Abstract
The remarkable success of messenger RNA (mRNA) vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has propelled the rapid development of this vaccination technology in recent years. Over the last three decades, numerous studies have shown the considerable potential of mRNA vaccines that elicit protective immune responses against pathogens or cancers in preclinical studies or clinical trials. These effective mRNA vaccines usually contain specific adjuvants to obtain the desired immune effect. Vaccine adjuvants traditionally are immunopotentiators that bind to pattern recognition receptors (PRRs) of innate immune cells to increase the magnitude or achieve qualitative alteration of immune responses, finally enhancing the efficacy of vaccines. Generally, adjuvants are necessary parts of competent vaccines. According to the existing literature, adjuvants in mRNA vaccines can be broadly classified into three categories: 1) RNA with self-adjuvant characteristics, 2) components of the delivery system, and 3) exogenous immunostimulants. This review summarizes the three types of adjuvants used in mRNA vaccines and provides a comprehensive understanding of molecular mechanisms by which adjuvants exert their functions in mRNA vaccines.
Similar content being viewed by others
Introduction
The mRNA vaccines have emerged as a promising vaccine format compared to conventional vaccines because of their advantages: high potency, rapid development, safe administration, and low-cost manufacture1. Since the 1990s, researchers have made successive attempts to use mRNA to produce target proteins and develop mRNA vaccines against infectious diseases in preclinical models2,3. Nowadays, mRNA vaccines have shown great potential in many preclinical and clinical studies against multiple diseases, such as autoimmune encephalomyelitis, tumors, infectious diseases including Zika virus disease, influenza A, coronavirus disease 2019 (COVID-2019), rabies, and so on4,5,6,7. It’s well known that mRNA vaccines have protected millions of people from the threat of SARS-Cov-2 because of their superior efficacy. This unprecedented success has proved that mRNA vaccines are safe, reliable, and potent weapons against infectious diseases. Interestingly, two recent clinical studies have shown that mRNA vaccines based on individualized neoantigens can potentiate the therapeutic efficacy of immunotherapy drugs like pembrolizumab and atezolizumab and delay the recurrence of melanoma and pancreatic cancer via inducing neoantigen-specific CD8+ cell responses8,9. These studies further highlight the promising future of mRNA vaccines in treating malignant diseases.
The mRNA vaccine usually comprises three parts: the synthetic RNA molecules that direct the production of antigens, the material for mRNA delivery, and the adjuvant component. Adjuvants generally are additional immunostimulants besides nonliving antigens in vaccines, which activate innate immunity and provide the “help” needed to enhance the magnitude and quality of the adaptive responses to provide maximum protection against specific pathogens10. Different adjuvants tend to induce various immunological reactions that can tailor the outcome of vaccines. For example, commercialized adjuvant AS03 prefers to stimulate a mixed T helper-1 (Th1) and Th2 cell response, while adjuvant AS37 stimulates a Th1-biased response and adjuvant alum induces a Th2-biased response11. It is important to note that mRNA vaccines should produce customized immune responses in order to treat different diseases. For instance, strong T follicular helper (TFH) cell and germinal center (GC) B cell responses are needed to induce high titers of neutralizing antibodies against the infectious diseases caused by viruses12. On the other hand, treating other diseases such as tumors and malaria needs potent antigen-specific T cell responses to produce cytotoxic cytokines like interferon-γ (IFNγ) to kill target cells13,14. One feasible way to modulate the immune responses elicited by mRNA vaccines is to use different adjuvants to tailor the immune responses and thus meet the therapeutic requirements of treating different diseases. For example, an ionizable cationic lipid DLinDMA can act as a potent adjuvant for mRNA vaccines to induce robust TFH cell and GC B cell responses to generate neutralizing antibodies, while Toll-like receptor 7/8 (TLR7/8) agonist R848 can elicit an effective anti-tumor immunity by significantly enhancing the antigen-specific CD8+ T cells responses15,16. Agonists for innate immune pathways beyond TLRs have been under test for their adjuvant activities in therapeutic vaccines for diseases with the complex immune microenvironment, such as tumors17,18. Agonists for the stimulator of interferon genes (STING) pathway, like CF501 and ADU-S100, exhibited potent adjuvant effect for mRNA vaccines in preclinical and clinical studies19,20. Ligands for the C-type lectin receptors pathway or nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) pathway also show robust adjuvant activity and deserve further investigation21,22. To summarize, adjuvants can influence the immune response types of mRNA vaccines and determine whether to improve the level of neutralizing antibodies or enhance antigen-specific cytotoxicity. Thus, it is vital to deepen our understanding of adjuvants in mRNA vaccines, which could provide valuable insights for designing next-generation mRNA vaccines.
Despite the rapid development of mRNA vaccine technology, there still is a lack of clear characterization of the adjuvants used in mRNA vaccines. Here, we overview the adjuvants used in mRNA vaccines in recent years by summarizing their common features and the relevant molecular mechanisms, expecting to improve the understanding of mRNA vaccine adjuvants for better vaccine design and preparation.
Adjuvants based on the intrinsic characteristics of mRNA
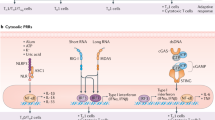
Adjuvants often are a kind of immunostimulatory agent that elicit immune responses by binding with PRRs of innate immunity to enhance the efficacy of vaccines. It is well known that foreign RNA molecules have the potential to induce immune responses in mammalian cells. Exogenous nucleoside-unmodified mRNA used to express antigens in the mRNA vaccines has intrinsic adjuvant activity by triggering innate immune signaling. In particular, double-stranded RNA (dsRNA) can activate TLR3, single-stranded RNA can activate murine TLR7, and RNA oligonucleotides with phosphorothioate internucleotide linkages are ligands for human TLR823. Poly uracil (U) and short dsRNA with 5’ triphosphate at the blunt end could act as adjuvants for mRNA vaccines by enhancing the immune responses through TLR3 and retinoic acid-inducible gene (RIG)-I signal pathways without hampering the antigen expression24,25. The activation of TLRs and RIG-I signaling could induce the production of proinflammatory cytokines such as tumor-associated factor-α (TNF-α), interleukin 6 (IL-6), IL-12, IL-1β and IFNα/β (Fig. 1), which on one side strengthen the desired protective immunity of mRNA vaccines, but on the other side may cause excessive inflammation18. The seminal work by Kariko et al. found that RNA molecules without nucleoside modifications activated TLR or RIG-I signaling to evoke antiviral-like immune responses, which may impair RNA translation and lead to RNA degradation26. Such immune activation can be avoided by nucleoside-modified mRNA, such as pseudouridine, which has been widely used in mRNA vaccines23,27. A recent study showed that the modified mRNA in the BNT162b2 mRNA vaccine from Pfizer-BioNTech may be recognized by melanoma differentiation-associated protein 5 (MDA-5) to trigger the production of IFNα, finally contributing to the magnitude of antigen-specific T cell and antibody responses28. It is intriguing that the host interferon responses in most scenario suppress mRNA translation and induce RNA degradation, but in some other cases promote antigen-specific responses induced by mRNA vaccines. Further work is warranted to delineate the underlying mechanism. Overall, RNA molecules in the mRNA vaccines can have adjuvant activity via intrinsic immune-stimulating characteristics, which should be considered when designing mRNA vaccines.
Adjuvants often act as ligands for PRRs in innate immunity to activate corresponding immune signaling pathways. These activated signaling pathways can promote the secretion of cytokines like IL-12, IL-1β, and IFNα/β and ultimately improve the immune effect of vaccines. IRFs, interferon regulatory factors; NF-κB, nuclear factor kappa light chain enhancer of activated B cells; ROS, reactive oxygen species; NLRP3, NOD-, LRR- and pyrin domain-containing protein 3; 5’-ppp, 5’ triphosphate.
Aside from the intrinsic self-adjuvant characteristic of ribonucleotide sequences, mRNA can be used for producing functional immune proteins such as cytokines and antibodies to promote antigen presentation and improve vaccine efficacy. Antigen-presenting cells (APCs) are critical for vaccinology, but how to efficiently deliver antigens to APCs has always been challenging. Fc receptors are widely expressed on APCs to recognize and bind with the Fc portion of antibodies29. Therefore, making antigens fused with the Fc portion is a helpful method to deliver the antigens to APCs. Recently, there has been a report that an mRNA vaccine encoding the human Fc-conjugated receptor binding domain of SARS-CoV-2 (RBD-hFc) can efficiently prevent SARS-CoV-2 infection in the K18 promoter-driven human angiotensin-converting enzyme 2 (K18-ACE2) transgenic mouse model30. In another study, a constitutively active mutant of STING encoded by mRNA was used as an adjuvant to amplify antigen-specific CD8+ T-cell responses induced by mRNA vaccine to inhibit TC-1 tumor growth31. In addition, restoring the immunological fitness of the tumor microenvironment by mRNA encoding immune stimulatory molecules and immune checkpoint blockade antibodies is also under test in mRNA cancer vaccines17. For example, TriMix is an mRNA cocktail composed of three mRNA molecules encoding CD40L, CD70, and a constitutively active form of TLR4, which is currently under clinical trials as an mRNA therapy in dendritic cells (DCs) vaccine to prevent melanoma progression32,33. The mRNA-encoding bispecific T-cell engager (BiTE) is another promising therapy for eliminating advanced tumors, which encodes a fusion protein with an antibody targeting CD3 on one fragment antigen-binding arm and a tumor antigen on the other arm to induce sustained endogenous synthesis of the bispecific T cell-engaging antibodies34. Therefore, using mRNA to produce antigens together with specific functional immune proteins as adjuvants, such like STING protein and anti-CD3 antibody, is a feasible way to boost the efficacy of mRNA vaccines. For mRNA vaccines in cancer immunotherapy, directing mRNA to produce immune modulators like antibodies against programmed cell death 1 (PD-1) may also help amplify the mRNA vaccine efficacy, which deserves further study.
Adjuvants based on the components of the delivery system
Due to the physiochemical features like biological macromolecule and negative charge, naked mRNA cannot efficiently enter the cells. Delivery tools are required to facilitate the transfer of mRNA into the cytoplasm for protein translation. Although there are a number of methods to promote the cytoplasmic entry of mRNA, lipid nanoparticle (LNP) has recently emerged as the optimal delivery material in this field35. LNP is often comprised of cationic lipids that form the complex with negatively charged mRNA and structural “helper” lipids such as cholesterol, phospholipids, or polyethylene glycol (PEG)-conjugated lipid17. Some lipid components in the LNP system have immunostimulatory characteristics that can act as adjuvants for mRNA vaccines (Table 1). Specifically, a lipid-coated calcium phosphate nanoparticle can act as an artificial nanosized Ca2+ reservoir to promote DC maturation by upregulating co-stimulatory molecules like CD80 and CD86 via providing extra Ca2+ in the cytoplasm36. In addition, the cationic lipid components may be crucial for the adjuvant activities of LNP. The LNP based on ionizable cationic lipid DLinDMA is immunostimulatory and can act as an adjuvant for nucleoside-modified mRNA vaccines to elicit potent TFH cell responses and GC B cell responses with abundant neutralizing antibodies15,37,38. Intriguingly, although the adjuvant activity of DLinDMA relies on IL-6 production instead of MyD88 or MAVS-dependent signal pathways, there is still a poor understanding of the specific molecular mechanism by which it triggers the production of IL-615. In addition, cationic lipid-like material C1 can deliver mRNA into the intracellular space, promote the release of inflammatory cytokines such as IL-1β, IL-6, and IL-12P70, and upregulate the expression of co-stimulatory molecules through the TLR4 signaling pathway39. Lipidoid C12-TLRa (composed of Epoxide C12 and amine-containing TLR7/8 agonist) can promote mRNA vaccine delivery and TLR response, together inducing high levels of neutralizing antibodies40. Another ionizable lipid-like material used for mRNA vaccine, A2-Iso5–2DC18 (A2), is composed of an unsaturated lipid tail, a dihydroimidazole linker, and a cyclic amine head group, which can activate STING signaling (Fig. 1) and the release of cytokines like C-X-C motif chemokine 10 (CXCL10), type I IFNs to enhance immune responses41. A non-nucleotide STING agonist-derived amino lipid, SAL12, can also formulate into LNP for mRNA delivery and induce the production of IFNβ to elicit potent neutralizing antibodies against SARS-Cov-242. Dimethyldioctadecylammonium (DDA) is a quaternary ammonium lipid that can conjugate with mRNA and elicit innate immunity and Th1 cell responses, thereby serving as a potent immune adjuvant for mRNA vaccines against rabies virus43. However, the inherent immune stimulatory features of the lipid materials used for mRNA vaccines are not always beneficial for the vaccine. A recent study reported that the lipid components (DOTMA and DOPE) instead of unmodified mRNA in mRNA vaccines would promote the generation of mitochondrial ROS in monocytes to activate NLRP3 inflammasome and release IL-1β, triggering the inflammatory side effect of mRNA vaccine in human beings44. These studies emphasize the importance of the independent immune-stimulatory effect of lipid components. Hence, screening suitable and feasible lipid components with proper immune activation function is valuable for the rational design and development of future mRNA vaccines.
In addition, the physical properties may influence the immunological activity of adjuvants. Recently, a study reported that the particulate form instead of the soluble form of mannans can act as adjuvants in vaccine formulations directed against respiratory viruses45. Moreover, a pathogen-mimicking hollow nanoparticle displaying mannan can activate the Dectin-2 and TLR4 receptors in DCs, and induce the differentiation of Th17 cells for anti-tumor responses46. Therefore, the physical characteristics of adjuvants, such as size and particle morphology, should also be considered when developing vaccines. Given that many mRNA vaccines are also based on nano-delivery like the LNP systems, it is important to consider the effect of the physical properties of nanoparticles on vaccine efficacy as well as potential undesired inflammatory side effects.
Adjuvants based on additional immunostimulants
Adjuvants used in mRNA vaccines can also be immunostimulants other than lipid components or mRNA-encoded immune proteins. Arginine-rich protamine peptides can condense and deliver mRNA in vivo by forming a complex with mRNA. The complex formed by protamine and mRNA can further activate TLR7/8 pathways to elicit B-cell and T-cell-dependent responses against influenza A or tumors47,48. A protamine-containing delivery platform for mRNA vaccine has been evaluated in clinical trials for treating melanoma, prostate cancer, and non-small-cell lung cancer49,50,51. Another peptide, the cholesterol-modified cationic peptide DP7 (VQWRIRVAVIRK), can activate the TLR2-MyD88-IKK-NF-κB pathway and improve the immune responses evoked by neoantigen vaccine, thereby serving as an effective adjuvant for individualized cancer vaccine52. The liposomes with DP7 in mRNA vaccines are efficacious in stimulating DCs maturation, generating CD103+ DCs (a major DC subtype for antigen presentation), and secreting proinflammatory cytokines such as TNFα, IL-6, IL-12p70 to facilitate the mRNA vaccine efficacy53.
A proof-of-concept adjuvant, palmitic acid-modified TLR7/8 agonist R848 (C16-R848), has been shown to promote the efficiency of mRNA delivery and induce an effective adaptive immune response16. A glycolipid antigen, α-GC, which could be presented in the MHC-I-like molecule CD1d on APCs to activate invariant natural killer T (NKT) cells, is also used in mRNA vaccines to boost the antitumor efficacy54. Taken together, the immunostimulants demonstrated with adjuvant activities in the mRNA vaccines are functional peptides, ligands for innate immune receptors such as TLRs, and agonists for innate immune cells such as NKT cells. These additional immunostimulants often work by activating the TLRs signaling. Looking for agonists that trigger innate immune pathways independent of TLRs may be worth further exploring as adjuvants in mRNA vaccines.
The adjuvant components in COVID-19 mRNA vaccines
The pandemic of COVID-2019 has expedited the development of mRNA vaccine research. During the battle against SARS-CoV-2, Moderna and BioNTech vaccines based on pseudouridine-modified mRNA and the LNP delivery system have achieved unprecedented success. The nano-delivery system for mRNA vaccines plays a dual role by acting as a carrier and an adjuvant. In addition, mRNA without pseudouridine modification has the potential self-adjuvant activity to induce inflammatory effects through activating TLRs signaling23. However, a recent clinical trial reports that the unmodified mRNA vaccine (CVnCoV) from CureVac showed only 47% efficacy in preventing SARS-CoV-2 infection in phase III trials, which was much weaker than the mRNA-modified vaccines (mRNA-1273 and BNT162b2)55,56. Moreover, the study of immunization dose and neutralization levels across CVnCoV, mRNA-1273, and BNT162b2 suggests the lowest neutralization of CVnCoV compared to the other two vaccines when the recommended dose was administered57. There are several possible explanations for the different efficacy across three mRNA vaccines. Firstly, the immunogenicity of mRNA plays a critical role in the vaccine efficacy23,58. CVnCoV uses an unmodified mRNA construct, which may act as a ligand for PRRs like TLRs to cause inflammatory reactions. A report points out that the mRNA vaccine-induced type I IFN response might establish a temporary state limiting the initial antigen expression, which is harmful to vaccine efficacy59. Secondly, the immunization dose of CVnCoV is 12 μg while mRNA-1273 and BNT162b2 are 30 μg and 100 μg, respectively56,60,61. The lower mRNA dose of CVnCoV may also weaken the vaccine’s effectiveness.
The mRNA vaccines BNT162b2 and mRNA-1273 are similarly effective in protecting people from COVID-19 infection by different viral variants. Although both vaccines are based on nucleoside-modified mRNA construct and the LNP technology, there are still some differences in how the two vaccines are designed. The administered dosage of mRNA-1273 is lower than BNT162b2. Moreover, mRNA-1273 uses the ionizable lipid SM-102 to achieve a stable nanoparticle structure, while BNT162b2 chooses a different lipid named ALC-031562. Intriguingly, nucleoside-modified mRNA vaccines based on LNP are not immunosilent and have potent immunogenicity15,28,44. The immunogenicity of BNT162b2 and mRNA-1273 could generally be attributed to some factors, including the immune recognition of nucleoside-modified mRNA, the adjuvant activity of LNP, and the manufacturing process of vaccine62. Modifications of nucleosides can help mRNA escape from the sensing of PRRs like TLRs and RIG-I. Intriguingly, BNT162b2 still activates the MDA-5 signaling pathway to induce type I IFNs to elicit antigen-specific T cell responses and generate neutralizing antibodies23,28. LNP could also serve as highly effective adjuvants besides delivery agents62. Empty LNP has enhanced B and T cell responses in subunit vaccines against hepatitis B or dengue virus63,64. Recently, the ionizable lipid component has been found responsible for LNP-elicited innate immune responses in vivo, as LNP with mRNA can induce the production of chemokines like CXCL10, cytokines IL-1β and IL-6, while empty LNP without the ionizable lipid loses its ability to induce efficient antibody responses15. Therefore, the adjuvant activity of LNP may also account for the immunogenicity of mRNA vaccines BNT162b2 and mRNA-1273. In addition, during the preparation of vaccines, dsRNA would be produced as a byproduct during the in vitro transcription (IVT) process when synthesizing mRNA. DsRNA could induce potent inflammatory responses via various intracellular PRRs sensors that contribute to the immunogenicity of mRNA vaccines65. These findings suggest that the nucleosides modification of mRNA construct, optimizing the preparation process of antigen-encoding mRNA by purification techniques, and screening for suitable ionizable lipids with delivery and adjuvant activities will be critical for successful mRNA vaccines.
Summary
In conclusion, the adjuvants in mRNA vaccines can be generally classified into three categories: (1) RNA with self-adjuvant activities from its nucleotide sequences or encoded protein; (2) the components of the delivery system, especially ionizable cationic lipids in LNP; (3) exogenous immunostimulants. Adjuvants can guide the immune response type of mRNA vaccines, thus selecting appropriate adjuvants is essential for designing mRNA vaccines to treat different diseases. An ideal adjuvant for mRNA vaccines should boost the vaccine efficacy by eliciting the desired potent immune response and helping overcome the drawbacks of mRNA, such as low stability and translation efficiency1. Ionizable cationic lipids in the LNP system can tremendously impact the efficacy of mRNA vaccines15,66,67. It is important to conduct further studies on the cationic lipid components by screening efficient delivery agents with adjuvant activity and low toxicity to prepare mRNA vaccines. Moreover, utilizing mRNA to encode specific functional immune proteins and screening agonists of innate immune receptors as applicable adjuvants are also promising approaches for the adjuvant development of mRNA vaccines. Searching for competent adjuvants will promote a better understanding of mRNA vaccines and help develop safer and more efficacious mRNA vaccines against different diseases.
Data availability
The data of this article are available from the corresponding author upon reasonable request.
References
Pardi, N., Hogan, M. J., Porter, F. W. & Weissman, D. mRNA vaccines - a new era in vaccinology. Nat. Rev. Drug Discov. 17, 261–279 (2018).
Wolff, J. A. et al. Direct gene transfer into mouse muscle in vivo. Science 247, 1465–1468 (1990).
Martinon, F. et al. Induction of virus-specific cytotoxic T lymphocytes in vivo by liposome-entrapped mRNA. Eur. J. Immunol. 23, 1719–1722 (1993).
Krienke, C. et al. A noninflammatory mRNA vaccine for treatment of experimental autoimmune encephalomyelitis. Science 371, 145–153 (2021).
Richner, J. M. et al. Modified mRNA vaccines protect against Zika virus infection. Cell 168, 1114–1125.e1110 (2017).
Chahal, J. S. et al. Dendrimer-RNA nanoparticles generate protective immunity against lethal Ebola, H1N1 influenza, and Toxoplasma gondii challenges with a single dose. Proc. Natl Acad. Sci. USA 113, E4133–E4142 (2016).
Alberer, M. et al. Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: an open-label, non-randomised, prospective, first-in-human phase 1 clinical trial. Lancet 390, 1511–1520 (2017).
Rojas, L. A. et al. Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer. Nature 618, 144–150 (2023).
mRNA Vaccine Slows Melanoma Recurrence. Cancer Discov 13, 1278, https://doi.org/10.1158/2159-8290.Cd-nb2023-0028 (2023).
Coffman, R. L., Sher, A. & Seder, R. A. Vaccine adjuvants: putting innate immunity to work. Immunity 33, 492–503 (2010).
Arunachalam, P. S. et al. Adjuvanting a subunit COVID-19 vaccine to induce protective immunity. Nature 594, 253–258 (2021).
Andreano, E. et al. B cell analyses after SARS-CoV-2 mRNA third vaccination reveals a hybrid immunity like antibody response. Nat. Commun. 14, 53 (2023).
Mallory, K. L. et al. Messenger RNA expressing PfCSP induces functional, protective immune responses against malaria in mice. NPJ Vaccines 6, 84 (2021).
Sahin, U. et al. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature 585, 107–112 (2020).
Alameh, M. G. et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity 54, 2877–2892. (2021).
Islam, M. A. et al. Adjuvant-pulsed mRNA vaccine nanoparticle for immunoprophylactic and therapeutic tumor suppression in mice. Biomaterials 266, 120431 (2021).
Van Hoecke, L. et al. mRNA in cancer immunotherapy: beyond a source of antigen. Mol. Cancer 20, 48 (2021).
Pulendran, B., Arunachalam, P. S. & O'Hagan, D. T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 20, 454–475 (2021).
Liu, Z. et al. A novel STING agonist-adjuvanted pan-sarbecovirus vaccine elicits potent and durable neutralizing antibody and T cell responses in mice, rabbits and NHPs. Cell Res. 1–19, https://doi.org/10.1038/s41422-022-00612-2 (2022).
Meric-Bernstam, F. et al. Phase I Dose-Escalation Trial of MIW815 (ADU-S100), an Intratumoral STING Agonist, in Patients with Advanced/Metastatic Solid Tumors or Lymphomas. Clin. Cancer Res. https://doi.org/10.1158/1078-0432.Ccr-21-1963 (2021).
Maisonneuve, C., Bertholet, S., Philpott, D. J. & De Gregorio, E. Unleashing the potential of NOD- and Toll-like agonists as vaccine adjuvants. Proc. Natl Acad. Sci. USA 111, 12294–12299 (2014).
Chiffoleau, E. C-Type Lectin-Like Receptors As Emerging Orchestrators of Sterile Inflammation Represent Potential Therapeutic Targets. Front. Immunol. 9, 227 (2018).
Karikó, K., Buckstein, M., Ni, H. & Weissman, D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity 23, 165–175 (2005).
Uchida, S. et al. Designing immunostimulatory double stranded messenger RNA with maintained translational activity through hybridization with poly A sequences for effective vaccination. Biomaterials 150, 162–170 (2018).
Tockary, T. A. et al. Comb-structured mRNA vaccine tethered with short double-stranded RNA adjuvants maximizes cellular immunity for cancer treatment. Proc. Natl. Acad. Sci. USA 120, e2214320120 (2023).
Kariko, K. et al. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol. Ther. 16, 1833–1840 (2008).
Barbier, A. J., Jiang, A. Y., Zhang, P., Wooster, R. & Anderson, D. G. The clinical progress of mRNA vaccines and immunotherapies. Nat. Biotechnol. 40, 840–854 (2022).
Li, C. et al. Mechanisms of innate and adaptive immunity to the Pfizer-BioNTech BNT162b2 vaccine. Nat. Immunol. 23, 543–555 (2022).
Wieland, A. & Ahmed, R. Fc Receptors in Antimicrobial Protection. Curr. Top. Microbiol Immunol. 423, 119–150 (2019).
Elia, U. et al. Lipid Nanoparticle RBD-hFc mRNA Vaccine Protects hACE2 Transgenic Mice against a Lethal SARS-CoV-2 Infection. Nano Lett. 21, 4774–4779 (2021).
Tse, S. W. et al. mRNA-encoded, constitutively active STING(V155M) is a potent genetic adjuvant of antigen-specific CD8(+) T cell response. Mol. Ther. 29, 2227–2238 (2021).
Bonehill, A. et al. Enhancing the T-cell stimulatory capacity of human dendritic cells by co-electroporation with CD40L, CD70 and constitutively active TLR4 encoding mRNA. Mol. Ther. 16, 1170–1180 (2008).
Wilgenhof, S. et al. Therapeutic vaccination with an autologous mRNA electroporated dendritic cell vaccine in patients with advanced melanoma. J. Immunother. 34, 448–456 (2011).
Stadler, C. R. et al. Elimination of large tumors in mice by mRNA-encoded bispecific antibodies. Nat. Med 23, 815–817 (2017).
Gebre, M. S. et al. Novel approaches for vaccine development. Cell 184, 1589–1603 (2021).
Wang, Y., Zhang, L., Xu, Z., Miao, L. & Huang, L. mRNA Vaccine with Antigen-Specific Checkpoint Blockade Induces an Enhanced Immune Response against Established Melanoma. Mol. Ther. 26, 420–434 (2018).
Morrissey, D. V. et al. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat. Biotechnol. 23, 1002–1007 (2005).
Pardi, N. et al. Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses. J. Exp. Med 215, 1571–1588 (2018).
Zhang, H. et al. Delivery of mRNA vaccine with a lipid-like material potentiates antitumor efficacy through Toll-like receptor 4 signaling. Proc. Natl. Acad. Sci. USA 118, https://doi.org/10.1073/pnas.2005191118 (2021).
Han, X. et al. Adjuvant lipidoid-substituted lipid nanoparticles augment the immunogenicity of SARS-CoV-2 mRNA vaccines. Nat. Nanotechnol. https://doi.org/10.1038/s41565-023-01404-4 (2023).
Miao, L. et al. Delivery of mRNA vaccines with heterocyclic lipids increases anti-tumor efficacy by STING-mediated immune cell activation. Nat. Biotechnol. 37, 1174–1185 (2019).
Zhang, Y. et al. STING Agonist-Derived LNP-mRNA Vaccine Enhances Protective Immunity Against SARS-CoV-2. Nano Lett. 23, 2593–2600 (2023).
Lou, G. et al. Delivery of self-amplifying mRNA vaccines by cationic lipid nanoparticles: The impact of cationic lipid selection. J. Control Release 325, 370–379 (2020).
Tahtinen, S. et al. IL-1 and IL-1ra are key regulators of the inflammatory response to RNA vaccines. Nat. Immunol. 23, 532–542 (2022).
Borriello, F. et al. An adjuvant strategy enabled by modulation of the physical properties of microbial ligands expands antigen immunogenicity. Cell 185, 614–629.e621 (2022).
Son, S. et al. Induction of T-helper-17-cell-mediated anti-tumour immunity by pathogen-mimicking polymer nanoparticles. Nat. Biomed. Eng. 7, 72–84 (2023).
Petsch, B. et al. Protective efficacy of in vitro synthesized, specific mRNA vaccines against influenza A virus infection. Nat. Biotechnol. 30, 1210–1216 (2012).
Kallen, K. J. et al. A novel, disruptive vaccination technology: self-adjuvanted RNActive(®) vaccines. Hum. Vaccin Immunother. 9, 2263–2276 (2013).
Weide, B. et al. Direct injection of protamine-protected mRNA: results of a phase 1/2 vaccination trial in metastatic melanoma patients. J. Immunother. 32, 498–507 (2009).
Kubler, H. et al. Self-adjuvanted mRNA vaccination in advanced prostate cancer patients: a first-in-man phase I/IIa study. J. Immunother. Cancer 3, 26 (2015).
Papachristofilou, A. et al. Phase Ib evaluation of a self-adjuvanted protamine formulated mRNA-based active cancer immunotherapy, BI1361849 (CV9202), combined with local radiation treatment in patients with stage IV non-small cell lung cancer. J. Immunother. Cancer 7, 38 (2019).
Zhang, R. et al. Cholesterol-modified DP7 enhances the effect of individualized cancer immunotherapy based on neoantigens. Biomaterials 241, https://doi.org/10.1016/j.biomaterials.2020.119852 (2020).
Zhang, R. et al. DP7-C-modified liposomes enhance immune responses and the antitumor effect of a neoantigen-based mRNA vaccine. J. Control Release 328, 210–221 (2020).
Verbeke, R. et al. Broadening the Message: A Nanovaccine Co-loaded with Messenger RNA and alpha-GalCer Induces Antitumor Immunity through Conventional and Natural Killer T Cells. ACS Nano. 13, 1655–1669 (2019).
Dolgin, E. CureVac COVID vaccine let-down spotlights mRNA design challenges. Nature 594, 483 (2021).
Kremsner, P. G. et al. Efficacy and safety of the CVnCoV SARS-CoV-2 mRNA vaccine candidate in ten countries in Europe and Latin America (HERALD): a randomised, observer-blinded, placebo-controlled, phase 2b/3 trial. Lancet Infect. Dis. 22, 329–340 (2022).
Cromer, D. et al. Relating In Vitro Neutralization Level and Protection in the CVnCoV (CUREVAC) Trial. Clin. Infect. Dis. 75, e878–e879 (2022).
Chaudhary, N., Weissman, D. & Whitehead, K. A. mRNA vaccines for infectious diseases: principles, delivery and clinical translation. Nat. Rev. Drug Discov. 20, 817–838 (2021).
Pepini, T. et al. Induction of an IFN-Mediated Antiviral Response by a Self-Amplifying RNA Vaccine: Implications for Vaccine Design. J. Immunol. 198, 4012–4024 (2017).
Thomas, S. J. et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine through 6 Months. N. Engl. J. Med. 385, 1761–1773 (2021).
Baden, L. R. et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 384, 403–416 (2021).
Verbeke, R., Hogan, M. J., Loré, K. & Pardi, N. Innate immune mechanisms of mRNA vaccines. Immunity 55, 1993–2005 (2022).
Swaminathan, G. et al. A novel lipid nanoparticle adjuvant significantly enhances B cell and T cell responses to sub-unit vaccine antigens. Vaccine 34, 110–119 (2016).
Swaminathan, G. et al. A Tetravalent Sub-unit Dengue Vaccine Formulated with Ionizable Cationic Lipid Nanoparticle induces Significant Immune Responses in Rodents and Non-Human Primates. Sci. Rep. 6, 34215 (2016).
Karikó, K., Muramatsu, H., Ludwig, J. & Weissman, D. Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA. Nucleic Acids Res. 39, e142 (2011).
Packer, M., Gyawali, D., Yerabolu, R., Schariter, J. & White, P. A novel mechanism for the loss of mRNA activity in lipid nanoparticle delivery systems. Nat. Commun. 12, 6777 (2021).
Ndeupen, S. et al. The mRNA-LNP platform’s lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience 24, 103479 (2021).
Acknowledgements
The authors thank Dr. Yu Sun (The Third Affiliated Hospital of Sun Yat-sen University) for language editing. This work was supported by the National Key R&D Program of China (2021YFC2400600/2021YFC2400601), National Natural Science Foundation of China (81972692), and the Guangdong Basic and Applied Basic Research Foundation (2020B1515120018).
Author information
Authors and Affiliations
Contributions
C.X. wrote the original draft and R.Y. revised it. X.X. reviewed and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xie, C., Yao, R. & Xia, X. The advances of adjuvants in mRNA vaccines. npj Vaccines 8, 162 (2023). https://doi.org/10.1038/s41541-023-00760-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41541-023-00760-5
This article is cited by
-
In silico designed novel multi-epitope mRNA vaccines against Brucella by targeting extracellular protein BtuB and LptD
Scientific Reports (2024)
-
Progress with COVID vaccine development and implementation
npj Vaccines (2024)