CuSO4·5H2O (0.3 g), KI (1.8 g), MnCl2·4H2O (0.6 g), Na2MoO4·2H2O (4.2 g), ZnSO4·7H2O (1.2 g), CoCl2·6H2O (1.5 g), and EDTA (1 g).
In the hybrid combination of Fenton and activated sludge process, CBZ was treated by Fenton pretreatment for 30 min and subsequently introduced into the SBR for biological treatment. The solution pH was adjusted to neutrality before introduction into SBR to ensure the sludge bacteria activity would not be inactivated by the acidic solution. Being introduced into SBR, samples were collected 1, 2, 3, and 4 h after the activated sludge degradation and were analyzed by HPLC, TOC, EEM, and MS.
Analytical methods
The CBZ concentrations were quantified by Thermo Scientific Ultimate 3000 HPLC with Agilent HC-C18(2) 250 × 4.6 mm, 5 μm column, and ultraviolet detector at a wavelength of 286 nm. The sample volume injected was 50 μL and the flow rate was 0.6 mL/min. The column temperature was maintained at 30 °C. Methanol and deionized water were the mobile phase reagents and consisted at 60:40 for 15 min detection duration. Mass spectrometer MicrOTOF-QII from Bruker was used to obtain the mass spectra for peak identifications. The following settings were used: carbamazepine and its degradation products were detected under electrospray ionization (ESI) in the positive ion mode. The dry gas flow rate was set to 6.9 L/min and the dry heater was at 180 °C. The capillary voltage was set to 4500 V and the end plate offset to −500 V. MS data were recorded in the full scan mode in the range from 50 to 1000 m/z.
Total organic carbon (TOC) was measured using OIA 1030W TOC analyzer by the proven heated persulfate wet oxidation technique to analyze organic contamination levels in liquid samples. The EEMs were illustrated using a Perkin Elmer LS-45 fluorescence spectrometer equipped with FL WINLAB software for data processing. The system comprised a Xenon arc lamp as a radiation source, and the excitation and emission slits were fixed at 10 nm. EEMs were recorded in the wavelength range of λem =250–600 nm, λex=200–500 nm both with a step width of 5 nm. The scan speed was 500 nm/min in the 3D emission scan mode.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its supplementary materials.
Code availability
No computing code was used in this study.
References
Esplugas, S., Bila, D. M., Krause, L. G. & Dezotti, M. Ozonation and advanced oxidation technologies to remove endocrine disrupting chemicals (EDCs) and pharmaceuticals and personal care products (PPCPs) in water effluents. J. Hazard Mater. 149, 631–642 (2007).
Wilkinson, J., Hooda, P. S., Barker, J., Barton, S. & Swinden, J. Occurrence, fate and transformation of emerging contaminants in water: An overarching review of the field. Environ. Pollut. 231, 954–970 (2017).
Luo, Y. et al. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 473-474, 619–641 (2014).
Hai, F. et al. Carbamazepine as a possible anthropogenic marker in water: occurrences, toxicological effects, regulations and removal by wastewater treatment technologies. Water 10, 107 (2018).
Monsalvo, V. M. et al. Application of Fenton-like oxidation as pre-treatment for carbamazepine biodegradation. Chem. Eng. J. 264, 856–862 (2015).
Balakrishna, K., Rath, A., Praveenkumarreddy, Y., Guruge, K. S. & Subedi, B. A review of the occurrence of pharmaceuticals and personal care products in Indian water bodies. Ecotoxicol. Environ. Saf. 137, 113–120 (2017).
Ferrer, I. & Thurman, E. M. Analysis of 100 pharmaceuticals and their degradates in water samples by liquid chromatography/quadrupole time-of-flight mass spectrometry. J. Chromatogr. A 1259, 148–157 (2012).
Lapworth, D. J. et al. Deep urban groundwater vulnerability in India revealed through the use of emerging organic contaminants and residence time tracers. Environ. Pollut. 240, 938–949 (2018).
Ramaswamy, B. R., Shanmugam, G., Velu, G., Rengarajan, B. & Larsson, D. G. J. GC–MS analysis and ecotoxicological risk assessment of triclosan, carbamazepine and parabens in Indian rivers. J. Hazard. Mater. 186, 1586–1593 (2011).
Zhang, Y., Geissen, S. U. & Gal, C. Carbamazepine and diclofenac: removal in wastewater treatment plants and occurrence in water bodies. Chemosphere 73, 1151–1161 (2008).
Kumar, A., Batley, G. E., Nidumolu, B. & Hutchinson, T. H. Derivation of water quality guidelines for priority pharmaceuticals. Environ. Toxicol. Chem. 35, 1815–1824 (2016).
Marcelo, V. O., Salette, R., Jose, L. F. C. L. & Marcela, A. S. Analytical features of diclofenac evaluation in water as a potential marker of anthropogenic pollution. Curr. Pharm. Anal. 13, 39–47 (2017).
Kårelid, V., Larsson, G. & Björlenius, B. Pilot-scale removal of pharmaceuticals in municipal wastewater: comparison of granular and powdered activated carbon treatment at three wastewater treatment plants. J. Environ. Manag. 193, 491–502 (2017).
Simon, A., Price, W. E. & Nghiem, L. D. Effects of chemical cleaning on the nanofiltration of pharmaceutically active compounds (PhACs). Sep. Purif. Technol. 88, 208–215 (2012).
Grover, D. P., Zhou, J. L., Frickers, P. E. & Readman, J. W. Improved removal of estrogenic and pharmaceutical compounds in sewage effluent by full scale granular activated carbon: impact on receiving river water. J. Hazard Mater. 185, 1005–1011 (2011).
Bernabeu, A. et al. Solar photo-Fenton at mild conditions to treat a mixture of six emerging pollutants. Chem. Eng. J. 198-199, 65–72 (2012).
Dolatabadi, M., Świergosz, T. & Ahmadzadeh, S. Electro-Fenton approach in oxidative degradation of dimethyl phthalate—the treatment of aqueous leachate from landfills. Sci. Total Environ. 772, 145323 (2021).
Dolatabadi, M., Ghaneian, M. T., Wang, C. & Ahmadzadeh, S. Electro-Fenton approach for highly efficient degradation of the herbicide 2,4-dichlorophenoxyacetic acid from agricultural wastewater: Process optimization, kinetic and mechanism. J. Mol. Liq. 334, 116116 (2021).
Fan, C., Tsui, L. & Liao, M.-C. Parathion degradation and its intermediate formation by Fenton process in neutral environment. Chemosphere 82, 229–236 (2011).
De la Cruz, N. et al. Degradation of emergent contaminants by UV, UV/H2O2 and neutral photo-Fenton at pilot scale in a domestic wastewater treatment plant. Water Res. 47, 5836–5845 (2013).
Ustün, G. E., Solmaz, S. K., Morsünbül, T. & Azak, H. S. Advanced oxidation and mineralization of 3-indole butyric acid (IBA) by Fenton and Fenton-like processes. J. Hazard Mater. 180, 508–513 (2010).
Hu, L. et al. Oxidation of carbamazepine by Mn(VII) and Fe(VI): reaction kinetics and mechanism. Environ. Sci. Technol. 43, 509–515 (2009).
Kosjek, T., Andersen, H. R., Kompare, B., Ledin, A. & Heath, E. Fate of carbamazepine during water treatment. Environ. Sci. Technol. 43, 6256–6261 (2009).
Prado, M. et al. Removal of emerging contaminant and fouling control in membrane bioreactors by combined ozonation and sonolysis. Int. Biodeterior. Biodegrad. 119, 577–586 (2017).
Khan, N. A., Khan, A. H., Tiwari, P., Zubair, M. & Naushad, M. New insights into the integrated application of Fenton-based oxidation processes for the treatment of pharmaceutical wastewater. J. Water Process Eng. 44, 102440 (2021).
Hermosilla, D., Cortijo, M. & Huang, C. P. The role of iron on the degradation and mineralization of organic compounds using conventional Fenton and photo-Fenton processes. Chem. Eng. J. 155, 637–646 (2009).
Ye, Z., Sirés, I., Zhang, H. & Huang, Y.-H. Mineralization of pentachlorophenol by ferrioxalate-assisted solar photo-Fenton process at mild pH. Chemosphere 217, 475–482 (2019).
Rahim Pouran, S., Abdul Aziz, A. R. & Wan Daud, W. M. A. Review on the main advances in photo-Fenton oxidation system for recalcitrant wastewaters. J. Ind. Eng. Chem. 21, 53–69 (2015).
Vedrenne, M. et al. A ferrous oxalate mediated photo-Fenton system: Toward an increased biodegradability of indigo dyed wastewaters. J. Hazard. Mater. 243, 292–301 (2012).
Monteagudo, J. M., Durán, A., Martín, I. S. & Aguirre, M. Catalytic degradation of Orange II in a ferrioxalate-assisted photo-Fenton process using a combined UV-A/C–solar pilot-plant system. Appl. Catal. B: Environ. 95, 120–129 (2010).
Metelitsa, D. I. Mechanisms of the hydroxylation of aromatic compounds. Russian Chem. Rev. 40, 563–580 (1971).
Christensen, H., Sehested, K. & Corfitzen, H. Reactions of hydroxyl radicals with hydrogen peroxide at ambient and elevated temperatures. J. Phys. Chem. 86, 1588–1590 (1982).
Stuglik, Z. & PawełZagórski, Z. Pulse radiolysis of neutral iron(II) solutions: oxidation of ferrous ions by OH radicals. Radiat. Phys. Chem. (1977) 17, 229–233 (1981).
Walling, C. & Goosen, A. Mechanism of the ferric ion catalyzed decomposition of hydrogen peroxide. Effect of organic substrates. J. Am. Chem. Soc. 95, 2987–2991 (1973).
Xiao, R. et al. Experimental and theoretical insight into hydroxyl and sulfate radicals-mediated degradation of carbamazepine. Environ. Pollut. 257, 113498 (2020).
Cui, K. et al. Fenton oxidation kinetics and intermediates of nonylphenol ethoxylates. Environ. Eng. Sci. 31, 217–224 (2014).
Chan, K. H. & Chu, W. Model applications and mechanism study on the degradation of atrazine by Fenton’s system. J. Hazard Mater. 118, 227–237 (2005).
Boye, B., Dieng, M. M. & Brillas, E. Degradation of herbicide 4-chlorophenoxyacetic acid by advanced electrochemical oxidation methods. Environ. Sci. Technol. 36, 3030–3035 (2002).
Badawy, M. I., Ghaly, M. Y. & Gad-Allah, T. A. Advanced oxidation processes for the removal of organophosphorus pesticides from wastewater. Desalination 194, 166–175 (2006).
Popa, P. et al. Study of physico-chemical characteristics of wastewater in an urban agglomeration in Romania. Sci. World J. 2012, 549028 (2012).
Xiang, W. et al. Efficient degradation of carbamazepine in a neutral sonochemical FeS/persulfate system based on the enhanced heterogeneous-homogeneous sulfur-iron cycle. Sep. Purif. Technol. 282, 120041 (2022).
Yang, S. et al. Understanding the factors controlling the removal of trace organic contaminants by white-rot fungi and their lignin modifying enzymes: a critical review. Bioresour. Technol. 141, 97–108 (2013).
Pelzer, K. M., Cheng, L. & Curtiss, L. A. Effects of functional groups in redox-active organic molecules: a high-throughput screening approach. J. Phys. Chem. C. 121, 237–245 (2017).
Wijekoon, K. C. et al. The fate of pharmaceuticals, steroid hormones, phytoestrogens, UV-filters and pesticides during MBR treatment. Bioresour. Technol. 144, 247–254 (2013).
Martínez-Alcalá, I., Guillén-Navarro, J. M. & Fernández-López, C. Pharmaceutical biological degradation, sorption and mass balance determination in a conventional activated-sludge wastewater treatment plant from Murcia, Spain. Chem. Eng. J. 316, 332–340 (2017).
min, X. et al. Sorption and biodegradation of pharmaceuticals in aerobic activated sludge system: A combined experimental and theoretical mechanistic study. Chem. Eng. J. 342, 211–219 (2018).
Urase, T. & Kikuta, T. Separate estimation of adsorption and degradation of pharmaceutical substances and estrogens in the activated sludge process. Water Res. 39, 1289–1300 (2005).
Tadkaew, N., Hai, F. I., McDonald, J. A., Khan, S. J. & Nghiem, L. D. Removal of trace organics by MBR treatment: the role of molecular properties. Water Res. 45, 2439–2451 (2011).
Nguyen, H. T., Adil, S., Cho, K., Jeong, S. & Kim, E.-J. Improvement of carbamazepine removal through biodegradation coupled with peroxymonosulfate-based Fenton oxidation. J. Environ. Chem. Eng. 10, 108150 (2022).
Cui, Y., Wu, Q., Yang, M. & Cui, F. Three-dimensional excitation-emission matrix fluorescence spectroscopy and fractions of dissolved organic matter change in landfill leachate by biological treatment. Environ. Sci. Pollut. Res. 23, 793–799 (2016).
Valencia, S., Marín, J. M., Restrepo, G. & Frimmel, F. H. Application of excitation–emission fluorescence matrices and UV/Vis absorption to monitoring the photocatalytic degradation of commercial humic acid. Sci. Total Environ. 442, 207–214 (2013).
Henderson, R. K. et al. Fluorescence as a potential monitoring tool for recycled water systems: a review. Water Res. 43, 863–881 (2009).
Vogna, D., Marotta, R., Andreozzi, R., Napolitano, A. & d’Ischia, M. Kinetic and chemical assessment of the UV/H2O2 treatment of antiepileptic drug carbamazepine. Chemosphere 54, 497–505 (2004).
Zhu, S., Dong, B., Wu, Y., Bu, L. & Zhou, S. Degradation of carbamazepine by vacuum-UV oxidation process: kinetics modeling and energy efficiency. J. Hazard. Mater. 368, 178–185 (2019).
Sauvêtre, A., May, R., Harpaintner, R., Poschenrieder, C. & Schröder, P. Metabolism of carbamazepine in plant roots and endophytic rhizobacteria isolated from Phragmites australis. J. Hazard. Mater. 342, 85–95 (2018).
Aukema, K. G. et al. In silico identification of bioremediation potential: carbamazepine and other recalcitrant personal care products. Environ. Sci. Technol. 51, 880–888 (2017).
De Laurentiis, E. et al. Photochemical fate of carbamazepine in surface freshwaters: laboratory measures and modeling. Environ. Sci. Technol. 46, 8164–8173 (2012).
Xu, J., Li, L., Guo, C., Zhang, Y. & Meng, W. Photocatalytic degradation of carbamazepine by tailored BiPO4: efficiency, intermediates and pathway. Appl. Catal. B: Environ. 130-131, 285–292 (2013).
Zhou, S. et al. Degradation of carbamazepine by UV/chlorine advanced oxidation process and formation of disinfection by-products. Environ. Sci. Pollut. Res. 23, 16448–16455 (2016).
Badakhshan, S., Ahmadzadeh, S., Mohseni-Bandpei, A., Aghasi, M. & Basiri, A. Potentiometric sensor for iron (III) quantitative determination: experimental and computational approaches. BMC Chem. 13, 131 (2019).
Chen, H.-Y. Why the reactive oxygen species of the fenton reaction switches from oxoiron(IV) species to hydroxyl radical in phosphate buffer solutions? A computational rationale. ACS Omega 4, 14105–14113 (2019).
Yoshimura, Y. et al. Effects of buffer solutions and chelators on the generation of hydroxyl radical and the lipid peroxidation in the Fenton reaction system. J. Clin. Biochem. Nutr. 13, 147–154 (1992).
Author information
Authors and Affiliations
Contributions
Y.-Y.L.: Methodology, software, formal analysis, investigation, visualization, writing-original draft. C.F.: Conceptualization, methodology, investigation, validation, funding acquisition, software, formal analysis, data curation, visualization, writing-review & editing, project administration, supervision, resources. F.H.: Data Curation, Visualization, Writing-review & Editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, YY., Fan, C. & Haque, F. Hybrid combination of advanced oxidation and biological processes for the micropollutant removal of carbamazepine. npj Clean Water 5, 60 (2022). https://doi.org/10.1038/s41545-022-00203-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41545-022-00203-z
This article is cited by
-
Roles and mechanisms of carbonaceous materials in advanced oxidation coupling processes for degradation organic pollutants in wastewater: a review
Biochar (2023)
-
Pharmaceuticals in hospital wastewaters: an analysis of the UBA’s pharmaceutical database
Environmental Science and Pollution Research (2023)