Abstract
Combining electrocoagulation with another process is a potential strategy for increasing the efficiency of water and wastewater pollutant removal. The integration of advanced oxidation processes (AOPs) and electrocoagulation (EC) demonstrates improved performance. The mechanism of the EC combined with ozone (O3), hydrogen peroxide (H2O2), sulfate radicals, electrooxidation (EO), Fenton/electro-Fenton, and UV is discussed. This review sheds light on EC-AOP hybrid processes in terms of their mechanisms, development, challenges, and their potential application for the removal of contaminants of emerging concern (CECs). The majority of the articles claimed improved performance of the EC process when combined with AOP as a pre-treatment, especially in terms of removing recalcitrant contaminants. For instance, the integrated EC-Fenton/photo-Fenton processes have been shown to be a promising treatment to virtually complete removal of the phenolic compounds in oil refinery wastewater. In EC-EO process, boron doped diamond (BDD) anode, despite being costly electrode, has the highest oxidation potential and is therefore the most suitable type for the mineralization of organic pollutants. PFASs are more effective at being removed from water through zinc and Ti4O7 electrodes in EC-EO treatment. Furthermore, the peroxone and synergistic effects between O3 and coagulants played almost equal dominant role to removal of ibuprofen using hybrid EC-O3. However, enough data for conducting these integrated processes at industrial scale or with real wastewaters do not exist, and so there is a lack for comprehensive and systematic approaches to address complexity of such systems. Although a great number of papers were focused on the degradation of effluents from different industries, viruses, and pharmaceuticals, there is not sufficient research in terms of the removal of herbicides, pesticides, microplastics, and micropollutants.
Similar content being viewed by others
Introduction
Over the past few decades, a rise in the number of harmful pollutants has been observed as a result of industrial expansion and population growth. Contaminants of emerging concern (CECs), including personal care products, medicines, pesticides, flame-retardants, plasticizers, endocrine disruptors, surfactants, and polycyclic aromatic hydrocarbons (PAHs), and food additives have become an indispensable requirement. Despite their wide-ranging uses, CECs can also be detrimental to human health and other animals. Recently, the scientific community and regulatory authorities have become increasingly concerned about CECs’ widespread presence in surface and ground water sources due to their extensive use1.
The utility of electrochemistry in drinking/waste water treatment can be a cost-effective option. The majority of contaminants in water or wastewater may be eliminated or transformed to non-toxic forms using one or a combination of electrochemical methods. Electrochemical technologies have reappeared in the last three decades, due to cheaper power costs and stronger environmental rules and norms. The electrocoagulation process is the oldest and most widespread of the electrochemical processes used in water and wastewater treatment. Many water pollutants, such as anionic contaminants1, phenol2, sulfite and sulfate3, oil emulsions4,5,6, and anionic toxic colors7,8, were successfully removed using this process. It has also been shown to be a capable method for removing heavy metal ions9,10,11 from industrial effluents, including chromium12,13, copper14, nickel15 and arsenic16, boron17, zinc, manganese and mercury18, cadmium19, lead and silver20. Numerous reviews highlight the strengths of electrocoagulation (EC) technology for water and wastewater treatment21,22,23,24,25,26,27. This method has many advantages such as a minimal setup time, no requirement for chemicals to improve removal rate, high filterability of the generated sludge, high removal efficiency, and a simple and quick control procedure. On the other hand, it has several disadvantages, including: a reliance on non-renewable resources for electrical energy, which can be mitigated with the aid of using renewable resources like solar power28 and biogas29, and also the use of a costly medium for oxidation (sacrificial anode) in the treatment process. According to the literature30, adequate information for scaling up this technique from lab- to bench/industrial- scale is not provided. As a result, combining this process with other methods can enhance its effectiveness31,32,33,34.
CECs’ degradation can be enhanced by advanced oxidation processes (AOPs)35. Recalcitrant pollutants in environmental matrices can be effectively treated by AOPs36. Because of their ability to generate strong reactive oxygen species, advanced degradation of plentiful categories of pollutants by different AOPs has attracted tremendous attention from various researchers37,38. AOPs can convert contaminants into biodegradable intermediates or result in the complete/ultimate mineralization of contaminants39,40. Despite their advantages, AOPs have some drawbacks, such as forming more stable and toxic intermediates, the use of energy and chemicals, and the increase in treatment time and costs41,42, which can be reduced by adding electrocoagulation to the treatment process (different AOPs coupled with EC can be seen in Fig. 1).
Nevertheless, in terms of the removal of CECs and recalcitrant pollutants electrocoagulation may lack the required efficiency potential. For instance, it is declared that penicillin-based drugs cannot be degraded completely by EC, while AOPs are very effective for this reason43. It can thus be considered that, for treating highly polluted effluents and complete degradation of recalcitrant contaminants, EC integration with AOPs such as electrochemical oxidation, ozonation, Fenton, Electro-Fenton, hydrogen peroxide or sulfate radical addition, and UV irradiation can be a potential treatment option. As can be seen in Fig. 2, the terms associated with the applications of different AOPs and emerging contaminants removal are illustrated with the use of the VOS Viewer software. The data was recorded by ScienceDirect using the keywords “electrocoagulation”, “advanced oxidation processes”, “emerging contaminants”, and “water/wastewater treatment”. This issue has also attracted the attention of many researchers in the recent decade, especially since 2019 as it can be seen in Fig. 3a. What’s more, the percentage of research papers published in the recent decade, based on the AOPs that was incorporated into EC, is presented in Fig. 3b.
However, there are not much available data for the performance of such processes regarding the removal of newly concerned pollutants including microplastics, viruses, and pharmaceuticals since the topic is a state-of-art. Therefore, this comprehensive review focuses on integrated EC-AOPs processes in the last decade to shed light on the improvement in reactors design, mechanisms involved in the removal of emerging contaminants, as well as techno-economic analysis, crucial operating parameters, and kinetic models.
Hybrid electrocoagulation-electrooxidation
Typically, the electrocoagulation process (Fig. 4) is accompanied by flotation, in which the anodic reaction of coagulation is followed by the hydrogen production at the cathode and brings the flocs to the top of the water/wastewater. In this case, the process is called electrocoagulation/flotation. Anode and cathode materials are usually iron or aluminum and the reactions occurring around the anode are as follows44:
For aluminum anode:
At alkaline conditions:
At acidic conditions:
For iron anode:
At alkaline conditions:
At acidic conditions:
Cathodic reaction:
Moreover, throughout these reactions there is also the possibility of oxygen evolution nearby the anode according to the following the reaction:
It should be noted that for the iron electrode, another mechanism may also occur during which Fe3+ is produced. Al3+ and Fe2+ ions are strong coagulants that absorb most of the pollutants.
Electrooxidation is the oxidation of contaminants either by the formation of hydroxyl radicals (OH.) on the surface of anode or producing oxidants in the solution45, as shown in Eq. 945:
The anode material is the most important factor in electro-oxidation46. According to the anode material, the mechanism of oxidation can be divided into indirect electro-oxidation and direct electrochemical oxidation. In the former partial oxidation occurs whereas the latter can advance complete mineralization29,47. In the direct electrolysis, also named electrochemical oxygen transfer reaction, contaminants are oxidized after adsorption on the anode surface only by help of the removed electron and then an adsorbed organic radical is generated. Conversely, in the indirect electro-oxidation, also called mediated anodic oxidation, chemical oxidants are formed in-situ either by anodic oxidation such as generation of active chlorine, ozone and persulphate, or by cathodic reduction such as generation of hydrogen peroxide48.
The first stage of both mechanisms is the release of water molecules at the anode surface to produce adsorbed hydroxyl radicals as presented in Eq. 1045 where MOx represents the metal oxide anode.
The subsequent steps depend strongly on the nature of the anode materials, which is classified into “active” and “non-active” anodes for indirect electrochemical oxidation and direct electro-oxidation, respectively. The active anodes, used in indirect electro-oxidation, have higher oxidation states resulting in firmer interaction with the adsorbed hydroxyl radicals by further oxidation, forming higher oxides (Eq. 1145).
The surface redox couple MOx+1/MOx, denoted as chemisorbed active oxygen, is the mediator of the partial oxidation of organics on active anodes. As demonstrated in Eq. 1245, it is obvious that the anode returns to its original oxidation state, thus these are called non-sacrificial anodes. Generally, anodes with low overpotential of oxygen growth such as amorphous carbon, graphite, platinum, ruthenium dioxide, and iridium dioxide are categorized as “active” electrodes.
On the contrary, the non-active anodes are defined by a weak electrode hydroxyl radical interaction creating physiosorbed active oxygen, which promotes the complete mineralization of the organics to carbon dioxide (Eq. 1345).
Though, a competitive oxygen evolution side reaction (Eqs. 14–1545) happens in both of the chemisorbed and physiosorbed active oxygen evolution mechanisms and results in reduced anodic process efficiency and waste of energy. Mainly, electrodes with high over potential of oxygen evolution such as lead dioxide, antimony-doped tin oxide, or boron-doped diamond (BDD) are entitled as “non-active” anodes.
The whole reactions of both mechanisms of electro-oxidation are represented in Fig. 5 schematically as a full oxidation cycle49. The weak involvement of hydroxyl radical with non-active anodes imposes a need for great anodic potential for water oxidation (approximately 2.0 V per SHE)50. On the other hand, oxidation states cycle repeatedly in the electrochemical oxidation of the active electrodes. The BDD anode has the highest oxidation potential and is therefore the most suitable type for the mineralization of organic pollutants. The higher the over potential, the weaker the hydroxyl radicals in the anode will be and less energy will be used for the side reaction of water oxidation. The activity of “Oxygen evolution reactions” for Pt, IRO2-Ta2O5 and RuO2-TiO2 are higher which makes them less effective in pollutant mineralizing51.
Such mechanisms including formation of hydroxyl radicals (OH*), oxygen evolution by electrochemical oxidation of OH*, formation of the higher metal oxide (MO), oxygen evolution by chemical decomposition of the higher MO, electrochemical combustion of the organic compound via OH*, and electrochemical conversion of the organic compound (R) via the higher MO. Reproduced with permission from ref. 49. Copyright 2006 Royal Society of Chemistry.
As noted above anode material is the most important parameter in the process. In summary, advantages and disadvantages of each anode type is as follows50:
-
Graphite is cheap but ineffective and unstable.
-
Platinum is expensive with low-impact.
-
IrO2 and RuO2 are only effective for the mediated anodic oxidation.
-
Due to the low conductivity, SnO2 must be doped with Sb which is a toxic substance.
-
PbO2 is available and easily made, but unstable and should be doped with Bi, Co or Fe or mingled with Teflon.
-
TiO2 has low conductivity and often Ebonex® is used in electrooxidation. Ebonex® is a non-stoichiometric titanium oxide mixture contained of Magneli phase titanium oxides Ti4O7 and Ti5O9. It has a rather difficult and expensive way to be made and surface passivation under extreme settings may occur.
-
BDD is stable and effective but very expensive.
Another drawback of electrooxidation is that it works poorly when purifying effluents with a high concentration of suspended solids. In this case, suspended solids must first be treated using other methods, which can be accomplished by combining it with other techniques. Coupling electrocoagulation with electrooxidation provides a feasible combination that takes advantage of both processes. When colloidal particles are present, electrooxidation becomes impractical due to its significant operating time consumption. Electrocoagulation is used to speed up the process and remove colloidal elements and charged types from the water. While electrocoagulation may remove pollutants rapidly, it cannot do so entirely, whereas electrooxidation is a slow process, but it removes contaminants consistently52. Combining the benefits of two processes that provides quick and total pollutant removal is possible through sequential and simultaneous hybrid systems.
Electrocoagulation and electrooxidation are the most promising electrochemical treatment methods due to their low or no chemical needs and ease of use53. To study the effectiveness of the hybrid EC-EO process for the treatment of each type of water/wastewater, usually a batch mode is used. Once a batch system is employed to find the ideal operating parameters, a continuous mode can be used to find out the effects of pollutant concentration and flow rate and to see whether there is a correlation54. A few studies have compared batch and continuous reactors for integrated form of electrocoagulation and electrooxidation55. For instance, Lakshmi Kruthika et al.56 used a hybrid method with continuous and batch reactor systems. Using continuous and batch reactor systems, the highest removal of total organic carbon (TOC) was attained in the range of 38–54% and 80%, respectively. Linares Hernandez et al.57, on the other hand, investigated soft drink wastewater treatment in a batch-type reactor using an integrated of successive electrocoagulation and electrooxidation processes. As a result, at pH 8, only 27% and 85% of TOC could be eliminated by EC and EO, respectively.
For the treatment of a textile wastewater, Afaha et al.58 disclosed that the hybrid treatment method of EC-EO is a promising method since the COD reduction percentage rose from 76% to 97%. In a study for treating soluble coffee industrial plant wastewater59, an amazing formulation was also derived for estimating the total cost of the sequential process of EC-EO. In addition, anode material consumption (AMC), energy consumption, and sludge formation have all been taken into account not only for electrocoagulation but for electrooxidation. A linear increase in AMC was observed with the extension of the EC operation time (tEC). Anode material consumption increases linearly with applied current, according to Faraday’s Law (Eq. 16):
Another example of treatment by sequential EC-EO is the treatment of container washing wastewater by Nayr and Kara53. They observed that EC performed less well in the removal of soluble chemical oxygen demand (sCOD) than expected and so EO was used as a post-treatment to EC. As removal efficiency of color declined at the same length of time in the hybrid process, they inferred that EO was not practical and related it with aqueous solution’s colloidal particles. However, a very recent study in Canada on a similar wastewater, reached different results60. Azarian et al.61 observed that solo EC process was unable to treat the tannery effluent completely, but when electrooxidation was used as the post-treatment, results were very satisfactory. The question of whether EC should be utilized as the pre-treatment or post-treatment to EO was examined for treating poultry slaughterhouse/dairy wastewaters62. Descriptions of reviewed paper in this scope are provided in Table 1.
Landfill leachates as one of the most concerning problems of the current era are proved to be very toxic and difficult to degrade. In landfill leachate treatment, electrochemical techniques have gotten a lot of interest during the last five years63. In a recent work, to remove and degrade polyfluoroalkyl substances (PFASs) from water, Shi et al.64 combined electrocoagulation with electrooxidation, using zinc and stainless-steel in the former and Ti4O7 electrode in the latter. In Fig. 5, concentration profiles of three different PFAS-containing solutions that went through EC under various conditions are shown. PFASs elimination through EO is obvious for all of the EC-derived solutions. More than 95% PFAS removal was attained in 60 min of EO for Solution I (Fig. 6a), excluding PFBS (73.4%), and it was over 90% for Solution III (Fig. 6c) bar PFBS (52.3%), PFHxA (74.2%) and 4:2 FtS (85.9%). Degradation of all kinds of PFAS in Solution II (Fig. 6b) was satisfactorily high. Overall, they found that long-chain PFASs (C7–C10) was more effective at being removed from water by electrocoagulation with zinc anodes. As a consequence of the high current density and/or the high PFAS concentration during EC, foam was produced, promoting the separation of PFASs from the bulk solution, specifically for the long-chain PFASs. Also, viruses and bacteria in water and wastewater can be inactivated by sequential EC-EO65,66.
a Solution I: PFASs-laden flocs formed by EC at 0.3 mA.cm−2 with dissolved acid; b Solution II: PFASs-laden flocs prepared by EC at 5 mA.cm−2 with dissolved acid; c Solution III: foam made throughout EC at 5 mA.cm−2. Reprinted with permission from ref. 64. Copyright © 2021 Elsevier Ltd.
Maher et al.67 investigated four resistant estrogenic compounds, namely estrone (E1), 17β-estradiol (E2), estriol (E3), and 17α-ethynylestradiol (EE2) and found that for each estrogenic compound, the sequential EC and EO method was efficient by more than half over EO alone. The results revealed that EC as the pretreatment also reduced the energy consumption. Pesticide-containing effluent is another concern that has been shown to be treated very well by electrochemical processes68,69,70,71.
Hybrid electrocoagulation-ozone
The oxidation of numerous organic and inorganic compounds by ozone can be performed via two main mechanisms: direct oxidation as well as indirect oxidation using the free radicals’ generation including the hydroxyl radical (OH.), as shown by the Eqs. 17–2272:
Electrocoagulation (EC) coupled with ozone (O3) bubbles involves redox processes in which ozone decomposes into FeO2+ and hydroxyl radical (OH.) via reactions with Fe2+ ions and Fe3+ ions73. The following are the redox reactions that take place:
By adding radicals to organic compounds, hydrogen abstraction, and transferring electrons to the hydroxyl radicals, as a result the organic compounds of the medium are oxidized due to the hydroxyl radicals74. Equations 23–25 demonstrate a mutual activation between Fe2+ and O3. In EC mediums containing Fe2+, ozone decomposes more quickly to hydroxyl radicals. O3 bubbles within an EC system produce a catalytic O3/Fe2+ that forms FeO2+ which is an intermediate species reacting with water to produce hydroxyl radicals, HO ions, and Fe3+ covalent ions. Organic pollutants can decompose more rapidly through these mutual activation products, thus improving process efficiency75. The combination of O3 and EC treatment processes, especially for the removal of organic pollutants, has therefore been the subject of a wide range of research, the first of which was that of He et al.76 who tested the elimination of C.I. Reactive Yellow 84 by adding ozone to electrocoagulation process.
In order to treat real distillery industrial effluents, Asaithambi et al.77 used three distinct methods: EC, O3 and integrated EC-O3. In the hybrid process of EC-O3, in-situ active radicals attack and oxidize the organic substance as a result of the interaction between ozone and Fe2+ ions created by iron electrode oxidation, as indicated by Eqs. 6–8. In addition to chemical oxygen demand (COD), color was significantly lowered as a result. They reported similar observations for the COD removal from actual distillery wastewater78 and landfill leachate79. It is of concern that EC or the combinations of O3-EC processes outperformed ozonation standalone in removing both color and COD. The inadequate mass transfer of ozone from the gaseous phase to the liquid in which the organic contaminants might be the cause.
Ahangarnokolaei et al.80 proposed EC-O3 reactor (Fig. 7), which is enhanced coagulation under O3-induced rapid mixing in the first side, as well as flocculation under flow-induced slow mixing on the other side. It lowered floc degradation at high O3 dosages and improved removal rate. For integrated processes, especially simultaneous combination, superior performance was observed with less treatment times. When utilizing Al electrodes, the synergistic impact of EC and O3 on dye, COD and TOC removal was found to be more pronounced because of the increased ozone activation and decreased anode corrosion. They suggested the simultaneous process of EC (Al)–O3 and sequential process of EC (Fe)→O3 in their suggested reactor design as an innovative and appropriate solution for treating dye wastewater.
The EC-ozonation reactor with a middle wall was used to reduce floc breakage under ozonation-induced excessive turbulence. Reproduced with permission from ref. 80. Copyright © 2021 Elsevier Ltd.
The O3-assisted EC process was investigated by Bilinska et al.81 for the removal of Reactive Black 5. The high pH value of the water in the EC process lowered the efficacy of the aluminum electrodes, making the iron electrodes better conductors. In addition, a semi-batch stirred cell that held 1 L of 1 g/L ozone was tested and the color removal rate was 90% after 50 min. The O3 time was reduced from 50 to 10 min, saving five times as much as it would have saved had it not been pretreated with EC. Similarly, García-Morales et al.82 conducted a study for the dye removal in Denim wastewater and 66% color removal was reported when EC-O3 was run. According to their findings, second-order kinetics for color and turbidity removal in the O3-EC pulses method indicated that the efficiency achieved in the integrated process was dependent on the coupling of treatments (O3 and EC). Tanveer et al.83 also investigated the efficacy and practicality of EC/O3, EC/Fenton, and EC/photo-Fenton processes for the treatment of textile wastewater and demonstrated effective decolorization and removal of TDS, TSS, and COD from textile dye-bath effluents compared to the solo EC process. EC alone was able to eliminate 57% COD, but when combined with ozone the removal percentage reached to 77%. Aseman-Bashiz and Sayyaf84 concluded that solely EC could not profoundly effect on ofloxacin removal (only 19% after 90 min). Therefore, integrated process through O3 and peroxydisulfate was achieved higher removal rate of 98% under optimum conditions. Most importantly, such process easily coped with conventional issues of EC such as sludge production and electrode corrosion.
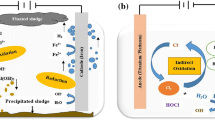
To investigate the effectiveness of EC/O3 in treating greywater, Barzegar et al.85 used a hybrid approach and observed that when the pH of greywater was near to neutral, the system worked effectively. In contrast to EC with Al electrode, EC with Fe electrode displayed strong catalytic activity for O3 activation. Also, when used in combination with the EC method for greywater treatment, O3 performed better than other chemical oxidants (peroxidisulfate, peroxymonosulfate, and hydrogen peroxide). Jin et al. conducted a research86 on the removal of ibuprofen by EC-O3 hybrid system showed that the peroxone (O3/H2O2) and synergistic effects between O3 and coagulants (SOC) had almost equal contribution to ibuprofen removal (Fig. 8). Also of note, the study of Das et al.87 that concentrated on the removal of COD, BOD, cyanide, and chloride from biological oxidation treated effluent of the steel plant. Results revealed that a rise in the volumetric mass transfer coefficient, Kla, as ozone production rate rose.
Based on the reactive oxygen species (ROS) detection and reactions on the electrodes, the synergistic effects between ozone and coagulants (SOC) were found to be involved in the E-HOC process. Reproduced with permission from ref. 86. Copyright © 2020 Elsevier Ltd.
It is also of notice that the electro-peroxone (E-peroxone) technique is an innovative update of the ozonation that generates hydrogen peroxide in-situ during ozonation from cathodic oxygen reduction at carbon-based cathodes (Eq. 26)88. The in-situ produced H2O2 can then be used through the peroxone reaction in order to boost the transformation of O3 into OH• (Eq. 27)88. Furthermore, compared to conventional O3, the E-peroxone process can dramatically reduce bromate formation in the treatment of bromide-containing water because O3 possesses shortened lifespan and quenching of hypobromous acid, which is a key intermediate for bromate formation in the O3-induced process, by electro-generated H2O2 (Eq. 28)89. When compared to ozonation, the E-peroxone technique can considerably enhance efficiency and decrease energy consumption for the removal of O3-resistant pollutants owing to the improvement of O3 transition to hydroxyl radicals by electro-generated H2O2. As a result, the E-peroxone method can significantly strengthen the performance of water and wastewater treatment by simply putting electrodes in O3 reactors90. Wang et al. treated shale gas fracturing flowback water by the sequential EC-Epeoxone (Fig. 9) and concluded that adding E-Peroxone after the EC process increased both current efficiency and enhancement factor (Eq. 55) 88. The latter implies that hydrolyzed Al species could have a synergistic effect in the ECP process, accelerating O3 decomposition to create hydroxyl radical. Hydrogen peroxide can also be added to the system instead of being generated in-situ91. All papers reviewed in this area are summarized in Table 2.
The transformation of enhanced ozone (O3) to hydroxyl radicals (OH.) by electro-generated H2O2, the E-peroxone process can considerably increase the efficiency and decrease the energy demand for the abatement of ozone-resistant ECs. Reproduced with permission from ref. 88. Copyright © 2018 Elsevier Ltd.
Many obstacles remain, however, preventing large-scale implementation of ozone-assisted electrocoagulation since there are far more significant issues regarding mass transfer of ozone gas to the liquid phase. As a result, finding a proper reactor design that facilitates high rates of ozone mass transfer to the liquid phase is the next step. The novel reactor system was employed by Ahangarkolei et al.80 (Fig. 7) are good attempts at achieving an appropriate reactor design, as demonstrated by the results. However, in a circular cross section reactor, mixing among the phases will be more effective than in a square cross section, which might lead to findings that are more positive. Secondly, the lack of any scale-up attempts for ozone-assisted electrocoagulation process is a major problem because the reactor design is crucial in optimizing the overall performance of the process and it often incorporates several phases, including the gas phase. Ozone generation on a large scale is also another problem that might be overcome by the use of electrochemical technology to create oxidants and simultaneous anodic oxidation92.
Peroxi-coagulation, and hybrid electrocoagulation-Fenton/electro-Fenton processes
In the Peroxi-electrocoagulation process, pollutants are eliminated by generating hydroxyl radicals owing to adding of H2O2 during the conventional electrocoagulation (EC). In this technique, organic pollutants are converted to H2O, CO2 and inorganic ions93. When hydrogen peroxide is introduced to the anode and cathode surfaces during electrocoagulation, in which Al electrodes are used, the following reactions occur94:
This causes chain reaction between the hydroxyl radical and an organic matter (R) and so oxidizes organic pollutants according to the following reactions95:
When iron is used instead of aluminum, the mechanism is different. By incorporating the Fenton reagent into an oxidation process, which results in the reaction of hydrogen peroxide, H2O2, and ferrous salt, Fe2+, to oxidize organic matter96. An enhanced form of the Fenton process, electro-Fenton is a method in which H2O2 is produced electrochemically in-situ under acidic circumstances. Cathodic Fenton processes and anodic Fenton processes are two different ways of describing the process, depending on how iron enters the system. One or both reagents Fe(II) and H2O2 are created in-situ in cathodic Fenton reactions, when iron is supplied as Fe (III) salt. The source of the ferrous ion involved in Fenton reaction in anodic Fenton processes (peroxi-coagulation) is a sacrificial iron anode97,98. The followings are the primary reactions that occur throughout the electro-Fenton process99:
Fe2+ and H2O2 are generated between the anode and cathode (Eqs. 35–38) and then hydroxyl radical can be electrogenerated in the bulk solution through the Fenton reaction (Eq. 39). Due to powerful attacks on aromatic rings or long chains, the hydroxyl radical can non-selectively transform difficult-to-degrade organic contaminants into small micro molecules. Furthermore, this process allows for coagulation100, which is precipitated by iron hydroxides produced as Fe(OH)n (n = 2, 3) (Eqs. 40–42). As a result of the combined actions of oxidation and coagulation, persistent contaminants may be more easily converted into biodegradable chemicals and even eliminated. A number of studies have shown that electro-Fenton process is more effective in acidic conditions, particularly at pH levels of 3 or below101. However, if pH is less than three, H2O2 availability may be reduced. This leads to coagulate contaminants by Fe(OH)n produced at higher pH because of the enhanced decomposition of H2O2 into O2 and H2O due to an increase in pH102.
When hydrogen peroxide is not produced in situ and is added to the EC system, following reactions take place:
Reduction of the ferric species formed by the ferrous ion regeneration with hydrogen peroxide is the primary means of propagating this process. More ferrous ions are consumed than they are created in a certain period. Hydroxyl radicals, on the other hand, have the ability to swiftly degrade ferrous ions. In order to keep hydroxyl radical generation at a manageable level, greater ferrous ion dose is required. The neutralization stage of the Fenton process generates a substantial volume of ferric hydroxide sludge that must be separated and disposed later on103. Persulfate has also been considered as an efficient oxidant because its redox potential is 2.6 V104. Additionally, it has a longer half-life compared with hydroxyl radical due to its preference for electron transfer reaction, whereas OH. is capable of taking part in a variety of reaction with equal preference. As an example, 2,4,6-trinitrotoluene was degraded to 88% by the EC-persulfate method105. Thus, it is less selective than SO4.- regarding the oxidation of organic pollutants106. In the integrated EC/Persulfate system, Fe2+ is produced from the sacrificial iron electrode and also hydrogen peroxide may be formed. Thus, according to Eqs. 46–49107 both OH. and SO4.− are generated.
Enhanced electro-Fenton processes have been created to advance the treatment process regarding water reclamation. Peroxi-coagulation, sonoelectro-Fenton, photoelectron-Fenton, solar photo-electro-Fenton, bioelectro-Fenton, and Fered-Fenton are only a few examples108,109. The efficacy of these EF-based techniques as a robust wastewater treatment approach has absorbed the interest of academic researchers and industry companies, who are constantly investing in developing these technologies. A number of challenges must be overcome in order to make this process more attractive for large-scale industrial use, such as the need for a suitable electrolyte, high energy usage, very low pH, and the possible evolution of hazardous intermediates in the treated water, among other things110.
The EF method is believed to be environmentally friendly despite its drawbacks, as it does not generate any harmful byproducts such as secondary pollutants111. It is possible, however, that harmful organic or inorganic compounds will be formed if the overall pollutant mineralization goal is not met. However, it should be noted that the focus of this section is on the combination of EC with H2O2, Fenton, and/or electro-Fenton processes. Thus, the application of solo electro-Fenton processes, in which only mixed metal oxides (MMO), graphite, graphene, boron doped diamond (BDD), and dimensionally stable anodes (DSA) electrodes are used (which are very similar to the electrooxidation processes) are not mentioned here. Nevertheless, electro-Fenton process using mentioned materials as electrodes are proven to be a great candidate for removing difficult-to-degrade pollutants and also contaminants of emerging concerns such as pharmaceuticals112 like Metoprolol113, Levofloxacin114, Ciprofloxacin115, Tramadol116, Chloroquine117, Imatinib118, Sulfathiazole119, 5-fluorouracil120, Acetaminophen121 and pesticides122,123,124,125.
For the treatment of distillery wastewater conducted by Asaithambi et al.126, a variety of parameters, including current density, pH, and concentration of H2O2 were measured. Dubey et al.127 conducted an experimental work using H2O2 assisted EC process by both Al and Fe electrodes for the treatment of the effluent from a biodigester. They concluded that iron electrodes were a better choice and the cost estimation for the operation was 1.56 US $/m3 in this case. Electro-Fenton was also used for treating an actual brewery effluent with high organic content and toxicity and great results were achieved (90% TOC removal)128.
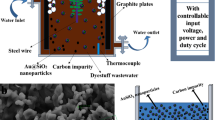
Because the main focus of this paper is whether hybrid EC processes are useful for removing emerging contaminants, it should be mentioned that the peroxi-EC process was found to be useful for removing Azithromycin from synthetic wastewater129. In terms of disinfection of bacterial contamination, peroxi-EC was more successful than the mere EC process130, which was also shown for cyanobacterial removal131. By using a sacrificial iron anode, air breathing cathode, and Ti/IrO2 electrode, An et al. demonstrated that the simultaneous EC-EF system produced iron ions and H2O2 in situ, thereby eliminating the need for chemical reactants to be purchased, transported, and stored (Fig. 10a). Various current intensities were applied to alter the release rate and quantity of oxidizing reagents to suit different requirements for degrading persistent organic contaminants. As it can be seen in Fig. 10b, increasing current intensity from 7 mA to 35 mA, favored microcystins’ removal up to 99%. A large number of coagulants nearly completely covered all algae cells in their SEM images (Fig. 10c). Various Fe valences resulted in different floccule morphologies. In addition, air bubbles produce shear forces that scatter floccules and shape them rounder and more regularly (Fig. 10c). In aerated ECs, coagulation performance was better due to three reasons: 1) Aeration improved contact rates between the cyanobacterial cells and the coagulant, resulting in better removal efficiency; 2) Unaerated ECs had slower pH increases that weakened charge neutralization and lowered cyanobacterial cell removal; 3) Nanosized Fe(III) floccules with higher specific surfaces formed in-situ and easily adsorbed to algal surface.
a EC-Fenton mechanism in (i) EC approach and (ii) EF approach. b The degradation of microcystins within 30 min at various current intensities (Conditions: pH = 3, 0.05 M Na2SO4, stirring rate: 600 rpm). c SEM images of the generated algal flocs at distinct magnifications: Floccules produced under aerated condition (i); Floccules produced under unaerated state (ii)). Reproduced with permission from ref. 131. Copyright © 2019 Elsevier Ltd.
The removal of Perfluorocarboxylic acids (PFCAs) and Perfluorooctanoic acid (PFOAs), which are commonly consumed in domestic products, semiconductor industrial, as a surfactant in medical activities, fire retardant, and metal coating, was conducted using a combination of aerated EC and modified peroxi-coagulation132. It was shown that aeration significantly increased the EC process’s performance. Because hydroxyl radicals are created on site, peroxi-coagulation excels aerated EC. Figure 11 illustrates their suggested pathway of mixed valent Fe-oxide formation and Fe-hydroxide phases at different pH values during EC process. However, pourbaix diagram of iron confirms that pH conditions change during treatment.
The schematic shows mixed valent Fe-oxide formation and Fe-hydroxide phases at different pH using EC process of PFPAs degradation. Reproduced with permission from ref. 132. Copyright © 2021 Elsevier Ltd.
The removal rate of atorvastatin, a drug for lowering the cholesterol, by the peroxi-coagulation process with Fe electrodes was higher than the solo EC process133 and so was for cefixime, an antibiotic, with both iron134 and aluminum electrodes135, and also for the degradation of lamivudine, which is an antiretroviral drug136. The amount of electrode consumption also can be calculated according to Eq. 50133:
Bisphenol A as another hazardous compound and endocrine-disruptor was successfully removed by peroxi-coagulation process137,138. Akbari et al.137 proposed Eq. 51 and Eq. 52 for the mineralization of Bisphenol A by hydroxyl radical and persulfate, respectively. They also suggested recycling electrochemical sludge in its suspended form as a promising way to decrease the generated sludge since it was seen that hydrogen peroxide and persulfate were effectively activated by the sludge.
In another study139, the degradation of pentachlorophenol applying electrochemical activation of peroxodisulfate (PDS), H2O2 and peroxomonosulfate (PMS) with iron electrodes demonstrated that PMS was more effective than two other oxidants (Eqs. 53–57); because chloride ions can also activate PMS to produce oxidizing agents including hypochlorite (HOCl) and chlorine gas.
Persistent mixed industrial wastewater from numerous industries such as cotton, textile, rubber, chemical plants, oil, and plastic factories was treated by EC-EF system140. It was found that heterogeneous EF catalysts made from alkali modified laterite soil were superior to those made from pure laterite regarding removal performance. COD removal rate after 1 h of the EF treatment was 54.57%, while it increased to 85.27% after 2 h of EC-EF treatment. Conducting EC after EF process was a better option in spite of similar performance. The reason was mentioned to be because of the need to nullify neutralization after EF process not only mineralization efficacy was high, but improved biodegradability, and lower sludge formation as well. Also, it should be noted that the higher current density would not favor the removal rate necessarily as the concentration of hydroxyl radical can be decreased because of side reactions141.
Also, the combination of EF/Fenton and EC process was proven to be successful for treating different types of wastewaters. Gunawan et al.142 treated a yarn dyed wastewater (also done by143) in a batch mode using EC to lessen Total Suspended Solid (TSS), followed by Fenton process in a sequential continuous system to reduce COD level. In another study, different electrochemical oxidation processes were examined for the treatment of an actual textile effluent and the sequential process of EC-EF was chosen as the best method, regarding higher degradation effectiveness and lesser consuming energy144. Kumar et al.93 treated composite wastewater using aerated EC and modified peroxi-coagulation processes. The improved peroxi-coagulation process has a much greater efficacy than aerated EC due to the superior oxidant activity of in-situ produced hydroxyl radicals. For treating landfill leachate, simultaneous EC and EF achieved the concurrent removal of organics and ammonia145. Electro-disinfection of primary and secondary effluents from municipal wastewater treatment plants showed that EC with iron electrodes was better for the treatment of the primary effluent, with total removal of coliphages, eukaryotes and E. coli., while EF with BDD anode was better for the treatment of the secondary effluent146. Conducting EF at pH of 7 had almost same results as that of EF at pH 3. Chen et al.147 applied integrated EC/EF processes to degrade selected antibiotic resistance genes namely sul1, sul2, tetM, tetW and 16 S rRNA from Swine Wastewater. As can be seen from Fig. 12, they found that removal efficiency of selected ARGs accounted for 2.94 logs (sul1), 2.49 logs (sul2), 3.25 logs (tetM), 2.64 logs (tetW), and 2.86 logs (16 S rRNA) during 60 min which is shown sequencing EC/EF processes were more efficient than standalone EC. The results proved that EC as a pretreatment could remove bacterial cells through adsorption and enmeshment of precipitated flocs to enhance the removal efficiency in integrated processes. Sequential EC/EF process was proposed as the best disinfection treatment. The details of reviewed papers are mentioned in Table 3.
The removal efficiency of sequencing EC/EF processes for selected ARGs were 2.94 logs (sul1), 2.49 logs (sul2), 3.25 logs (tetM), 2.64 logs (tetW), and 2.86 logs (16 S rRNA) during 60 min. Reproduced with permission from ref. 147. Copyright © 2021 Mary Ann Liebert, Inc.
Because wastewater contains inorganic ions, the Fenton and photo Fenton processes become more complicated. With the existence of anions such as NO3-, PO4 3-, SO4 2-, and Cl- the reaction rate of H2O2 with ferrous ion is different148. To decrease the production of reactive hydroxyl radicals and the removal rate of pollutants, these ions can either form indirect chloride ion-scavenging HO. or less reactive complexes with iron and ferric ions149. Chloride ions, chlorate and perchlorate oxyanions, (oxy) chlorinated radicles, chloramines, hydroxyl amines, and trihalomethanes can all be produced as well as refractory organochlorinated byproducts as undesirable byproducts150.
Hybrid electrocoagulation-UV
Reactive oxygen species are generated when a semiconductor surface is exposed to high-intensity light, which is used in photocatalysis151. When a semiconductor is stimulated using light of energy larger than or equivalent to its band gap, production of electron-hole pair occurs. As a result, reactive oxygen species can be produced in the aqueous phase when oxygen is present152 (Fig. 13). An interaction occurs between UV light and the contaminant molecule, thus the molecule is degraded and mineralized as a direct result. Hydrogen peroxide that can boost the release of hydroxyl radicals, enhances this direct photolysis even more153,154. Photoelectrochemical systems consist of an electrolytic reactor containing an illumination source for the photoanode, a photocatalyst and an electrical power supply or potentiostat. Even though, using various reactors including stirred tank and flow reactors, as well as different light sources within/outside the cell, such as a quartz window, UVC (300 nm > λmax), and UV/Vis lamps (λmax from 320 to 400 nm) have all been considered. Xe lamp can replicate sunlight to allow UV/solar irradiation on the anode surface. In photo-Fenton, Fenton reagents are irradiated with UV-Visible light. In this process hydroxyl radical generation is enhanced within two mechanisms: (i) the reproduction of active Fe2+ ions by reducing Fe3+ (UV < 580 nm), and (ii) the photolysis of hydrogen peroxide (UV < 310 nm)155.
In this mechanism, when a semiconductor is irradiated by light, electron–hole pairs are generated. An electron (e−) will jump into the conduction band, leaving a hole (h+) in the valence band. Then, the photo-generated electron–hole (e−–h+) pairs are separated, and migrate to the surface or interface of the photocatalyst. Reprinted with permission from ref. 226. Copyright © 2015 Elsevier Ltd.
When Peroxi-EC process is integrated with UV photolysis, the number of photoactive sites has a positive impact on the EC process. Fe(OH)2+ photoreduction and the photodecomposition of complexes produced during Fe3+ reactions help increasing the rate of synthesis of OH. (Eqs. 58–59):
The sequential EC and photooxidation methods were used to remediate high-strength tannery effluents156,157,158. Aside from direct photolysis of organic contaminants, UV had a significant impact on degradation pathways. Coagulation formed flocs that helped remove total chromium from the photooxidation process, and the simultaneous oxidation of organic molecules and reduction of Cr (VI) occurred. Iron electrodes were found to be improper due to production of Iron(II) sulfide flocs resulted in turbid appearance of the treated wastewater. UV irradiation and EC with aluminum electrodes can also remove microbial content and turbidity from urban treated wastewaters at low current densities159. When the pH during the process stayed around 8, insoluble aluminum hydroxides formed. Also, free and mixed chlorine disinfectants were found in wastewater. Urban wastewater contains chlorides that could be oxidized on the anode surface, generating hypochlorite. Electrogenerated hypochlorite reacts with wastewater ammonium to make chloramines. Both species had disinfection abilities and eliminated E. coli. The interaction of UV light with E. coli’s cell membrane enhanced the eradication of bacteria. UV light during EC promoted free radical production from oxidizing species and they boosted E. coli removal performance and also favored dissolution of the sacrificial electrode, enhancing turbidity removal.
The simultaneous EC-photo-Fenton was performed in order to treat steel industry effluent using a single reactor with a UV-C lamp and aluminum electrodes160. Yahiaoui et al.161 observed a decrease in the removal of metribuzin when H2O2 was introduced to a UV aided EC system. The authors believe that increasing turbidity might be the cause. Módenes et al.162, on the other hand, treated tannery wastewater through a batch mode incorporation of solar photo Fenton assisted EC and reached significant enhancement in the reduction of COD and also sludge generation. In a work conducted by Farhadi et al.163 EC, photoelectrocoagulation, peroxi-coagulation and peroxi-photoelectrocoagulation processes were compared in terms of treating a pharmaceutical wastewater.
The integrated EC-Fenton/photo-Fenton processes have been shown to be a promising treatment to nearly complete removal of the phenolic compounds in oil refinery wastewater164. Also, adding UVA-LED photo-Fenton as post-treatment after the EC process for the treatment of mature landfill leachate was very successful165. Zhang et al.166 investigated the efficacy of EC standalone and persulfate-assisted and UV-activated electrocoagulation technique using Fe foam electrode for treating PFOA from water. As illustrated in Fig. 14, they observed that EC only reduced 56.4% of PFOA, whilst it soared to 87.5% during 60 min by EC/UV/persulfate. On the other hand, defluorination of PFOA for such combined process (60%) accounted for 10 times higher than EC (6%). This because of sulfate radical generation by the activation of ferrous ions under the synergy of UV light and electric energy. The summary of reviewed articles is given in Table 4.
Synergistic effect (SE) (also called enhancement factor167) plays a crucial role in developing a hybrid approach for wastewater treatment168. Synergy is the amplified result produced by the combination of two or more processes or variables, as opposed to the sum of their individual impacts. Equation 60169 can be employed to get the SE by comparing the integrated process’s pollutant removal efficiency to the sum of the separate processes’ pollutant removal rate.
Where kAOP+EC, kAOP, and kEC are the rate constants of the hybrid process, the individual AOP, and the individual EC process, respectively. When the SE value is positive, it indicates a positive synergistic effect. The combined process is more effective because of: i) enhanced mass transfer and electrode activation, ii) no passive layer on the electrode surface, and iii) release of more hydroxyl radicals.
Another important issue is the scale-up of the hybrid processes, as it is a vital aspect in the practical use of every process for drinking/waste water treatment170. The surface area to volume ratio (S/V) is a critical scale-up factor. It is important to note that the electrode area has a significant effect on the current density, the rate of cation dosing, bubble development, and the bubble paths’ length. According to Mameri et al.171, the ideal current density declines with increasing S/V. However, there was little information available on the S/V ratio. Following dimensionless scale-up criteria have been recognized for EC to progress from experimental to industrial levels172:
-
Reynolds number for describing the fluid flow regime
-
Weber number for indicating the surface tension
-
Froude number as an index of buoyancy
-
Geometric resemblance
-
Gas saturation comparison
Nevertheless, scaling-up of EC-integrated processes from laboratory to industrial scale is of course more complicated and needs more research.
Life cycle assessment of integrated EC/AOPs processes: The way forward
Urban water infrastructure, including wastewater treatment plants (WWTP), has extensively been quantified using life cycle assessment (LCA) during the last 20 years173. This shift from a pollution-removal paradigm into one that incorporates resource recovery is where LCA can play a significant role in evaluating the environmental sustainability of new technologies and processes and capturing trade-offs across various environmental categories174. Life cycle assessment is an effective decision support tool for water sector strategic planning because of its quantitative nature as an environmental evaluation approach175. Due to the involvement of multiple processes that are interdependent (e.g., effluent from one process is often influent for the next), elucidating the environmental implications of WWTP variations via LCA can be rather complicated. Furthermore, as the paradigm change continues, WWTPs are becoming multi-functional, with aims that go beyond standard effluent quality criteria (BOD5, TSS, etc.) to include resource recovery, energy management, emerging pollutant removal, and so on. Addressing such complications in the application of LCA to wastewater management can be aided by the creation of a set of best practices customized particularly to this field of research176.
Ozonation is one of the most studied technologies for the removal of contaminants of emerging concern. It is thought to be more environmentally friendly than UV in terms of pharmaceuticals’ removal177. According to some research, it has a greater environmental impact than it prevents. Hoibye et al.178, on the other hand, determined that the prevented effects are overestimated since these technologies remove more than simply the identified pollutants. Thus, when more compounds are included, ozonation produces less consequences than it avoids, a finding validated by another study179. In contrast to Tarpani and Azapagic180, Rahman et al.181 concluded that ozonation had a lower impact on global warming and ozone depletion than activated carbon. Because chemical and energy production contribute more to these categories, the disparity may be due to differing energy mixes. According to Li et al.182, ozonation had one of the lowest impacts on the categories of acidification potential, human toxicity non-cancer, global warming potential, fossil depletion, smog, and eutrophication. Rodriguez et al.183 suggest that lower O3 intakes are preferable if a balance between toxicity reduction and greenhouse gas emissions is desired.
When employing light-based processes, the source of photon production determines total environmental implications. Both natural and simulated solar light have limited efficiency, and as a result, the treated effluent discharge has significant consequences. In a comparison of UV-A and UV-C without the use of H2O2 or any catalyst, Foteinis et al.184 determined that UV-C is about three times less dangerous than UV-A. That is because it is substantially more efficient for eliminating micropollutants, while requiring more power. When H2O2 is added to the UV-C treatment, the environmental footprint is decreased by up to 88%. In their study, UV light (both UV-A and UV-C) had the greatest influence on the endpoint category of human health, followed by resources, and finally ecosystems. TiO2 was also used to evaluate the possibilities of improving the UV-A process. While it was discovered that UV-A was the least environmentally friendly option when compared to UV-C, and that UVC + H2O2 was even more environmentally friendly, when UVA is combined with TiO2, the environmental performance of the process outpaces that of UV-C with or without H2O2 (despite TiO2 having a slightly larger footprint than H2O2).
LCA has not been performed on Fenton reaction without light for micropollutant and emerging contaminats’ elimination. Most LCA research were conducted on photo-Fenton and so energy usage is once again an important issue. Solar photo-Fenton is more sustainable than photo-Fenton using artificial/simulated light since it does not require energy (note that external electricity may be needed for equipment, such as pumps). Electro-Fenton has higher repercussions than solar photo-Fenton and solar photo-electro Fenton. After energy generation, H2O2 production is the greatest strain in photo-Fenton process. Ioannou-Ttofa et al.185 found that NaOH contributed most to total effects, followed by H2O2 and H2SO4. Solar photo-Fenton at stoichiometric H2O2 is the most environmentally sound solution, with the smallest footprint. Another study186 found that integrating solar photo-Fenton with nanofiltration boosts operating energy consumption while reducing chemical use, lowering total life cycle impacts. Solar photo-Fenton results in reduced loads since it removes micropollutants faster, however at larger scale the findings may be influenced by the decreased reaction volume. Farre et al.187 found that the best option in environmental terms was the coupling of solar photo-Fenton with the biological treatment, which resulted in less or up to 50% less impacts than photo-Fenton in all categories except for aquatic eutrophication potential. Only one publication188 on the life cycle assessment of the electrooxidation process was found, in which it was claimed that electrooxidation is an environmentally viable method since it generates reduced total environmental consequences, including CO2 emissions to the atmosphere.
In terms of electrocoagulation process, there is very few studies about LCA, one of which focused on life cycle assessment for the selection of electrodes for the treatment of a paper industry wastewater189. Factors like environmental impact of the electrode production, the consumption of materials and the power usage were considered and Fe-Fe arrangement was found to be more environmentally friendly. Moreover, only one paper was found about LCA for the combination of electrocoagulation and advanced oxidation processes (Fig. 15). Ahangarnokolaei et al.190 studied life cycle assessment of sequential and simultaneous combination of electrocoagulation and ozonation for the treatment of a textile wastewater. ReCiPe midpoint and endpoint approaches were used to compare lab-scale processes. As can be seen in Fig. 15, due to decreased energy usage, the EC process with Al electrodes had the lowest environmental effect. In terms of human health, O3 had a stronger effect than EC with Al electrodes. High ozonation time makes simultaneous combination (EC-O3) environmentally harmful, but in sequential combination, O3 treatment duration was very short and environmental consequences dropped dramatically. LCA demonstrated that the sequential combination of EC and O3 is the most sustainable textile wastewater treatment process and can be implemented industrially.
Reproduced with permission from ref. 190. Copyright © 2021 Elsevier Ltd.
Outlook and summary
The combination of AOPs with EC is helpful for treating water and wastewater and markedly improves performance. It is both extremely promising and very challenging. This review focused on this topic, giving the researchers a roadmap for covering the knowledge gap and tackling the obstacles, and investigating different aspects. Various mechanisms, pathways for pollutant removal, kinetic models, properties of experiments, and also scale-up issues, have been reviewed in this paper. These features differentiate this review from the previous ones as it can be a guideline, especially for those who are vaguely familiar with this topic. More importantly, the chief focus of this review is the application of the hybrid processes for the removal of contaminants of emerging concern, such as pharmaceuticals, pesticides, herbicides, and recalcitrant pollutants. EC-AOP integration has demonstrated to be highly effective for this purpose as a wide range of these contaminants has been removed efficiently by various combinations of EC-AOP. Combining EC with AOPs also led to faster and more cost-effective water and wastewater treatment (less energy consumption). Moreover, the integrated system overwhelms the disadvantages of each method when used standalone, such as lower sludge production.
In order to choose the best course of action, it is essential to determine if existing research on these technologies is tackling the most significant concerns. The following recommendations must be addressed for future studies:
-
Future works should be scaled up employing continuous operation and real wastewaters to be presented as an effective and trustworthy water and wastewater treatment method.
-
Combination selection could be determined solely on wastewater properties. If the wastewater includes substances that are more resistant and hazardous, especially contaminants of emerging concern, EC followed by an AOP can be a fruitful option.
-
Integrated processes can be conducted in a single reactor. External addition of oxidants such as hydrogen peroxide can also increase the initial rate of pollutant degradation. However, it is proposed that heterogenous Fenton process combined with EC is preferable to homogenous Fenton process. Also, aerated EC combined with AOP processes, particularly electro-Fenton, as it has been recognized as an effective method.
-
Concerning the combination of EC and electrooxidation, the creation of very stable, inexpensive electrodes with a large surface area is still an issue. The cost of purchasing and replacing BDD anodes influences the total expenses of the treatment procedure for particularly persistent pollutants.
-
CECs’ removal potential for reduced absolute toxicity must overcome a shortage of empirical data on its toxicity. Regarding combating micro and nano plastics, there has not been significant research. In terms of pharmaceuticals’ removal, peroxi-coagulation as well as the combination of EC with electrooxidation and electro-Fenton has confirmed to be outstanding, and also there has been acceptable results by integrated EC/UV. When it comes to removing pesticides, EC, electro-Fenton, and electrooxidation are proven to be very effective.
-
There are few papers that investigated the combination of EC with AOP processes for removing pesticides, especially in terms of ozone and electro-Fenton. Therefore, it is suggested that future works focus on performance of these hybrid processes for removing pesticide-containing effluents.
-
More reviews are needed to compare the performance of other EC-integrated processes including adsorption, chemical coagulation, membrane and biological methods for the removal of CECs.
-
In the EC processes aided by AOPs, the electrical energy generated by non-renewable resources is demanded. This could result in two main negatives, the high operational costs and the indirect contamination created by the fossil fuels combustion. Using renewable energy sources like wind, solar, and tidal energy or biogas is essential, so authors recommend future works to use these renewable energy sources instead of electricity.
-
Regarding life cycle assessment studies, there are still a lot of work to be done as there are only two papers that investigated this issue for hybrid EC/AOPs. According to them, integrating EC with ozone and also solar power are both environmentally friendly and cost effective. Also, ozonation is regarded as one of the most efficient methods for the removal of organic micropollutants in this regard.
Overall, hybrid treatment processes that successfully combine economic feasibility, high removal efficacy, and environmental sustainability are urgently needed in today’s world. However, further process optimization, modeling, and scaling-up research is required to verify these integrated processes’ reliability for large-scale wastewater treatment.
Data availability
The authors declare that the data supporting the findings of this study are available within the manuscript.
References
Pulkka, S., Martikainen, M., Bhatnagar, A. & Sillanpää, M. Electrochemical methods for the removal of anionic contaminants from water—a review. Sep Pur. Tech. 132, 252–271 (2014).
Uğurlu, M., Gürses, A., Doğar, Ç. & Yalçın, M. The removal of lignin and phenol from paper mill effluents by electrocoagulation. J. Env. Manag 87, 420–428 (2008).
Murugananthan, M., Raju, G. B. & Prabhakar, S. Removal of sulfide, sulfate and sulfite ions by electro coagulation. J. Hazard. Mat. 109, 37–44 (2004).
Calvo, L. S. et al. An electrocoagulation unit for the purification of soluble oil wastes of high COD. Env. Prog. Sustain. Energy 22, 57–65 (2003).
Ogutveren, U. B. & Koparal, S. Electrocoagulation for oil-water emulsion treatment. J. Env. Sci. Health 32, 2507–2520 (1997).
Oladzad, S., Fallah, N. & Nasernejad, B. Combination of novel coalescing oil/water separator and electrocoagulation technique for treatment of petroleum compound contaminated groundwater. Wat. Sci. Tech. 76, 57–67 (2017).
Butler, E., Hung, Y.-T., Yeh, R. Y.-L. & Al Ahmad, M. S. Electrocoagulation in wastewater treatment. Wat 3, 495–525 (2011).
Daneshvar, N., Oladegaragoze, A. & Jafarzadeh, N. Decolorization of basic dye solutions by electrocoagulation: an investigation of the effect of operational parameters. J. Hazard. Mat. 129, 116–122 (2006).
Al-Shannag, M., Al-Qodah, Z., Bani-Melhem, K., Qtaishat, M. R. & Alkasrawi, M. Heavy metal ions removal from metal plating wastewater using electrocoagulation: kinetic study and process performance. Chem. Eng. J. 240, 749–756 (2015).
Bazrafshan, E., Mohammadi, L., Ansari-Moghaddam, A. & Mahvi, A. H. Heavy metals removal from aqueous environments byelectrocoagulation process—a systematic review. J. Env. Health Sci. Eng. 13, 1–16 (2015).
Kim, T., Kim, T. K. & Zoh, K. D. Removal mechanism of heavy metal (Cu, Ni, Zn, and Cr) in the presence of cyanide during electrocoagulation using Fe and Al electrodes. J. Wat. Process Eng. 33, 101109 (2020).
Mahmad, M. K. N., Rozainy, M. R., Abustan, I. & Baharun, N. Removal of iron and total chromium contaminations in landfill Leachate by using electrocoagulation process. Key Eng. Mat. 660, 279–283 (2015).
Mouedhen, G., Feki, M., De Petris-Wery, M. & Ayedi, H. Electrochemical removal of Cr (VI) from aqueous media using iron and aluminum as electrode materials: towards a better understanding of the involved phenomena. J. Hazard. Mat. 168, 983–991 (2009).
Amarasinghe, B. M. W. P. K. & Williams, R. Tea waste as a low-cost adsorbent for the removal of Cu and Pb from wastewater. Chem. Eng. J. 132, 299–309 (2007).
Costa, J.M., Grisente dos Reis DaCosta, J. & Florêncio deAlmeida Neto, A. Techniques of nickel (II) removal from electroplating industry wastewater: Overview and trends. J. Wat. Process Eng. 46, 102593 (2022).
Bibi, S., Kamran, M. A., Sultana, J. & Farooqi, A. Occurrence and methods to remove arsenic and fluoride contamination in water. Env. Chem. Lett. 15, 125–149 (2017).
Kartikaningsih, D., Shih, Y. J. & Huang, Y. H. Boron removal from boric acid wastewater by electrocoagulation using aluminum as sacrificial anode. Sustain Env. Res. 26, 150–155 (2016).
Nanseu-Njiki, C. P., Tchamango, S. R., Ngom, P. C., Darchen, A. & Ngameni, E. Mercury (II.) removal from water byelectrocoagulation using aluminium and iron electrodes. J. Hazard. Mat. 168, 1430–1436 (2009).
Singh, K. K., Singh, A. K. & Hasan, S. H. Low-cost bio-sorbent ‘wheat bran’ for the removal of cadmium from wastewater: kinetic and equilibrium studies. Biores. Tech. 97, 994–1001 (2006).
Heidmann, I. & Calmano, W. Removal of Zn (II), Cu (II), Ni (II), Ag(I) and Cr (VI) present in aqueous solutions by aluminium electrocoagulation. J. Hazard. Mat. 15, 934–941 (2008).
Al-raad, A. A. & Hanafiah, M. M. Removal of inorganic pollutants using electrocoagulation technology: A review of emerging applications and mechanisms. J. Env. Manag 300, 113696 (2021).
Al-Qodah, Z. & Al-Shannag, M. Heavy metal ions removal from wastewater using electrocoagulation processes: a comprehensive review. Sep. Sci. Tech. 17, 2649–2676 (2017).
Hakizimana, J. N. et al. Electrocoagulation process in water treatment: A review of electrocoagulation modeling approaches. Desalin 404, 1–21 (2017).
López-Guzmán, M., Flores-Hidalgo, M. A. & Reynoso-Cuevas, L. Electrocoagulation Process: An Approach to Continuous Processes, Reactors Design, Pharmaceuticals Removal, and Hybrid Systems—A Review. Processes 9, 1831 (2021).
Moussa, D. T., El-Naas, M. H., Nasser, M. & Al-Marri, M. J. A comprehensive review of electrocoagulation for water treatment: potentials and challenges. J. Env. Manag 186, 24–41 (2016).
Sahu, O., Mazumdar, B. & Chaudhari, P. Treatment of wastewater by electrocoagulation: a review. Env. Sci. Pollut. Res. 21, 2397–2413 (2014).
Sandoval, M. A., Fuentes, R., Thiam, A. & Salazar, R. Arsenic and fluoride removal by electrocoagulation process: A general review. Sci. Tot. Env. 753, 142108 (2021).
Dominguez-Ramos, A., Aldaco, R. & Irabien, A. Photovoltaic solar electrochemical oxidation (PSEO) for treatment of lignosulfonate wastewater. J. Chem. Tech. Biotech. 85, 821–830 (2010).
Fernandes, A., Pacheco, M. J., Ciriaco, L. & Lopes, A. Review on the electrochemical processes for the treatment of sanitary landfill leachates: present and future. Appl. Catal. B Env. 176, 183–200 (2015).
Mousazadeh, M. et al. Systematic diagnosis of state of the art in the use of electrocoagulation as a sustainable technology for pollutant treatment: An updated review. Sustain. Energy Tech. Assess. 47, 101353 (2021).
Almukdad, A., Hafiz, M., Yasir, A. T., Alfahel, R. & Hawari, A. H. Unlocking the application potential of electrocoagulation process through hybrid processes. J. Wat. Process Eng. 40, 101956 (2021).
Das, P. P., Sharma, M. & Purkait, M. K. Recent progress on electrocoagulation process for wastewater treatment: A review. Sep. Pur. Tech. 292, 121058 (2022).
Nidheesh, P. V., Scaria, J., Syam Babu, D. & Suresh Kumar, M. An overview on combined electrocoagulation-degradation processes for the effective treatment of water and wastewater. Chemosphere 263, 127907 (2021).
Akter, S., Kabir Suhan, B. & Islam, S. Recent advances and perspective of electrocoagulation in the treatment of wastewater: A review. Env. Nanotech. Monitor. Manag 17, 100643 (2022).
Hernández-Maldonado, A. J. & Blaney, L. Contaminants of Emerging Concern in Water and Wastewater. 299–365 (Butterworth-Heinemann, 2020).
Nidheesh, P. V. et al. Potential role of biochar in advanced oxidation processes: a sustainable approach. Chem. Eng. J. 405, 126582 (2021).
Nidheesh, P. V., Divyapriya, G., Oturan, N., Trellu, C. & Oturan, M. A. Environmental applications of boron-doped diamond electrodes: 1. Applications in water and wastewater treatment. Chem. ElectroChem 6, 2124–2142 (2019).
Trellu, C., Chakraborty, S., Nidheesh, P. V. & Oturan, M. A. Environmental applications of boron-doped diamond electrodes: 2. Soil remediation and sensing applications. Chem. ElectroChem 6, 2143–2156 (2019).
Hayati, F., Isari, A. A., Anvaripour, B., Fattahi, M. & Kakavandi, B. Ultrasound-assisted photocatalytic degradation of sulfadiazine using MgO@CNT heterojunction composite: effective factors, pathway and biodegradability studies. Chem. Eng. J. 381, 122636 (2020).
Surenjan, A., Pradeep, T. & Philip, L. Application and performance evaluation of a cost-effective vis- LED based fluidized bed reactor for the treatment of emerging contaminants. Chemosphere 228, 629–639 (2019).
Ferrag-Siagh, F. et al. Tetracycline degradation and mineralization by the coupling of an electro-Fenton pretreatment and a biological process. J. Chem. Tech. Biotech. 88, 1380–1386 (2013).
Ramteke, L. P. & Gogate, P. R. Treatment of toluene, benzene, naphthalene and xylene (BTNXs) containing wastewater using improved biological oxidation with pretreatment using Fenton/ultrasound based processes. J. Ind. Eng. Chem. 28, 247–260 (2015a).
Ighalo, J. O., Igwegbe, C. A., Aniagor, C. O. & Oba, S. N. review of methods for the removal of penicillins from water. J. Wat. Proc. Eng. 39, 101886 (2021).
Bani Salameh, W. K. M., Ahmad, H. & Al-Shannag, M. Treatment of olive mill wastewater by electrocoagulation processes and water resources management. Int. J. Env. Chem. Eco. Geo. Geophys. Eng. 9, 288–299 (2015).
Özyurt, B. & Camcıoğlu, S. Applications of Combined Electrocoagulation and Electrooxidation Treatment to Industrial Wastewaters (ed. Yonar, T.) 71–89 (IntechOpen, 2018) https://www.intechopen.com/chapters/60826.
Chen, G. Electrochemical technologies in wastewater treatment. Sep Pur. Tech. 38, 11–41 (2004).
Comninellis, C. Electrocatalysis in the electrochemical conversion/combustion of organic pollutants for waste water treatment. Electrochem. Acta 39, 1857–1862 (1994).
Sarkka, H., Bhatnagar, A. & Sillanpaa, M. Recent developments of electro-oxidation in water treatment –A. Rev. J. Electroanal. Chem. 754, 46–56 (2015).
Martínez-Huitle, C. A. & Ferro, S. Electrochemical oxidation of organic pollutants for the wastewater treatment: Direct and indirect processes. Chem. Soc. Rev. 35, 1324–1340 (2006).
Chaplin, B. Critical review of electrochemical advanced oxidation processes for water treatment applications. Env. Sci. Process. Impact 16, 1182–1203 (2014).
Shestakova, M., Graves, J., Sitarz, M. & Sillanpaa, M. Optimization of Ti/Ta2O5–SnO2 electrodes and reaction parameters for electrocatalytic oxidation of methylene blue. J. Appl. Electrochem 46, 349–358 (2016).
Bhagawan, D., Poodari, S., Golla, S., Himabindu, V. & Vidyavathi, S. Treatment of the petroleum refinery wastewater using combined electrochemical methods. Desalin. Wat. Treat. 57, 3387–3394 (2016).
Yılmaz Nayır, T. & Kara, S. Container washing wastewater treatment by combined electrocoagulation–electrooxidation. Sep. Sci. Tech. 53, 1592–1603 (2018).
Samir Naje, A., Chelliapan, S., Zakaria, A., Ajeel, M. A. & Alaba, P. A. A review of electrocoagulation technology for the treatment of textile wastewater. Rev. Chem. Eng. 33, 263–292 (2017).
Ardhan, N., Ruttithiwapanich, T., Songkasiri, W. & Phalakornkule, C. Comparison of performance of continuous-flow and batch electrocoagulators: a case study for eliminating reactive blue 21 using iron electrodes. Sep. Pur. Tech. 146, 75–84 (2015).
Lakshmi Kruthika, N., Karthika, N., Bhaskar Raju, G. & Prabhakar, S. Efficacy of electrocoagulation and electrooxidation for the purification of wastewater generated from gelatin production plant. J. Env. Chem. Eng. 1, 183–188 (2013).
Linares Hernandez, I. et al. Soft drink wastewater treatment by electrocoagulation–electrooxidation processes. Env. Tech. 38, 433–442 (2017).
Afaha, Y. G., Zewge, F., Yohannes, T. & Kebede, S. Application of hybrid electrocoagulation and electrooxidation process for treatment of wastewater from the cotton textile industry. Chemosphere 302, 134706 (2022).
Ibarra-Taquez, H. N., GilPavas, E., Blatchley, E. R., Gómez-García, M. A. & Dobrosz-Gómez, I. Integrated electrocoagulation-electrooxidation process for the treatment of soluble coffee effluent: Optimization of COD degradation and operation time analysis. J. Env. Manag 200, 530–538 (2017).
Sanni, I., Estahbanati, Karimi, Carabin, M. R., Drogui, A. & Coupling, P. electrocoagulation with electro oxidation for COD and phosphorus removal from industrial container wash water. Sep. Pur. Tech. 282, 119992 (2022).
Azarian, G., Miri, M. & Nematollahi, D. Combined electrocoagulation/electrooxidation process for the COD removal and recovery of tannery industry wastewater. Env Prog. Sustain. Energy 37, 637–644 (2017).
Ghazouani, M., Akrout, H., Jellali, S. & Bousselmi, L. Comparative study of electrochemical hybrid systems for the treatment of real wastewaters from agri-food activities. Sci. Total Env. 647, 1651–1664 (2019b).
Guo, Z. et al. Electrochemical methods for landfill leachate treatment: A review on electrocoagulation and electrooxidation. Sci. Total Env 806, 150529 (2022).
Shi, H. et al. An electrocoagulation and electrooxidation treatment train to remove and degrade per- and polyfluoroalkyl substances in aqueous solution. Sci. Tot. Env. 788, 147723 (2021).
Ghatak, H. R. Comparative removal of commercial diclofenac sodium by electro-oxidation on platinum anode and combined electro-oxidation and electrocoagulation on stainless steel anode. Env. Tech. 35, 2483–2492 (2014).
Sanni, I., Estahbanati, M. K., Carabin, A. & Drogui, P. Coupling electrocoagulation with electro-oxidation for COD and phosphorus removal from industrial container wash water. Sep. Pur. Tech. 1, 119992 (2022).
Maher, E. K., O’Malley, K. N., Dollhopf, M. E., Mayer, B. K. & McNamara, P. J. Removal of Estrogenic Compounds from Water Via Energy Efficient Sequential Electrocoagulation-Electrooxidation. Env. Eng. Sci. 37, 99–108 (2020).
Behloul, M. et al. Removal of malathion pesticide from polluted solutions by electrocoagulation: modeling of experimental results using response surface methodology. Sep. Sci. Tech. 48, 664–672 (2013).
Danial, R., Abdullaha, L. C., Mobarekeh, M. N., Sobri, S. & Mohd Adnan, N. A comparison between aluminium and iron electrodes in electrocoagulation process for glyphosate removal. J. Teknol. 77, 21–26 (2015).
Kamaraj, R., Davidson, D. J., Sozhan, G. & Vasudevan, S. Adsorption of 2,4- dichlorophenoxyacetic acid (2,4-D) from water by in situ generated metal hydroxides using sacrificial anodes. J. Taiwan Inst. Chem. Eng. 45, 2943–2949 (2014).
Rao, S., Srikanth, M., Neelima, P. & Vangalapati, M. Optimisation parameters for dicofol pesticide removal by electro-coagulation. Int. Adv. Res. J. Sci. Eng. Tech. 4, 258–261 (2017).
Hernández-Ortega, M. et al. Use of a combined electrocoagulation—ozone process as a pre-treatment for industrial wastewater. Desalin 250, 144–149 (2010).
Bernal-Martínez, L. A., Barrera-Díaz, C., Solís-Morelos, C. & Natividad, R. Synergy of electrochemical and ozonation processes in industrial wastewater treatment. Chem. Eng. J. 165, 71–77 (2010).
Huang, C., Dong, C. & Tang, Z. Advanced chemical oxidation: its present role and potential future in hazardous waste treatment. Waste Manag 13, 361–377 (1993).
Piera, E., Calpe, J. C., Brillas, E., Domènech, X. & Peral, J. 2,4-Dichlorophenoxyacetic acid degradation by catalyzed ozonation: TiO2/UVA/O3 and Fe (II)/UVA/O3 systems. Appl. Catal. B 27, 169–177 (2000).
He, Z. et al. Decolorization of CI Reactive Yellow 84 in aqueous solution by electrocoagulation enhanced with ozone: influence of operating conditions. Env. Tech. 28, 1257–1263 (2007).
Asaithambi, P., Aziz, A. R. A. & Daud, W. M. A. B. W. Integrated ozone-electrocoagulation process for the removal of pollutant from industrial effluent: optimization through response surface methodology. Chem. Eng. Process 105, 92–102 (2016a).
Asaithambi, P., Susree, M., Saravanathamizhan, R. & Matheswaran, M. Ozone assisted electrocoagulation for the treatment of distillery effluent. Desalin 297, 1–7 (2012).
Asaithambi, P., Govindarajan, R., Busier Yesuf, M., Selvakumar, P. & Alemayehu, E. Enhanced treatment of landfill leachate wastewater using sono(US)-ozone(O3)–electrocoagulation(EC) process: Role of process parameters on color, COD and electrical energy consumption. Process saf. Env. Prot. 142, 212–218 (2020).
Ahangarnokolaei, M. A., Ayati, B. & Ganjidoust, A. Simultaneous and sequential combination of electrocoagulation and ozonation by Al and Fe electrodes for DirectBlue71 treatment in a new reactor: Synergistic effect and kinetics study. Chemosphere 285, 1–10 (2021).
Bilińska, L., Blus, K., Gmurek, M. & Ledakowicz, S. Coupling of electrocoagulation and ozone treatment for textile wastewater reuse. Chem. Eng. J. 385, 992–1001 (2019).
García-Morales, M. A. et al. Integrated Advanced Oxidation Process (Ozonation) and Electrocoagulation Treatments for Dye Removal in Denim Effluents. Int. J. Electrochem. Sci. 8, 8752–8763 (2013).
Tanveer, R. et al. Comparison of ozonation, Fenton, and photo-Fenton processes for the treatment of textile dye-bath effluents integrated with electrocoagulation. J. Wat. Process Eng. 40, 102547 (2022).
Aseman-Bashiz, E. & Sayyaf, H. Synthesis of nano-FeS2 and its application as an effective activator of ozone and peroxydisulfate in the electrochemical process for ofloxacin degradation: A comparative study. Chemosphere 1, 129772 (2021).
Barzegar, G., Wu, J. & Ghanbari, F. Enhanced treatment of greywater using electrocoagulation/ozonation: Investigation of process parameters. Process Saf. Env. Prot. 121, 125–132 (2019).
Jin, X. et al. The role of synergistic effects between ozone and coagulants (SOC) in the electrohybrid ozonation-coagulation process. Wat. Res. 177, 115800 (2020).
Das, P. P. et al. Integrated ozonation assisted electrocoagulation process for the removal of cyanide from steel industry wastewater. Chemosphere 263, 128370 (2021).
Wang, Y., Yu, G., Deng, S., Huang, J. & Wang, B. The electro-peroxone process for the abatement of emerging contaminants: Mechanisms, recent advances, and prospects. Chemosphere 208, 640–654 (2018).
Von Gunten, U. & Oliveras, Y. Kinetics of the reaction between hydrogen peroxide and hypobromous acid: implication on water treatment and natural systems. Wat. Res. 31, 900–906 (1997).
Lee, Y. & von Gunten, U. Advances in predicting organic contaminant abatement during ozonation of municipal wastewater effluent: reaction kinetics, transformation products, and changes of biological effects. Env. Sci. Wat. Res. 2, 421–442 (2016).
Dadban Shahamat, Y., Hamidi, F., Mohammadi, H., Ghahrchi, M. Optimisation of COD removal from the olive oil mill wastewater by combined electrocoagulation and peroxone process: modelling and determination of kinetic coefficients. Int. J. Env. Analyt. Chem. 1–14 (2021). https://doi.org/10.1080/03067319.2021.1937615
Cañizares, P., Sáez, C., Sánchez-Carretero, A. & Rodrigo, M. Synthesis of novel oxidants by electrochemical technology. J. Appl. Electrochem 39, 2143 (2009).
Kumar, A., Nidheesh, P. V. & Kumar, M. S. Composite Wastewater Treatment by Aerated Electrocoagulation and Modified Peroxi-Coagulation Processes. Chemosphere 205, 587–593 (2018).
Bard, A.J. Standard Potential in Aqueous Solution. (Marcel Decker Inc., 1985).
Eckenfelder, W.W. Industrial Water Pollution Control. Serial in Water, Resources and Environmental Engineering. 3 edn, (McGraw-Hill, 1988).
Casado, J. Towards industrial implementation of Electro-Fenton and derived technologies for wastewater treatment: a review. J. Env. Chem. Eng. 7, 102823 (2019).
Stefan, M. I. Advanced Oxidation Processes for Water Treatment – Fundamentals and Applications. (IWA publishing, 2017).
Nidheesh, P. V. Removal of organic pollutants by peroxicoagulation. Env. Chem. Lett. 16, 1283–1292 (2018).
Martínez-Huitle, C. A. & Brillas, E. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: a general review. Appl. Catal. B. Env. 87, 105–145 (2009).
Ren, G. et al. Highly energy-efficient removal of acrylonitrile by peroxi-coagulation with modified graphite felt cathode: influence factors, possible mechanism. Chem. Eng. J. 343, 467–476 (2018).
Li, H. et al. Improved BDD anode system in electrochemical degradation of p-nitrophenol by corroding electrode of iron. Electrochim. Acta 291, 335–342 (2018).
Nidheesh, P. V. & Gandhimathi, R. Trends in electro-Fenton process for water and wastewater treatment: an overview. Desalin 299, 1–15 (2012).
Sun, Y. & Pignatello, J. J. Photochemical reactions involved in the total mineralization of 2,4-D by Fe3+/H2O2/UV. Env. Sci. Tech. 27, 304–310 (1993).
Waclawek, S., Antos, V., Hrabak, P., Cernik, M. & Elliott, D. Remediation of hexachlorocyclohexanes by electrochemically activated persulfates. Env. Sci. Pollut. Res 23, 765–773 (2016).
Mirshafiee, A. & Darvish, M. Degradation of 2,4,6-trinitrotoluene (TNT) from aqueous solution by coupled electrocoagulation process with persulfate salt. J. Env. Health Sci. Eng. 19, 1035–1041 (2021).
Bu, L. et al. Degradation of oxcarbazepine by UV-activated persulfate oxidation: kinetics, mechanisms, and pathways. Env. Sci. Pollut. Res. 23, 2848–2855 (2016).
Yuan, S., Liao, P. & Alshawabkeh, A. N. Electrolytic manipulation of persulfate reactivity by iron electrodes for trichloroethylene degradation in groundwater. Env. Sci. Tech. 48, 656–663 (2014).
Li, X., Chen, S., Angelidaki, I. & Zhang, Y. Bio-electro-Fenton processes for wastewater treatment: advances and prospects. Chem. Eng. J. 354, 492–506 (2018).
Oturan, M. A. & Aaron, J. J. Advanced oxidation processes in water/wastewater treatment: principles and applications. A review. Crit. Rev. Env. Sci. Tech. 44, 2577–2641 (2014).
Ismail, S. A., Ang, W. L. & Mohammad, A. W. Electro-Fenton technology for wastewater treatment: A bibliometric analysis of current research trends, future perspectives and energy consumption analysis. J. Wat. Process Eng. 40, 101952 (2021).
Oonnittan, A. & Sillanpaa, M. E. T. Water treatment by electro-fenton process. Curr. Org. Chem. 16, 2060–2072 (2012).
Olvera-Vargas, H., Gore-Datar, N., Garcia-Rodriguez, O., Mutnuri, S. & Lefebvre, O. Electro-Fenton treatment of real pharmaceutical wastewater paired with a BDD anode: reaction mechanisms and respective contribution of homogeneous and heterogeneous OH. Chem. Eng. J. 404, 126524 (2021).
Olvera-Vargas, H., Cocerva, T., Oturan, N., Buisson, D. & Oturan, M. A. Bioelectro-Fenton: A sustainable integrated process for removal of organic pollutants from water: Application to mineralization of metoprolol. J. Hazard. Mat. 319, 13–23 (2016).
Gong, Y. et al. Partial degradation of levofloxacin for biodegradability improvement by electro-Fenton process using an activated carbon fiber felt cathode. J. Hazard. Mat. 304, 320–328 (2015).
Shoorangiz, M., Nikoo, M. R., Salari, M., Rakhshandehroo, G. R. & Sadegh, M. Optimized electro-Fenton process with sacrificial stainless steel anode for degradation/mineralization of ciprofloxacin. Process Saf Env. Prot. 132, 340–350 (2019).
Montei, H., Oturan, N., Péchaud, Y. & Oturan, M. A. Electro-Fenton treatment of the analgesic tramadol: Kinetics, mechanism and energetic evaluation. Chemosphere 247, 125939 (2020).
Midassi, S., Bedoui, A. & Bensalah, N. Efficient degradation of chloroquine drug by electro-Fenton oxidation: Effects of operating conditions and degradation mechanism. Chemosphere 260, 127558 (2020).
Yang, W., Zhou, M., Oturan, N., Li, Y. & Oturan, M. A. Electrocatalytic destruction of pharmaceutical imatinib by electro-Fenton process with graphene-based cathode. Electrochim. Acta 305, 285–294 (2019).
Zhu, Y. et al. Enhanced degradation of sulfathiazole by electro-Fenton process using a novel carbon nitride modified electrode. Carbon 145, 321–332 (2019).
Ganzenko, O. et al. Fast and complete removal of the 5-fluorouracil drug from water by electro-Fenton oxidation. Env. Chem. Lett. 16, 281–286 (2018).
Ahmadzadeh, S. & Dolatabadi, M. Removal of acetaminophen from hospital wastewater using electro-Fenton process. Env. Earth Sci. 77, 53 (2018).
Zhang, Y. et al. Optimized terbium doped Ti/PbO2 dimensional stable anode as a strong tool for electrocatalytic degradation of imidacloprid waste water. Ecotoxic Env. Saf. 188, 109921 (2020).
Al-Zubaidi, D. K. & Pak, K. S. Degradation of parachlorophenol in synthetic wastewater using Batch Electro-Fenton process. Mater. Today.: Proceed. 20, 414–419 (2020).
Dominguez, C. M., Oturan, N., Romero, A., Santos, A. & Oturan, M. A. Optimization of electro-Fenton process for effective degradation of organochlorine pesticide lindane. Catal. Today 313, 196–202 (2018).
Diaw, P. A. et al. Removal of the herbicide monolinuron from waters by the electro-Fenton treatment. J. Electroanal. Chem. 864, 114087 (2020).
Asaithambi, P., Sajjadi, B., Aziz, A. R. A. & Daud, W. M. A. B. W. Performance Evaluation of Hybrid Electrocoagulation Process Parameters for the Treatment of Distillery Industrial Effluent. Process Saf. Env. Prot. 104, 406–412 (2016).
Dubey, S. et al. Hydrogen peroxide assisted electrocoagulation treatment of rice gain based biodigester effluent: mechanism, performance, and cost analysis. Int. J. Chem. React. Eng. 19, 969–980 (2021).
Espinoza-Quinones, F. R. et al. Insights into brewery wastewater treatment by the electro-Fenton hybrid process: How to get a significant decrease in organic matter and toxicity. Chemosphere 263, 128367 (2021).
Yazdanbakhsh, A. R., Massoudinegad, M. R., Eliasi, S. & SheikhMohammadi, A. The influence of operational parameters on reduce of azithromyin COD from wastewater using the peroxi -electrocoagulation process. J. Wat. Process Eng. 6, 51–57 (2015).
Kourdali, S., Badis, A., Boucherit, A., Boudjema, K. & Saiba, A. Electrochemical disinfection of bacterial contamination: Effectiveness and modeling study of E. coli inactivation by electro-Fenton, electro-peroxi-coagulation and electrocoagulation. J. Env. Manag 226, 106–119 (2018).
An, J. et al. A novel electro-coagulation-Fenton for energy efficient cyanobacteria and cyanotoxins removal without chemical addition. J. Hazard. Mat. 365, 650–658 (2019).
Singh, S., Lo, S. L., Srivastava, V. C., Qiao, Q. & Sharma, P. Mineralization of perfluorooctanoic acid by combined aerated electrocoagulation and Modified peroxi-coagulation methods. J. Taiwan Inst. Chem. Eng. 118, 169–178 (2021).
Barisci, S. & Turkay, O. The performance of electrosynthesised ferrate (VI) ion, electrocoagulation and peroxi-electrocoagulation processes for degradation of cholesterol-lowering drug atorvastatin. Desalin. Wat. Treat. 57, 25561–25571 (2016).
Lalwani, J., Sangeetha, C. J., Thatikonda, S. & Subrahmanyam, C. Sequential treatment of crude drug effluent for the elimination of API by combined electro-assisted coagulation-photocatalytic oxidation. J. Wat. Proc. Eng. 28, 195–202 (2019).
Asadi‑Ghalhari, M. et al. Removal of Cefixime from aqueous solutions via proxy electrocoagulation: modeling and optimization by response surface methodology. Reac. Kinet. Mech. Cat. 134, 459–471 (2021).
Fekadu, S., Alemayehu, E., Dewil, R. & Van der Bruggen, B. Electrochemical degradation of antivirus drug lamivudine formulation: photoelectrocoagulation, peroxi‑electrocoagulation, and peroxi‑photoelectrocoagulation processes. J. Appl. Electrochem 51, 607–618 (2021).
Akbari, S., Ghanbari, F. & Moradi, M. Bisphenol A degradation in aqueous solutions by electrogenerated ferrous ion activated ozone, hydrogen peroxide and persulfate: Applying low current density for oxidation mechanism. Chem. Eng. J. 294, 298–307 (2016).
Boyasan, F. & Çavunt, A. Investigation of bisphenol A removal using peroxy electrocoagulation method. Desalin. Wat. Treat. 222, 426–433 (2021).
Govindan, K., Raja, M., Noel, M. & James, E. J. Degradation of pentachlorophenol by hydroxyl radicals and sulfateradicals using electrochemical activation of peroxomonosulfate,peroxodisulfate and hydrogen peroxide. J. Hazard. Mat. 272, 42–51 (2014).
Nidheesh, P. V., Behera, B. B., Babu, D. S., Scaria, J. & Kumar, M. S. Mixed industrial wastewater treatment by the combination of heterogeneous electro-Fenton and electrocoagulation processes. Chemosphere 290, 133348 (2022).
Guan, W., Zhang, B., Tian, S. & Zhao, X. The synergism between electro-Fenton and electrocoagulation process to remove Cu-EDTA. Appl. Catal. B 227, 252–257 (2018).
Gunawan, D., Kuswadi, V. B., Sapei, L. & Riadi, L. Yarn dyed wastewater treatment using hybrid electrocoagulation-Fenton method in a continuous system: Technical and economical viewpoint. Env. Eng. Res. 23, 114–119 (2018).
Riadi, L., Sapei, L., Lidiawati, T. & Agustin, Y. E. IOP. Conf. Ser. Mater. Sci. Eng. 012026.
Zazou, H. et al. Treatment of textile industry wastewater by electrocoagulation coupled with electrochemical advanced oxidation process. J. Wat. Process Eng. 28, 214–221 (2019).
Ding, J. et al. Treatment of leachate concentrate by electrocoagulation coupled with electro-Fenton-like process: Efficacy and mechanism. Sep. Pur. Tech 255, 117668 (2021).
Anfruns-Estrada et al. Inactivation of microbiota from urban wastewater by single and sequential electrocoagulation and electro-Fenton treatments. Wat. Res. 126, 450–459 (2017).
Chen, L., Gao, S., Lou, L. & Zhou, Z. Removal of intracellular and extracellular antibiotic resistance genes from swine wastewater by sequential electrocoagulation and electro-Fenton processes. Env. Eng. Sci. 38, 74–80 (2021).
Bouasla, C., Ismail, F. & Samar, M. E. H. Effects of operator parameters, anions and cations on the degradation of AY99 in an aqueous solution using Fenton’s reagent. Optimization and kinetics study. Int. J. Ind. Chem. 3, 15 (2012).
Machulek, A. et al. Abatement of the Inhibitory Effect of Chloride Anions on the Photo-Fenton Process. Env. Sci. Tech. 41, 8459–8463 (2007).
Brillas, E. & Sirés, I. Hybrid and Sequential Chemical and Electrochemical Processes for Water Decontamination, in: Electrochemical Water and Wastewater Treatment. 267–304 (Elsevier, 2018).
Carp, O., Huisman, C. L. & Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 32, 33–177 (2004).
Nakata, K. & Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C. Photochem. Rev. 13, 169–189 (2012).
Klavarioti, M., Mantzavinos, D. & Kassinos, D. Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes. Env. Int. 35, 402–417 (2009).
Ryan, C. C., Tan, D. T. & Arnold, W. A. Direct and indirect photolysis of sulfamethoxazole and trimethoprim in wastewater treatment plant effluent. Wat. Res. 45, 1280–1286 (2011).
Divyapriya, G., Nambi, I. M. & Senthilnathan, J. Nanocatalysts in fenton based advanced oxidation process for water and wastewater treatment. J. Bionanosci. 10, 356–368 (2016).
Borba, F. H. et al. Desirability function applied to the optimization of the Photoperoxi-Electrocoagulation process conditions in the treatment of tannery industrial wastewater. J. Wat. Process Eng. 23, 207–216 (2018).
Jallouli, S. et al. Efficient and sustainable treatment of tannery wastewater by a sequential electrocoagulation-UV photolytic process. J. Wat. Process Eng. 38, 101642 (2020).
Moradi, M. & Moussavi, G. Enhanced treatment of tannery wastewater using the electrocoagulation process combined with UVC/VUV photoreactor: Parametric and mechanistic evaluation. Chem. Eng. J. 358, 1038–1046 (2019).
Cotillas, S. et al. Electrocoagulation-UV Irradiation Process for Urban Wastewater Reuse. Chem. Eng. Trans. 41, 133–138 (2014).
Malakootian, M. & Heidari, M. R. Removal of phenol from steel wastewater by combined electrocoagulation with photo-Fenton. Wat. Sci. Tech. 78, 1260–1267 (2018).
Yahiaoui, O. et al. Evaluating removal of metribuzin pesticide from contaminated groundwater using an electrochemical reactor combined with ultraviolet oxidation. Desalin 270, 84–89 (2011).
Módenes, A. N., Espinoza-Quiñones, F. R., Borba, F. H. & Manenti, D. R. Performance evaluation of an integrated photo-Fenton – Electrocoagulation process applied to pollutant removal from tannery effluent in batch system. Chem. Eng. J. 197, 1–9 (2012).
Farhadi, S., Aminzadeh, B., Torabian, A., Khatibikamal, V. & Alizadeh Fard, M. Comparison of COD removal from pharmaceutical wastewater by electrocoagulation, photoelectrocoagulation, peroxi-electrocoagulation and peroxi-photoelectrocoagulation processes. J. Hazard. Mat. 219-220, 35–42 (2012).
Hernández-Francisco, E., Peral, J. & Blanco-Jerez, L. M. Removal of phenolic compounds from oil refinery wastewater by electrocoagulation and Fenton/photo-Fenton processes. J. Wat. Process Eng. 19, 96–100 (2017).
Tejera, J., Hermosilla, D., Gascó, A., Negro, C. & Blanco, A. Combining Coagulation and Electrocoagulation with UVA-LED Photo-Fenton to Improve the Efficiency and Reduce the Cost of Mature Landfill Leachate Treatment. Molecules 26, 6425 (2021).
Zhang, D. et al. Efficient degradation of PFOA in water by persulfate-assisted and UV-activated electrocoagulation technique using Fe foam electrode. Electrochim. Acta 434, 141296 (2022).
Wang, Y. et al. The synergistic effect of electrocoagulation coupled with E-peroxone process for shale gas fracturing flowback water treatment. Chemosphere 262, 127968 (2021).
Ren, Y. Z. et al. Sonoelectrochemical degradation of phenol in aqueous solutions. Ultrason. Sonochem. 20, 715–721 (2013).
Zhou, M. H., Wu, C. Z. & Wang, D. H. Synergistic effects of UV/Fe3+ combined with electrocatalysis for p-Nitrophenol degradation. Chinese. Chem. Lett. 13, 375–378 (2002).
AlJaberi, F. Y. et al. Can electrocoagulation technology be integrated with wastewater treatment systems to improve treatment efficiency? Env. Res. 214, 113890 (2022).
Mameri, N. et al. Defluoridation of septentrional Sahara water of north Africa by electrocoagulation process using bipolar aluminium electrodes. Wat. Res. 32, 1604–1612 (1998).
Holt, P. K. Electrocoagulation: unravelling and synthesising the mechanisms behind a water treatment process. (The University of Sydney-Department of chemical engineering, 2002).
Byrne, D. M., Lohman, H. A. C., Cook, S. M., Peters, G. M. & Guest, J. S. Life cycle assessment (LCA) of urban water infrastructure: Emerging approaches to balance objectives and inform comprehensive decision-making. Env. Sci. Wat. Res. Tech. 3, 1002–1014 (2017).
Guest, J. S. et al. A new planning and design paradigm to achieve sustainable resource recovery from wastewater. Env. Sci. Tech. 43, 6126–6130 (2009).
Lundie, S., Peters, G. M. & Beavis, P. C. Life cycle assessment for sustainable metropolitan water systems planning. Env. Sci. Tech. 38, 3465–3473 (2004).
Corominas, L. et al. The application of life cycle assessment (LCA) to wastewater treatment: A best practice guide and critical review. Wat. Res. 184, 116058 (2020).
Igos, E., Benetto, E., Venditti, S., Kohler, C. & Cornelissen, A. Comparative and integrative environmental assessment of advanced wastewater treatment processes based on an average removal of pharmaceuticals. Wat. Sci. Tech. 67, 387–394 (2012a).
Hoibye, L. et al. Sustainability assessment of advanced wastewater treatment technologies. Wat. Sci. Tech. 58, 963–968 (2008).
Munoz, I., Rodriguez, A., Rosal, R. & Fernandez-Alba, A. R. Life Cycle Assessment of urban wastewater reuse with ozonation as tertiary treatment: a focus on toxicity-related impacts. Sci. Total Env. 407, 1245–1256 (2009).
Tarpani, R. R. Z. & Azapagic, A. Life cycle environmental impacts of advanced wastewater treatment techniques for removal of pharmaceuticals and personal care products (PPCPs). J. Env. Manag 215, 258–272 (2018).
Rahman, S. M., Eckelman, M. J., Onnis-Hayden, A. & Gu, A. Z. Comparative life cycle assessment of advanced wastewater treatment processes for removal of chemicals of emerging concern. Env. Sci. Tech. 52, 11346–11358 (2018).
Li, Y. et al. Life cycle assessment of advanced wastewater treatment processes: involving 126 pharmaceuticals and personal care products in life cycle inventory. J. Env. Manag 238, 442–450 (2019).
Rodriguez, A. et al. Environmental optimization of continuous flow ozonation for urban wastewater reclamation. Sci. Total Env. 437, 68–75 (2012).
Foteinis, S., Borthwick, A. G. L., Frontistis, Z., Mantzavinos, D. & Chatzisymeon, E. Environmental sustainability of light-driven processes for wastewater treatment applications. J. Clean. Prod. 182, 8–15 (2018).
Ioannou-Ttofa, L., Foteinis, S., Chatzisymeon, E., Michael-Kordatou, I. & Fatta- Kassinos, D. Life cycle assessment of solar-driven oxidation as a polishing step of secondary-treated urban effluents. J. Chem. Tech. Biotech. 92, 1315–1327 (2017).
Gallego-Schmid, A. et al. Environmental assessment of solar photo-Fenton processes in combination with nanofiltration for the removal of micro-contaminants from real wastewaters. Sci. Total Env 650, 2210–2220 (2019).
Farre, M. J. et al. Life cycle assessment of the removal of Diuron and Linuron herbicides from water using three environmentally friendly technologies. Env. Tech. 28, 819–830 (2007).
Chatzisymeon, E., Foteinis, S., Mantzavinos, D. & Tsoutsos, T. Life cycle assessment of advanced oxidation processes for olive mill wastewater treatment. J. Clean. Prod. 54, 229–234 (2013).
Lopez Grimau, V., Amante García, B., Canals Casals, L. 21st Int. Cong. Project Manag. Eng. (Cádiz, 2017).
Ahangarnokolaei, M. A., Attarian, P., Ayati, B., Ganjidoust, H. & Rizzo, L. Life cycle assessment of sequential and simultaneous combination of electrocoagulation and ozonation for textile wastewater treatment. J. Env. Chem. Eng. 9, 106251 (2021).
Ilhan, F., Apaydin, O., Kurt, U., Arslankaya, E. & Gonullu, M. T. in Third Int. Conf. Env. Sci. Tech. (ICEST). 1-6 (Houston, Texas, USA, 2007).
Özyurt, B., Camcıoğlu, Ş. & Hapoglu, H. A consecutive electrocoagulation and electrooxidation treatment for pulp and paper mill wastewater. Desalin. Wat. Treat. 9, 214–228 (2017).
Heffron, J., Ryan, D. R. & Mayer, B. K. Sequential electrocoagulation-electrooxidation for virus mitigation in drinking water. Wat. Res 160, 435–444 (2019).
Ghanbari, F., Wu, J., Khatebasre, M., Ding, D. & Lin, K. Y. A. Efficient treatment for landfill leachate through sequential electrocoagulation, electrooxidation and PMS/ UV/CuFe2O4 process. Sep. Pur. Tech. 242, 116828 (2020).
Isik, Z., Arikan, E. B., Ozay, Y., Bouras, H. D. & Dizge, N. Electrocoagulation and electrooxidation pre-treatment effect on fungal treatment of pistachio processing wastewater. Chemosphere 244, 125383 (2020).
Özyonar, F. & Korkmaz, M. U. Sequential use of the electrocoagulation-electrooxidation processes for domestic wastewater treatment. Chemosphere 290, 133172 (2022).
Hsing, H. J., Chiang, P. C., Chang, E. E. & Chen, M. Y. The decolorization and mineralization of Acid Orange 6 azo dye in aqueous solution by advanced oxidation processes: a comparative study. J. Hazard. Mat. 141, 8–16 (2007).
Song, S., Yao, J., He, Z. Q., Qiu, J. & Chen, J. Effect of operational parameters on the decolorization of CI Reactive Blue 19 in aqueous solution by ozone-enhanced electrocoagulation. J. Hazard. Mat. 152, 204–210 (2008).
Wu, C. H., Chang, C. L. & Kuo, C. Y. Decolorization of Procion Red MX-5B in electrocoagulation (EC), UV/TiO2 and ozone-related systems. Dyes Pigm 76, 187–194 (2008).
Barrera-Díaz, C., Bernal-Martínez, L. A., Natividad, R. & Peralta-Hernández, J. M. Synergy of Electrochemical/O3 Process with Aluminum Electrodes in Industrial Wastewater Treatment. Ind. Eng. Chem. Res 51, 9335–9342 (2012).
García-García, P., Arroyo-López, F. N. & Rodríguez-Gómez, F. Partial purification of iron solutions from ripe table olive processing using ozone and electro-coagulation. Sep. Pur. Tech. 133, 227–235 (2014).
Roa-Morales, G. et al. Removal of color and chemical oxygen demand using a coupled coagulation-electrocoagulation-ozone treatment of industrial wastewater that contains offset printing dyes. J. Mex. Chem. Soc. 58, 362–368 (2014).
Barrera Díaz, C.E. & González-Rivas, N. The Use of Al, Cu, and Fe in an Integrated Electrocoagulation-Ozonation Process. J. Chem. 12, 1–7 (2015).
Behin, J., Farhadian, N., Ahmadi, M. & Parvizi, M. Ozone assisted electrocoagulation in a rectangular internal-loop airlift reactor: application to decolorization of acid dye. J. Wat. Process Eng. 8, 171–178 (2015).
Daghrir, R., Gherrou, A., Noel, I. & Seyhi, B. Hybrid process combining electrocoagulation, electroreduction, and ozonation processes for the treatment of grey wastewater in batch mode. J. Env. Eng. 142, 04016008 (2016).
Wagh, M. P., Nemade, P. D., Naik, U. & Sengupta, A. Enhancing color and chemical oxygen demand degradation in distillery spent wash by electrocoagulation and ozone assisted electrocoagulation. Desalin. Wat. Treat. 197, 213–223 (2020).
Wagh, M. P., Nemade, P. D. & Sengupta, A. Augmentation with Ozone‑Assisted Electrochemical degradation of distillery spent wash for the removal of color and chemical oxygen demand. Int. J. Env. Sci. Tech. 18, 619–630 (2021).
Mehralian, M., Khashij, M. & Dalvand, A. Treatment of cardboard factory wastewater using ozone-assisted electrocoagulation process: optimization through response surface methodology. Env. Sci. Pollut. Res. 28, 45041–45049 (2021).
Roa-Morales, G., Campos-Medina, E., Aguilera-Cotero, J., Bilyeu, B. & Barrera-Diaz, C. Aluminum electrocoagulation with peroxide applied to wastewater from pasta and cookie processing. Sep Pur. Tech. 54, 124–129 (2007).
Rangel-Peraza, J. G., Prado, M. A., Amabilis-Sosa, L. E., Bustos-Terrones, Y. A. & Ramírez-Pereda, B. Malathion removal through peroxi-electrocoagulation and photocatalytic treatments optimization by statistical analysis. Int. J. Electrochem. Sci. 15, 8253–8264 (2020).
Kamarehie, B. et al. Experimental data of electric coagulation and photo-electro-phenton process efficiency in the removal of metronidazole antibiotic from aqueous solution. Data Brief. 1, 96–101 (2018).
Aziz, A. R. A., Asaithambi, P. & Daud, W. M. A. B. W. Combination of Electrocoagulation with Advanced Oxidation Processes for the Treatment of Distillery Industrial Effluent. Process Saf. Env. Prot. 99, 227–235 (2016).
Yang, B. et al. Highly efficient removal of perfluorooctanoic acid from aqueous solution by H2O2-enhanced electrocoagulation-electroflotation technique. Emerg. Cont. 2, 49–55 (2016).
Gong, C., Zhang, Z., Zhang, J. & Li, S. The addition of hydrogen peroxide in the electrocoagulation treatment for improving toxic organic matters removal: A comparative study. Sep. Sci. Tech. 52, 1404–1411 (2017).
Bashir, M. J. K., Lim, J. H., Abu Amr, S. S., Wong, L. P. & Sim, Y. L. Post treatment of palm oil mill effluent using electro-coagulation-peroxidation (ECP) technique. J. Clean. Prod. 208, 716–727 (2019).
Malakootian, M. & Ahmadian, M. Removal of ciprofloxacin from aqueous solution by electro-activated persulfate oxidation using aluminum electrodes. Wat. Sci. Tech. 80, 587–596 (2019).
Sharma, S. & Simsek, H. Treatment of canola-oil refinery effluent using electrochemical methods: A comparison between combined electrocoagulation + electrooxidation and electrochemical peroxidation methods. Chemosphere 221, 630–639 (2019).
Asha, A., Muthukrishnaraj, A. & Balasubramanian, N. Improvement of biodegradability index through electrocoagulation and advanced oxidation process. Int. J. Ind. Chem. 5, 4 (2014).
Thiam, A., Zhou, M., Brillas, E. & Sirés, I. Two-step mineralization of Tartrazine solutions: Study of parameters and by-products during the coupling of electrocoagulation with electrochemical advanced oxidation processes. Appl. Catal. B 150-151, 116–125 (2014).
Akyol, A., Can, O. T. & Bayramoglu, M. Treatment of hydroquinone by photochemical oxidation and electrocoagulation combined process. J. Wat. Process Eng. 8, 45–54 (2015).
Suárez-Escobar, A., Pataquiva-Mateus, A. & López-Vasquez, A. Electrocoagulation -Photocatalytic process for the treatment of lithographic wastewater. Optimization using response surface methodology (RSM) and kinetic study. Catal. Today 266, 120–125 (2016).
Flores, N. et al. Treatment of olive oil mill wastewater by single electrocoagulation with different electrodes and sequential electrocoagulation/electrochemical Fenton-based processes. J. Hazard. Mat. 347, 58–66 (2018).
Azerrad, S. P., Isaacs, M. & Dosoretz, C. G. Integrated treatment of reverse osmosis brines coupling electrocoagulation with advanced oxidation processes. Chem. Eng. J. 356, 771–780 (2019).
Dindaş, G. B. et al. Treatment of Pharmaceutical Wastewater by Combination of Electrocoagulation, Electro-Fenton and Photocatalytic Oxidation Processes. J. Env. Chem. Eng. 8, 103777 (2020).
Asaithambi, P. et al. Distillery industrial wastewater(DIW) treatment by the combination of sono(US), photo(UV) and electrocoagulation(EC) process. J. Env. Manag. 320, 115926 (2022).
Wang, W., Huang, G., Yu, J. C. & Wong, P. K. Advances in photocatalytic disinfection of bacteria: Development of photocatalysts and mechanisms. J. Env. Sci. 34, 232–247 (2015).
Acknowledgements
The support of the Research Vice-Chancellor of Qazvin University of Medical Sciences is wholeheartedly appreciated.
Author information
Authors and Affiliations
Contributions
Z.H.: Data curation, Methodology, Software, Validation, Visualization, Formal analysis, Investigation, Writing—original draft, Writing—review and editing. M.M.: Conceptualization, Validation, Visualization, Funding acquisition, Resources, Supervision, Project administration, Writing—original draft, Writing—review and editing. S.K.: Data curation, Writing—original draft, modeling and simulation, Writing—review and editing. N.K.: Software, Formal analysis, Writing—original draft, Writing—review and editing. M.H.J.: Investigation, Writing—original draft, Writing—review and editing. M.S.: Supervision, Writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hajalifard, Z., Mousazadeh, M., Khademi, S. et al. The efficacious of AOP-based processes in concert with electrocoagulation in abatement of CECs from water/wastewater. npj Clean Water 6, 30 (2023). https://doi.org/10.1038/s41545-023-00239-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41545-023-00239-9