Abstract
Since the discovery of messenger RNA (mRNA), there have been tremendous efforts to wield them in the development of therapeutics and vaccines. During the COVID-19 pandemic, two mRNA vaccines were developed and approved in record-breaking time, revolutionizing the vaccine development landscape. Although first-generation COVID-19 mRNA vaccines have demonstrated over 90% efficacy, alongside strong immunogenicity in humoral and cell-mediated immune responses, their durability has lagged compared to long-lived vaccines, such as the yellow fever vaccine. Although worldwide vaccination campaigns have saved lives estimated in the tens of millions, side effects, ranging from mild reactogenicity to rare severe diseases, have been reported. This review provides an overview and mechanistic insights into immune responses and adverse effects documented primarily for COVID-19 mRNA vaccines. Furthermore, we discuss the perspectives of this promising vaccine platform and the challenges in balancing immunogenicity and adverse effects.
Similar content being viewed by others
Introduction
For more than 100 years, public vaccination campaigns have remained our most successful interdictions into the prevention of human infectious diseases. Prophylactic vaccination has been implemented to increase life expectancy and improve public health, thereby saving countless lives1,2. However, while vaccine technology has significantly advanced over the past century3, the conceptualization of an “optimal” vaccine has only grown more complicated with the rapid progress in our understanding of the cellular components associated with protective immunity. Conventional vaccines have often failed against difficult-to-target and antigenically variable viruses such as human immunodeficiency virus (HIV) and hepatitis C virus (HCV)4, and due to both the time required for development processes and rapid mutation of the viral genome, some traditional vaccine technologies are poorly suited for sudden outbreaks that threaten global health and security, such as the recent coronavirus disease (COVID-19) pandemic. Considering these constraints, messenger RNA (mRNA) vaccines have represented an attractive alternative to conventional vaccines due to their cell-free, rapid, and scalable development and production5.
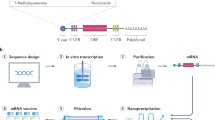
mRNA vaccine technology has been extensively studied for cancer treatment owing to its ability to trigger a potent T-cell response, well-tolerated nature, and suitability for personalized design. The details of mRNA-based cancer vaccine development are comprehensively described in recent reviews6,7,8. However, it was the COVID-19 pandemic that incited interest in mRNA for medical application, leading to expedited licensure of two mRNA vaccines9,10, with several others at various stages of development or in clinical trials11. The unprecedented pace of the SARS-CoV-2 vaccine rollout was accomplishable only because of the unique properties of mRNA vaccine platforms. Tremendous advancements have been made in the efficient delivery and expression of antigenic mRNA for current COVID-19 mRNA vaccines. Modifications such as N1-methylpseudouridine, 5′ capping, and codon optimization have been used to optimize mRNA production to maximize antigen availability12,13. To enable efficient delivery of mRNA to the cytosol of target cells, lipid nanoparticles (LNPs) comprised of ionizable cationic lipids, cholesterol, phospholipids, and polyethylene glycol (PEG) have been used. The LNP–mRNA complex is neutral at physiological pH but becomes positively charged when the LNP is sequestered and acidified in the endosome; this process is followed by fusion with the endosomal membrane and the release of mRNA into the cytosol13,14,15. Composed solely of modified mRNA encapsulated by an engineered LNP16, this approach eliminates the need for the massive pathogen culturing efforts required for attenuated or killed/split vaccines, such as the polio17 or influenza vaccine18. De novo DNA template synthesis and basic molecular biology propagation allow this technology to significantly outpace even protein subunit-based approaches, resulting in nimble, cost-effective platforms capable of rapidly deploying to the front line to improve public health19.
The COVID-19 pandemic has allowed for a real-time assessment of the strong potential of mRNA vaccines to rapidly reshape the worldwide landscape of a deadly emerging infectious disease. First-generation SARS-CoV-2 vaccines were fully designed within a few weeks of the publication of the full-length Wuhan strain spike protein sequence20 and rolled into phase one clinical trials initiated within 4 months of the virus’s identification21. Despite the rapid pace of development and limited opportunity for design optimization, the resulting vaccines performed admirably in phase three trials with initial reported efficacies of 94–95% in preventing general illness by triggering the robust production of neutralizing antibodies and moderate T-cell responses, thus placing them among some of the most successful vaccines ever developed22. The rapid production and distribution of the resulting vaccines resulted in an estimated prevention of 14.4 million deaths in the first year of their availability23.
Despite their function as rapid countermeasures to pandemic situations, various side effects of mRNA vaccines, ranging from relatively common local reactogenicity to rare serious disease outcomes, have been reported. This review summarizes the current understanding of the balance of immune stimulation and undesired adverse events induced by mRNA vaccines and speculates future directions for developing more effective and safer vaccines using this promising vaccine technology.
mRNA vaccine-induced immune responses and mechanism of action
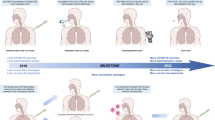
Recently, approved mRNA vaccines for COVID-19 from Pfizer/BioNTech and Moderna employed similar technological paths by utilizing antigenic mRNA encoding a nucleoside-modified prefusion form of the spike antigen (S-2P) packaged in ionizable cationic LNPs for delivery. Both formulations resulted in the strong induction of protective immunity in animal models and human vaccinees13. The immune response triggered by COVID-19 mRNA is characterized by robust production of spike-binding and neutralizing antibodies, as well as intermediate levels of T-cell responses (Fig. 1). Notably, after the first vaccination, moderate innate immune responses, including antiviral and interferon responses, were observed, while much broader and stronger inflammatory responses, such as sharp increases in inflammatory monocytes and IFN-γ, were observed after the boost immunization24.
After mRNA vaccination, secreted spike antigens are identified by cognate B-cells and induce potent neutralizing antibody responses with a strong germinal center reaction. Dendritic cells (DCs) uptake soluble spike antigens and stimulate antigen-specific CD4 and CD8 T-cells via the MHC II and cross-presentation pathways, respectively. In addition, endogenously expressed spike proteins in DCs can activate antigen-specific CD8 T-cells through the MHC I pathway. LNP, lipid nanoparticle; FDC, follicular dendritic cell; TFH, T follicular helper cell; TH1, type 1 T helper cell; CTL, cytotoxic T lymphocyte; PFN, perforin; GZB, granzyme B; IFN-γ, interferon gamma; TNF-α, tumor necrosis factor-alpha.
Despite clear-cut evidence of the efficacy of mRNA vaccination obtained throughout the pandemic9,25, challenges remain. Inefficient B-cell targeting has continued to hamper broadly neutralizing SARS-CoV-2 vaccination efforts26, as it has in HIV27 and influenza28. Characterization of independent pathways associated with B-cell activation and developmental biases in short-term effector, as well as long-term memory populations, has sparked interest in how differential activation of these pathways might contribute to vaccine potency29, targeting30, and reactogenicity31. Furthermore, the integration of robustly stimulated humoral responses with effective T-cell-mediated immunity, known to be critical in mounting primary immune responses, remains a currently unattainable goal in vaccine-induced protection, particularly at mucosal sites32. To address these challenges, it is crucial to fully understand the underlying mechanisms involved in immune responses to mRNA vaccination to maximize efficacy in the expanding arena of mRNA-based therapeutics.
Antibody production and targeting
The induction of neutralizing antibodies by SARS-CoV-2 mRNA vaccines is the main correlate of protection from infection and serious COVID-19 outcomes. Despite widespread success in stimulating robust antibody titers against the SARS-CoV-2 spike protein33, coupled with strong neutralizing reactivity against the receptor binding domain (RBD)34, first-generation mRNA vaccines have been insufficient in sustaining widespread protection. Indeed, the limitations of fixed-strain vaccination against a rapidly mutating RNA virus were obvious. The emergence of the SARS-CoV-2 Alpha strain (B.1.1.7) in South Africa during efficacy testing and the resulting decrease in efficacy raised an almost immediate red flag that small modifications in RBD primary structure might hamper overall vaccine effectiveness35. These fears were confirmed with the emergence of the Omicron subvariants, now boasting a heavily modified RBD, which resulted in a 30-fold reduction in neutralization capacity compared to that of the Wuhan strain-targeted mRNA vaccine responses36.
While unfortunate, this loss of reactivity against new and emerging viral variants is not entirely unexpected. Despite significant improvements in the elicitation of antibody titers through updated vaccine platforms, most recently in mRNA-based platforms, the ability to direct in vivo responses toward intended antigen epitopes is still lacking37. As a result, while current mRNA vaccination platforms show a strong ability to elicit robust humoral responses, scientists largely remain passive observers in the B-cell selection processes governing epitope targeting. Reactivity against undesired but immunodominant epitopes is likely to continue to challenge the development of cross-strain protective immunity, as has been shown in previous studies investigating HIV and influenza27,28.
The flexibility in mRNA vaccine design, coupled with new advances in the basic science of B-cell epitope selection, offers exciting new paths forward. In particular, the identification of a new governing principle in B-cell selection, rare epitope suppression, may provide a unique opportunity alongside mRNA vaccination to generate epitope-targeted responses38. By diversifying antigen species within a single vaccine dose, responding B-cells are placed in competition with each other for shared pools of T-cells. Thus, mRNA species may be diversified such that epitopes derived from conserved regions, likely to provide cross-protection against multiple variants, can be emphasized over strain-specific responses. Although still in its early phases of testing, paratope-focusing has been indirectly demonstrated as a plausible method for the development of chimeric nanoparticles that incorporate multiple influenza hemagglutinin species, resulting in increased generalized cross-reactivity39. Multivalent approaches in mRNA vaccination are under current investigation, with the currently available bivalent SARS-CoV-2 “updated” vaccine boosters as an important proof of concept40.
B-cell activation and memory development
In addition to challenges in B-cell selection and epitope targeting, response durability has become a primary concern in mRNA vaccination efforts against SARS-CoV-233. While early results suggested significant and persistent antigen-specific B-cell memory formation after the initial 2-dose series, analysis of initial vaccinated patient cohorts suggested a marked drop-off in circulating antibody response with an anti-spike IgG half-life of 30 days41. The result was tapering vaccine efficacy42, ultimately leading to the proposal and recommendation of an additional booster dose to maintain anti-viral titers and host protection43. Although this three-dose series is not uncommon among subunit vaccines, the durability profile of first-generation mRNA vaccines did not match that of historical vaccines of similar initial efficacy, such as polio and vaccinia vaccines, where titers are known to persist for decades without the need for additional booster doses44.
This is, perhaps, unsurprising, given the emerging complexity of B-cell responses responsible for the generation of lifelong humoral immunity. While much focus has been placed on the traditional germinal center (GC) responses assumed to be primarily responsible for vaccination response development45, an important emerging feature of primary immune responses, particularly those emerging in high-inflammation environments, is an emphasis on the extrafollicular (EF) B-cell pathway46. Initially, identified in mouse infection modeling47 and human autoimmune disease48, the COVID-19 pandemic has validated this pathway as a prominent component of early humoral immunity that is particularly emphasized in patients with severe disease. In stark contrast with GC-focused responses, EF responses undergo less somatic hypermutation, and despite being well-selected against foreign antigens, they are less frequently identified in the persisting immune memory months or years following infection, although evaluation in the context of vaccination is needed49. Identification and characterization of this pathway make it clear that the initial humoral response is not sufficient to produce well-targeted, long-lasting memory responsiveness. Indeed, it has become clearer in recent years that even the production of fully functional plasma cells is not sufficient for long-term bone marrow engraftment, as further maturation of these cells appears to be both required and contingent on unknown parameters50.
This need for careful B-cell response tuning during vaccination has led to significant investigation into the mimicry of infectious microenvironments through vaccine adjuvant delivery51. It is now well established that carefully selected combinations of microbe-associated molecular patterns and danger signals can greatly impact innate immune activation and downstream humoral immunity52,53,54. However, the unique properties of mRNA vaccine platforms have somewhat upended these fields of study due to the built-in adjuvant properties of mRNA and LNPs. Although previous subunit vaccines have shown strong dependence on Toll-like receptor (TLR) signaling and cell death pathways to drive sufficient reactivity, humoral responsiveness to mRNA vaccines might not be closely associated with these factors since the MyD88 pathway is partially responsible for optimal antibody and B-cell responses55,56. In addition to MyD88, functional studies in mice have identified antiviral pathways, particularly those governed by the RIG-I-like receptor melanoma differentiation-associated protein 5 (MDA5), as additional mediators of developing humoral immunity55. As these systems are type-I interferon dependent and dsRNA responsive, it is easy to find a connection between these responses and “natural” antiviral immunity. It is noteworthy that LNP formulation with ionizable lipids can strongly induce the proinflammatory cytokine IL-6, which, in turn, contributes to potent humoral immunity56. However, the lack of first-generation response durability in contrast to long-persisting antiviral responses suggests that additional factors are needed. The use of self-replicating mRNA vectors, an approach available uniquely to mRNA vaccination platforms, will be of high interest in continuing to push vaccination microenvironments toward true viral infection mimicry57.
Despite warranted excitement about continuing to push the edge of viral infection mimicry with innovative vaccine design, it is also clear that the introduction of inflammatory signaling into vaccination design must be carefully balanced with tolerability. It is now clear that the overstimulation of the EF response pathway—a process dependent on high TLR7 signaling and associated with an IFN-γ-induced cytokine milieu—leads not only to short-lived humoral responses but also to the emergence of cross-reactive antibodies capable of targeting self-tissue49. Thus, it is clear that the balance between localized and carefully tuned inflammatory signaling alongside systemic reactogenicity is both highly complex and critical in the conceptualization and design of second-generation mRNA platforms and beyond.
T-cell immunity by COVID-19 mRNA vaccines
Potent humoral immune responses, such as the production of neutralizing antibodies following vaccination, are considered to provide protective immunity against viral pathogens. However, a quality T-cell response is required for optimal vaccine efficacy both in fine-tuning B-cell activation and differentiation and in the removal of virus-infected cells58,59,60. For prophylactic COVID-19 vaccines, high titers of spike-binding neutralizing antibodies were closely associated with protective outcomes36,55,61. However, T-cell responses have also been linked to conferring protection against SARS-CoV-2 infection in certain contexts. For example, in convalescent rhesus macaques, the depletion of CD8 T-cells attenuated their protective immunity against subsequent challenge62. Moreover, uncomplicated recovery from COVID-19 in agammaglobulinemia patients or individuals receiving targeted anti-CD20 immunotherapy, as well as the preserved and durable responsiveness of T-cells to viral variants, imply that T-cell immunity plays a role in SARS-CoV-2 protection63,64,65.
In contrast to traditional vaccine technologies, including inactivated and protein subunit vaccines that rely on extracellular antigen capture, LNPs of current COVID-19 mRNA vaccines enable mRNAs to be released into the cytosol of target cells and produce spike proteins inside cells similar to that in the case of a viral infection13. Intracellular production of spike antigens promotes the classical loading of antigen-derived peptides on class I MHC and stimulates the activation of CD8 T-cells, while secreted antigens can be endocytosed and presented via class II MHC in antigen presenting cells and induce CD4 T-cell responses.
Follicular helper T (TFH) cells and T helper 1 (TH1) cell preference
Among CD4 T-cell subpopulations, TFH cells are specialized to support the GC reaction, where activated B-cells undergo massive proliferation and selection. Furthermore, they promote the differentiation of memory B and long-lived plasma cells and enhance antibody qualities through isotype switching and affinity maturation of B-cell receptors66. During the GC reaction, TFH cells interact with B-cells by recognizing cognate peptides presented on class II MHCs of the B-cell surface and promoting the expansion and survival of antigen-specific B-cells by providing costimulatory molecules and cytokines, such as IL-2166,67. Previous evaluations of mRNA vaccines demonstrated that such vaccines could trigger potent TFH cell responses displaying the characteristics of both TH1 and TH2 cells in mice and nonhuman primates68,69. Mechanistically, the MyD88 pathway is required for mRNA vaccine-induced potent TFH response, and intriguingly, LNP also works as a built-in adjuvant to mount normal TFH and GC B-cell responses56. COVID-19 mRNA vaccines, such as BNT162b2 and mRNA-1273, also generated efficient TFH cell responses with CD40L and IL-21 expression in rhesus macaques70,71. Notably, the COVID-19 mRNA vaccine induced higher levels of TFH cells than emulsion-adjuvanted RBD vaccines72.
In addition to TFH cells, CD4 T-cells are effectively activated and differentiate into effector T-cells that are strongly polarized to the TH1 response and secrete IFN-γ, TNF-α, and IL-2 upon restimulation by spike-derived peptides. A single immunization with the COVID-19 mRNA vaccine resulted in the effective generation of TH1-skewed polyfunctional CD4 T-cells in mice73. In nonhuman primates, potent IFN-γ and minimal IL-4 production was observed following BNT162b2 and mRNA-1273 administration70,71. The TH1-biased CD4 T-cell phenotype was also confirmed in several human mRNA vaccine studies74,75,76,77, in line with its assumed role in response against intracellular pathogens. Interestingly, TH2 response polarization has been linked to vaccine-associated enhanced immunity to respiratory disease in vaccines against other respiratory viral infections78. The aforementioned evidence implies that COVID-19 mRNA vaccines lead to desirable TH cell responses following SARS-CoV-2 infection.
Regulation of the CD8 T-cell response
Mounting the CD8 T-cell response by mRNA vaccine platforms is another relevant aspect due to intracellular antigen expression in target cells and an abundant track record of preclinical and clinical trials in the development of mRNA-based cancer immunotherapy where cytolytic T lymphocyte (CTL) activity is crucial6,19. Initially, divergent evidence was reported on the CD8 T-cell responses to COVID-19 mRNA vaccines, wherein detectable spike-specific CD8 T-cell responses were observed in BNT162b2-vaccinated rhesus macaques and humans. However, low CD8 T-cell responses were observed following mRNA-1273 vaccination of rhesus macaques and humans70,71. Nevertheless, more recent studies directly compared two approved mRNA vaccines and demonstrated similar induction of spike-specific CD8 T-cells in humans65,79. Notably, a mechanistic study in a mouse model revealed that the BNT162b2-induced CD8 T-cell response was conveyed by classical MHC I presentation, at least in part, as the CD8 T-cell response was not completely abrogated in cross-presentation-deficient Batf3 knockout mice55. Importantly, one of the cytosolic RNA-recognizing receptors, MDA5, and subsequent type I IFN signaling were required for the spike-specific CTL response following BNT162b2 vaccination55.
Adverse events of mRNA vaccines
Various vaccines against SARS-CoV-2 have been developed, and their efficacies have been compared; the effectiveness of mRNA vaccines is superior to that of those derived from previously existing technologies. However, as the effectiveness increases, adverse effects need to be carefully evaluated for the safer use and advancement of current vaccines. Recently, natural killer (NK)/monocyte subsets, dendritic cell (DC) subsets, and NK T-like cells have been shown to be involved in both mRNA vaccine effects (increasing neutralizing antibody titer) and side effects80. In addition, these cells are related to an increase in IFN-γ-inducible chemokines. These results suggest that mRNA-induced high vaccine efficacy can be closely related to several side effects and that maintaining an appropriate balance of the two opposing responses is paramount for developing a suitable vaccine. Here, we summarized the mRNA vaccine-induced side effects and their underlying mechanisms.
Local and systemic reactogenicity
In early clinical trials of COVID-19 mRNA vaccines, local side effects and symptoms, such as swelling, redness, pain, and heat, were more common in vaccine patients than in those receiving the placebo. Within one week of vaccination, one of the most common local reactions was pain at the injection site81,82. Systemic side effects, such as fatigue, headache, fever, myalgia, and arthralgia, were also more common following mRNA vaccine administration compared to those receiving the placebo. Additionally, it was reported that systemic side effects were more frequently observed in young vaccine recipients (16‒55 years of age) than in their older counterparts (>55 years of age). The higher systemic reactogenicity may imply that the immune responses are more robust in the younger population than in the older population. In comparison to the first dose, the second vaccine dose was associated with more severe side effects, such as fatigue and headache9,10.
Serious adverse events
A recent report showed that although the results were preliminary and had several limitations, 0.125% of mRNA-vaccinated individuals showed severe adverse effects, including acute myocardial infarction, Bell’s palsy, cerebral venous sinus thrombosis, Guillain–Barré syndrome, myocarditis/pericarditis (mostly at younger ages), pulmonary embolism, stroke, thrombosis with thrombocytopenia syndrome, lymphadenopathy, appendicitis, herpes zoster reactivation, neurological complications, and autoimmunity83.
Anaphylaxis is a severe adverse reaction that requires immediate medical treatment. According to a recent report, approximately 1:200,000 recipients experienced anaphylaxis as a result of the Pfizer/BioNTech vaccine10. More recently, the incidence of anaphylaxis was estimated at 2–11 cases per million after receiving the Moderna or Pfizer/BioNTech vaccines84. The rate for most vaccines is less than one per million doses. Although PEG has been considered a potential causative agent for anaphylaxis, the causative antigen and associated mechanisms remain under investigation81,85,86. Myocarditis and pericarditis are also reported serious adverse effects of mRNA vaccination, although the rate is extremely low (0.005%)87. A summary of the common mild and severe adverse effects and potential complications is shown in Fig. 2.
Risk factors and cytotoxic mechanisms associated with mRNA vaccines
mRNA vaccine safety is, at least in part, derived from the tolerability of the lipid and mRNA components included in the formulation. Lipids have been previously shown to induce host immune responses following vaccine administration, and reactivity to repeated administrations of PEG-based nanoparticles through complement-mediated mechanisms may negatively affect safety and efficacy profiles88. Another safety concern is the immunogenicity of in vitro transcript (IVT) mRNA, despite the potential advantage of stimulating cellular and humoral immunity for proper vaccination89,90,91.
As mRNA vaccines use single-stranded RNA, it was expected that the immune response would be induced through TLR7/8. However, recent reports have shown that mRNA vaccines lead to the production of type I interferon (IFN-I) via MDA5 and not TLR7 and proinflammatory cytokines, such as IL-1β and IL-655,56. These induced IFN-I and proinflammatory cytokines have the benefit of stimulating immune responses and improving vaccine efficacy, whereas they may also induce immunological adverse effects. Meanwhile, the complement system is activated when PEG, one of the LNP components, interacts with anti-PEG antibodies, which are already present in the body. However, it is merely postulated that complement-mediated phagocytosis may elicit adverse effects, such as rapid blood clearance and complement activation-related pseudoallergy (CARPA)92. In this section, we review the potential risk factors of mRNA vaccines consisting of LNPs and mRNA. A selection of adverse events with proposed associated mechanisms is delineated in Fig. 3.
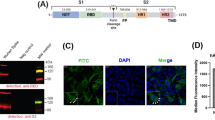
A LNP-induced immune activation. Schematic structure of mRNA encapsulated into LNP formulations composed of an ionizable cationic lipid, helper lipid, cholesterol, and PEG. LNPs can induce immune activation by stimulating Toll-like receptor (TLR) 2 and TLR4 and leading to NF-kB activation and cytokine secretion. Preexisting anti-PEG antibodies can lead to complement activation and subsequently complement-mediated phenomena, such as ABC or CARPA. B LNP-encapsulated mRNA is taken up by immune cells through endocytosis. In endosomes, TLR7/8 and TLR3 recognize ssRNA and dsRNA, respectively, and the receptors activate MyD88 and TLR3 in Toll-interleukin-1 domain-containing adapter-inducing interferon (TRIF). Eventually, the related signaling cascades transduce to the nucleus where type I IFN and pro-inflammatory cytokine production is promoted by transcription factors (NF-kB, IRF3, and IRF7). Endosomal escape is used to transport small amounts of mRNA and IVT reaction byproducts to the cytoplasm. The RNAs are recognized by RIG-I and MDA5 and then both signaling pathways activate the transcription factors for inflammatory gene expression.
LNP
Immunogenicity of LNP
The physical and chemical properties of LNP, such as its shape and charge, affect its interaction with the immune system. A previous study showed that LNP, as a built-in adjuvant, elicits potent antigen-specific CD4+ TFH cell and GC B-cell responses via induction of IL-6 production, independent of MAVS-mediated RNA-sensing pathways93. Strong immunogenicity is often responsible for reactogenicity (even toxicity) that causes local and systemic inflammation. Therefore, to ensure the efficacy and safety of mRNA vaccines, it is important to comprehend the molecular mechanism(s) by which adjuvants, such as lipid components, prompt the immune system94. Several recent studies have demonstrated that LNPs with ionizable lipids trigger proinflammatory cytokines, including IL-1β and IL-6, and subsequently, antibody and T-cell responses56,95. Moreover, the complement system and TLRs may participate in the activation of the innate immune system90.
Components and toxicity of LNP
LNP is a multicomponent lipid system that is comprised of cationic/ionizable lipids, cholesterol, helper lipids, and PEG-lipids, among others. Several studies have thus far investigated the structure-activity relationship of LNPs and the mechanisms through which LNPs interact with the immune system based on their particle size, charge, hydrophobicity, component molar fraction, and surface chemistry96,97. LNPs can cause various immune effects in vivo, including immune cell activation, inflammation, adaptive immune response, and complement activation, as well as CARPA, depending on their properties and method of delivery98,99,100.
Cationic/ionizable lipids
Cationic lipids, such as 1,2-di-O-octadecenyl-3-trimethylammonium-propane (DOTMA)101 or 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP), were used to facilitate mRNA encapsulation in the earliest liposomal delivery systems102. Cationic-based mRNA delivery systems have been proven to be related to innate immune responses in vitro and in vivo103,104. Cationic lipid nanocarriers induce the dimerization and activation of TLR2 and TLR4 proteins on antigen-presenting cells, such as DCs and macrophages, owing to their small size and electrical properties105,106,107. Consequently, the LNP–TLR complex triggers the secretion of various proinflammatory cytokines and chemokines through similar signaling pathways108 and promotes the formation of the NLRP3 inflammasome98,105. Therefore, based on safety concerns, cationic lipids have not been considered suitable materials for developing current mRNA vaccines. However, there have been attempts to develop ionizable lipids that are positively charged by the protonation of free amines at acidic pH, allowing for RNA complexation in an acidic buffer while remaining neutral at physiological pH. This ability of ionizable lipids plays a role in endosomal escape and RNA cytosolic delivery by forming destabilizing non‐bilayer structures, with a lowering of the pH103. Thus, pH-sensitive protonation or ionization properties of lipid materials in physiological and endosomal conditions are required for safe and effective LNP architecture in clinical use with systemic administration. These pH-sensitive ionizable lipids have little or no amphiphilicity at physiological pH but exhibit high amphiphilicity at endosomal pH, significantly reducing cytotoxicity and side effects in vivo. In addition, the destabilization of pH-sensitive amphiphilic cell membranes can be controlled by the gradient of LNP concentrations109. Furthermore, the pH-sensitive ionized lipids likely minimize cellular toxicity and adverse reactions through stable LNP formation and efficient intracellular delivery of nucleic acids at low N/P ratios, which can also be improved by surface modification of LNPs using biocompatible polymers110,111. Thus far, three ionizable cationic lipids, ALC-0315, SM-102, and DLin-MC3-DMA (MC3), have been approved for clinical use112. For the COVID-19 vaccines, Moderna used SM-102, and Pfizer/BioNTech employed ALC-0315 licensed from Acuitas113; these lipids are remarkably similar in structure. Recently, in a comparative study of ionizable cationic lipids for RNA therapy, ALC-0135 LNPs showed much higher siRNA knockdown efficiencies than MC-3 LNPs with markedly lower toxicities112. Taken together, these data reveal that ionizable cationic lipids are crucial components of RNA therapeutics required for maximal efficacy with limited toxicity.
PEG
PEGylation of LNPs is widely utilized to render stability and increase the plasma half-life. Currently, more than fifteen PEGylated drugs, including Doxil®, Onpattro®, Pfizer/BioNTech COVID-19 vaccines (COMIRNATY®), and MODERNA COVID-19 VACCINE®, have full approval or emergency authorizations worldwide114. Although PEG-based nanoparticles display low reactogenicity profiles115, the generation of anti-PEG antibodies is a potential issue that can be overcome with the repeated administration of PEGylated pharmaceuticals92,99.
For the first time in 1983, the immunogenicity of PEG was discovered through an assessment of the production of anti-PEG antibodies by injecting PEGylated proteins into rabbits116. The widespread use of PEG molecules in various industries has increased the percentage of positive healthy volunteers with anti-PEG antibodies, from approximately 0.2% in 1984 to 40% in 201698,99. The preexisting or de novo anti-PEG antibodies activate the complement system. As recently indicated in the case of the two LNP–mRNA vaccines for COVID-19, mRNA-1273 (Moderna) and BNT162b2 (Pfizer/BioNTech), PEGylated LNPs induced anti-PEG antibodies with the activation of the complement system; thus, their uses possibly put the vaccine recipients at risk of allergic reactions117.
Activation of the complement system can accelerate blood clearance (ABC) and significantly reduce the therapeutic efficacy of LNP–mRNA vaccines99,118. Additionally, anti-PEG antibodies have been linked to pseudo-allergic reactions in patients who received PEGylated drugs through intravenous administration, potentially leading to anaphylactic shock and death100,119. These hypersensitivity reactions are considered to occur through CARPA activation92,119, which is the primary mechanism of infusion reactions, including pseudo-anaphylactic shock98. Therefore, developing PEG-free delivery systems is necessary for future mRNA vaccine development. In this regard, exploring the properties of various materials, such as synthetic, natural, and zwitterionic polymers, is suggested for the development of more effective and safer mRNA delivery systems. These materials have been recognized for their potential to offer innovative and promising medicinal applications compared to PEG. However, whether they provide therapeutic benefits without eliciting pathological immune responses or side effects is uncertain. Therefore, guidelines should be established for screening and accurately determining the degree of immunogenicity of potential candidates120.
Toxicity of the mRNA platform
Regarding mRNA biomolecules, exogenous RNA can expose individuals to endothelial damage, intercellular junction relaxation, edema, increased viscosity, hypercoagulation, and thromboembolic events due to the immunogenicity of the exported RNA19. To abate exogenous RNA-induced immunogenicity, two technical approaches are commonly available. First, chemical modification of IVT mRNA molecules with pseudouridine (ψ) or N1-methylpseudouridine (m1ψ) allows the mRNA to avoid innate immune sensing that occurs through the interaction of exogenous mRNA with TLR7121,122. m1Ψ-modified mRNA has been shown to exhibit less cytotoxicity and immunogenicity than wild-type mRNA. The interference of intracellular innate immune signaling provides advantages for the replacement of conventional protein therapy with the mRNA platform. Second, chromatographic purification can attenuate immune reactions and enhance translational efficiency to eliminate double-stranded RNAs, such as analogs of the viral genome, during IVT mRNA preparations123. However, it may require optimization for mRNA vaccination, as inflammatory cytokines have been shown to boost adaptive immune responses. Therefore, further fine-tuning of the levels of base modifications may be required for mRNA vaccination to balance exogenous mRNA translation and innate immune stimulation.
Modified nucleosides, which have previously been applied in antiviral therapy, cannot be incorporated into the RNAs of natural organisms natively124. Theoretically, chemically modified unnatural molecules can improve the properties of natural mRNAs. However, intensive investigation of their safety is needed before therapeutic inclusion because the administration of unnaturally modified nucleoside molecules into human individuals has previously caused mitochondrial toxicity, liver failure, and even death during clinical trials125. Therefore, the use of base modifications naturally found in RNAs might be a safer strategy for therapeutic applications126.
Conclusions and future directions
mRNA vaccines gained considerable attention during the COVID-19 pandemic due to their great advantages associated with rapid manufacture, reasonable vaccine efficacy, acceptable tolerability, and broad applications relevant to therapeutic fields, including oncology and enzyme replacements, as well as prophylaxis. Their applicability can be further improved by preventing related adverse effects and reducing risk factors associated with the use of modified mRNA and LNP, as well as overcoming unstable durability and potentiating CD8 T-cell responses. Despite these challenges, the flexibility and cost-effectiveness of mRNA platforms offer unique strategies to further improve and solve long-standing challenges associated with vaccine design127. Due to their widespread current use128 and likely trajectory as a platform of choice in a variety of human vaccination efforts19, it is important to evaluate potential avenues for investigating both their ability to overcome these challenges and their potential impact on reactogenicity and tolerability in humans. In addition, it is important to simultaneously identify potential disease targets, as the mRNA vaccine platform may not be a universal solution. However, considering the immense potential of mRNA vaccines in mitigating human disease, significant efforts in clarifying all aspects of this exciting new technology are both warranted and needed.
References
Doherty, M., Buchy, P., Standaert, B., Giaquinto, C. & Prado-Cohrs, D. Vaccine impact: benefits for human health. Vaccine 34, 6707–6714 (2016).
Rappuoli, R., Mandl, C. W., Black, S. & De Gregorio, E. Vaccines for the twenty-first century society. Nat. Rev. Immunol. 11, 865–872 (2011).
Brisse, M., Vrba, S. M., Kirk, N., Liang, Y. & Ly, H. Emerging concepts and technologies in vaccine development. Front. Immunol. 11, 583077 (2020).
Kennedy, R. B., Ovsyannikova, I. G., Palese, P. & Poland, G. A. Current challenges in vaccinology. Front. Immunol. 11, 1181 (2020).
Kis, Z., Kontoravdi, C., Dey, A. K., Shattock, R. & Shah, N. Rapid development and deployment of high-volume vaccines for pandemic response. J. Adv. Manuf. Process 2, e10060 (2020).
Lorentzen, C. L., Haanen, J. B., Met, O. & Svane, I. M. Clinical advances and ongoing trials on mRNA vaccines for cancer treatment. Lancet Oncol. 23, e450–e458 (2022).
Miao, L., Zhang, Y. & Huang, L. mRNA vaccine for cancer immunotherapy. Mol. Cancer 20, 41 (2021).
Chen, J., Chen, J. & Xu, Q. Current developments and challenges of mRNA vaccines. Annu. Rev. Biomed. Eng. 24, 85–109 (2022).
Baden, L. R. et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 384, 403–416 (2021).
Polack, F. P. et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 383, 2603–2615 (2020).
Thi, T. T. H. et al. Lipid-based nanoparticles in the clinic and clinical trials: From cancer nanomedicine to COVID-19 vaccines. Vaccines (Basel) 9, 359 (2021).
Andries, O. et al. N(1)-methylpseudouridine-incorporated mRNA outperforms pseudouridine-incorporated mRNA by providing enhanced protein expression and reduced immunogenicity in mammalian cell lines and mice. J. Control Release 217, 337–344 (2015).
Hogan, M. J. & Pardi, N. mRNA vaccines in the COVID-19 pandemic and beyond. Annu. Rev. Med. 73, 17–39 (2022).
Buschmann, M. D. et al. Nanomaterial delivery systems for mRNA vaccines. Vaccines (Basel) 9, 65 (2021).
Hassett, K. J. et al. Optimization of lipid nanoparticles for intramuscular administration of mRNA vaccines. Mol. Ther. Nucleic Acids 15, 1–11 (2019).
Kanekiyo, M., Ellis, D. & King, N. P. New vaccine design and delivery technologies. J. Infect. Dis. 219, S88–S96 (2019).
Montagnon, B. J. Polio and rabies vaccines produced in continuous cell lines: a reality for Vero cell line. Dev. Biol. Stand. 70, 27–47 (1989).
Gerdil, C. The annual production cycle for influenza vaccine. Vaccine 21, 1776–1779 (2003).
Pardi, N., Hogan, M. J., Porter, F. W. & Weissman, D. mRNA vaccines—a new era in vaccinology. Nat. Rev. Drug Discov. 17, 261–279 (2018).
Wu, F. et al. A new coronavirus associated with human respiratory disease in China. Nature 579, 265–269 (2020).
Jackson, L. A. et al. An mRNA vaccine against SARS-CoV-2 - preliminary report. N. Engl. J. Med. 383, 1920–1931 (2020).
Amanna, I. J. & Slifka, M. K. Successful vaccines. Curr. Top. Microbiol. Immunol. 428, 1–30 (2020).
Watson, O. J. et al. Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. Lancet Infect. Dis. 22, 1293–1302 (2022).
Arunachalam, P. S. et al. Systems vaccinology of the BNT162b2 mRNA vaccine in humans. Nature 596, 410–416 (2021).
Thomas, S. J. et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. N. Engl. J. Med. 385, 1761–1773 (2021).
Weisblum, Y. et al. Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants. Elife 9, e61312 (2020).
Boutwell, C. L., Rolland, M. M., Herbeck, J. T., Mullins, J. I. & Allen, T. M. Viral evolution and escape during acute HIV-1 infection. J. Infect. Dis. 202, S309–S314 (2010).
Wu, N. C. et al. Different genetic barriers for resistance to HA stem antibodies in influenza H3 and H1 viruses. Science 368, 1335–1340 (2020).
Elsner, R. A. & Shlomchik, M. J. Germinal center and extrafollicular B cell responses in vaccination, immunity, and autoimmunity. Immunity 53, 1136–1150 (2020).
Goel, R. R. et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naive and recovered individuals following mRNA vaccination. Sci. Immunol. 6, eabi6950 (2021).
Sachinidis, A. & Garyfallos, A. COVID-19 vaccination can occasionally trigger autoimmune phenomena, probably via inducing age-associated B cells. Int. J. Rheum. Dis. 25, 83–85 (2022).
Kingstad-Bakke, B. et al. Vaccine-induced systemic and mucosal T cell immunity to SARS-CoV-2 viral variants. Proc. Natl Acad. Sci. USA 119, e2118312119 (2022).
Widge, A. T. et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N. Engl. J. Med. 384, 80–82 (2021).
Greaney, A. J. et al. Antibodies elicited by mRNA-1273 vaccination bind more broadly to the receptor binding domain than do those from SARS-CoV-2 infection. Sci. Transl. Med. 13, eabi9915 (2021).
Eyre, D. W. et al. Effect of Covid-19 vaccination on transmission of alpha and delta variants. N. Engl. J. Med. 386, 744–756 (2022).
Edara, V. V. et al. mRNA-1273 and BNT162b2 mRNA vaccines have reduced neutralizing activity against the SARS-CoV-2 omicron variant. Cell Rep. Med. 3, 100529 (2022).
Oscherwitz, J. The promise and challenge of epitope-focused vaccines. Hum. Vaccin. Immunother. 12, 2113–2116 (2016).
Woodruff, M. C., Kim, E. H., Luo, W. & Pulendran, B. B Cell competition for restricted T cell help suppresses rare-epitope responses. Cell Rep. 25, 321–327.e323 (2018).
Boyoglu-Barnum, S. et al. Quadrivalent influenza nanoparticle vaccines induce broad protection. Nature 592, 623–628 (2021).
Chalkias, S. et al. A bivalent omicron-containing booster vaccine against Covid-19. N. Engl. J. Med. 387, 1279–1291 (2022).
Goel, R. R. et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science 374, abm0829 (2021).
Khoury, D. S. et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 27, 1205–1211 (2021).
Barda, N. et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet 398, 2093–2100 (2021).
Taub, D. D. et al. Immunity from smallpox vaccine persists for decades: a longitudinal study. Am. J. Med. 121, 1058–1064 (2008).
Turner, J. S. et al. SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature 596, 109–113 (2021).
Jenks, S. A., Cashman, K. S., Woodruff, M. C., Lee, F. E. & Sanz, I. Extrafollicular responses in humans and SLE. Immunol. Rev. 288, 136–148 (2019).
Rothaeusler, K. & Baumgarth, N. B-cell fate decisions following influenza virus infection. Eur. J. Immunol. 40, 366–377 (2010).
Jenks, S. A. et al. Distinct effector B cells induced by unregulated Toll-like receptor 7 contribute to pathogenic responses in systemic lupus erythematosus. Immunity 52, 203 (2020).
Woodruff, M. C. et al. Dysregulated naive B cells and de novo autoreactivity in severe COVID-19. Nature 611, 139–147 (2022).
Nguyen, D. C., Joyner, C. J., Sanz, I. & Lee, F. E. Factors affecting early antibody secreting cell maturation into long-lived plasma cells. Front. Immunol. 10, 2138 (2019).
Pulendran, B., S. Arunachalam, P. & O’Hagan, D. T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 20, 454–475 (2021).
Kasturi, S. P. et al. Programming the magnitude and persistence of antibody responses with innate immunity. Nature 470, 543–547 (2011).
Kim, E. H. et al. Squalene emulsion-based vaccine adjuvants stimulate CD8 T cell, but not antibody responses, through a RIPK3-dependent pathway. Elife 9, e52687 (2020).
Lee, S. M., Kim, P., You, J. & Kim, E. H. Role of damage-associated molecular pattern/cell death pathways in vaccine-induced immunity. Viruses 13, 2340 (2021).
Li, C. et al. Mechanisms of innate and adaptive immunity to the Pfizer-BioNTech BNT162b2 vaccine. Nat. Immunol. 23, 543–555 (2022).
Alameh, M. G. et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity 54, 2877–2892.e2877 (2021).
Bloom, K., van den Berg, F. & Arbuthnot, P. Self-amplifying RNA vaccines for infectious diseases. Gene Ther. 28, 117–129 (2021).
Britto, C. & Alter, G. The next frontier in vaccine design: blending immune correlates of protection into rational vaccine design. Curr. Opin. Immunol. 78, 102234 (2022).
Plotkin, S. A. Vaccines: correlates of vaccine-induced immunity. Clin. Infect. Dis. 47, 401–409 (2008).
Pulendran, B. & Ahmed, R. Immunological mechanisms of vaccination. Nat. Immunol. 12, 509–517 (2011).
Gilbert, P. B. et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science 375, 43–50 (2022).
McMahan, K. et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature 590, 630–634 (2021).
Keeton, R. et al. T cell responses to SARS-CoV-2 spike cross-recognize Omicron. Nature 603, 488–492 (2022).
Shafqat, A. et al. Understanding COVID-19 vaccines today: Are T-cells key players? Vaccines (Basel) 10, 904 (2022).
Tarke, A. et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell 185, 847–859.e811 (2022).
Victora, G. D. & Nussenzweig, M. C. Germinal centers. Annu. Rev. Immunol. 40, 413–442 (2022).
Crotty, S. Follicular helper CD4 T cells (TFH). Annu. Rev. Immunol. 29, 621–663 (2011).
Lindgren, G. et al. Induction of Robust B Cell responses after influenza mRNA vaccination is accompanied by circulating hemagglutinin-specific ICOS+ PD-1+ CXCR3+ T follicular helper cells. Front. Immunol. 8, 1539 (2017).
Pardi, N. et al. Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses. J. Exp. Med. 215, 1571–1588 (2018).
Corbett, K. S. et al. Evaluation of the mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. N. Engl. J. Med. 383, 1544–1555 (2020).
Vogel, A. B. et al. BNT162b vaccines protect rhesus macaques from SARS-CoV-2. Nature 592, 283–289 (2021).
Lederer, K. et al. SARS-CoV-2 mRNA vaccines foster potent antigen-specific germinal center responses associated with neutralizing antibody generation. Immunity 53, 1281–1295.e1285 (2020).
Laczko, D. et al. A single immunization with nucleoside-modified mRNA vaccines elicits strong cellular and humoral immune responses against SARS-CoV-2 in mice. Immunity 53, 724–732.e727 (2020).
Anderson, E. J. et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N. Engl. J. Med. 383, 2427–2438 (2020).
Painter, M. M. et al. Rapid induction of antigen-specific CD4(+) T cells is associated with coordinated humoral and cellular immunity to SARS-CoV-2 mRNA vaccination. Immunity 54, 2133–2142.e2133 (2021).
Sahin, U. et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 586, 594–599 (2020).
Sahin, U. et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature 595, 572–577 (2021).
Bettini, E. & Locci, M. SARS-CoV-2 mRNA vaccines: Immunological mechanism and beyond. Vaccines (Basel) 9, 147 (2021).
Tarke, A. et al. Impact of SARS-CoV-2 variants on the total CD4(+) and CD8(+) T cell reactivity in infected or vaccinated individuals. Cell Rep. Med. 2, 100355 (2021).
Takano, T. et al. Distinct immune cell dynamics correlate with the immunogenicity and reactogenicity of SARS-CoV-2 mRNA vaccine. Cell Rep. Med. 3, 100631 (2022).
Anand, P. & Stahel, V. P. Review the safety of Covid-19 mRNA vaccines: a review. Patient Saf. Surg. 15, 20 (2021).
Moreira, E. D. Jr et al. Safety and efficacy of a third dose of BNT162b2 Covid-19 vaccine. N. Engl. J. Med. 386, 1910–1921 (2022).
Fraiman, J. et al. Serious adverse events of special interest following mRNA COVID-19 vaccination in randomized trials in adults. Vaccine 40, 5798–5805 (2022).
Li, L. L. et al. Impact of prior SARS-CoV-2 infection on incidence of hospitalization and adverse events following mRNA SARS-CoV-2 vaccination: a nationwide, retrospective cohort study. Vaccine 40, 1082–1089 (2022).
Repajic, M., Lai, X. L., Xu, P. & Liu, A. Bell’s Palsy after second dose of Pfizer COVID-19 vaccination in a patient with history of recurrent Bell’s palsy. Brain Behav. Immun. Health 13, 100217 (2021).
Caminati, M., Guarnieri, G. & Senna, G. Who is really at risk for anaphylaxis due to COVID-19 vaccine? Vaccines (Basel) 9, 38 (2021).
Barda, N. et al. Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N. Engl. J. Med. 385, 1078–1090 (2021).
Abu Lila, A. S., Kiwada, H. & Ishida, T. The accelerated blood clearance (ABC) phenomenon: clinical challenge and approaches to manage. J. Control Release 172, 38–47 (2013).
Heil, F. et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 303, 1526–1529 (2004).
Hou, X., Zaks, T., Langer, R. & Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 6, 1078–1094 (2021).
Kariko, K. et al. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol. Ther. 16, 1833–1840 (2008).
Estape Senti, M. et al. Anti-PEG antibodies compromise the integrity of PEGylated lipid-based nanoparticles via complement. J. Control Release 341, 475–486 (2022).
Alameh, M. G. et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity 55, 1136–1138 (2022).
Kobiyama, K. & Ishii, K. J. Making innate sense of mRNA vaccine adjuvanticity. Nat. Immunol. 23, 474–476 (2022).
Tahtinen, S. et al. IL-1 and IL-1ra are key regulators of the inflammatory response to RNA vaccines. Nat. Immunol. 23, 532–542 (2022).
Liu, Y., Hardie, J., Zhang, X. & Rotello, V. M. Effects of engineered nanoparticles on the innate immune system. Semin. Immunol. 34, 25–32 (2017).
Pham, C. T. et al. Variable antibody-dependent activation of complement by functionalized phospholipid nanoparticle surfaces. J. Biol. Chem. 286, 123–130 (2011).
Vlatkovic, I. Non-immunotherapy application of LNP-mRNA: Maximizing efficacy and safety. Biomedicines 9, 530 (2021).
Kozma, G. T., Shimizu, T., Ishida, T. & Szebeni, J. Anti-PEG antibodies: Properties, formation, testing and role in adverse immune reactions to PEGylated nano-biopharmaceuticals. Adv. Drug Deliv. Rev. 154–155, 163–175 (2020).
Szebeni, J. Complement activation-related pseudoallergy: a stress reaction in blood triggered by nanomedicines and biologicals. Mol. Immunol. 61, 163–173 (2014).
Malone, R. W., Felgner, P. L. & Verma, I. M. Cationic liposome-mediated RNA transfection. Proc. Natl Acad. Sci. USA 86, 6077–6081 (1989).
Wang, Y. et al. Systemic delivery of modified mRNA encoding herpes simplex virus 1 thymidine kinase for targeted cancer gene therapy. Mol. Ther. 21, 358–367 (2013).
Broudic, K. et al. Nonclinical safety evaluation of a novel ionizable lipid for mRNA delivery. Toxicol. Appl. Pharmacol. 451, 116143 (2022).
Lonez, C., Vandenbranden, M. & Ruysschaert, J. M. Cationic lipids activate intracellular signaling pathways. Adv. Drug Deliv. Rev. 64, 1749–1758 (2012).
Lonez, C. et al. Cationic lipid nanocarriers activate Toll-like receptor 2 and NLRP3 inflammasome pathways. Nanomedicine 10, 775–782 (2014).
Lonez, C. et al. Critical residues involved in Toll-like receptor 4 activation by cationic lipid nanocarriers are not located at the lipopolysaccharide-binding interface. Cell Mol. Life Sci. 72, 3971–3982 (2015).
Kedmi, R., Ben-Arie, N. & Peer, D. The systemic toxicity of positively charged lipid nanoparticles and the role of Toll-like receptor 4 in immune activation. Biomaterials 31, 6867–6875 (2010).
Abrams, M. T. et al. Evaluation of efficacy, biodistribution, and inflammation for a potent siRNA nanoparticle: effect of dexamethasone co-treatment. Mol. Ther. 18, 171–180 (2010).
Malamas, A. S., Gujrati, M., Kummitha, C. M., Xu, R. & Lu, Z. R. Design and evaluation of new pH-sensitive amphiphilic cationic lipids for siRNA delivery. J. Control Release 171, 296–307 (2013).
Sun, D. & Lu, Z. R. Structure and function of cationic and ionizable lipids for nucleic acid delivery. Pharm. Res. 40, 27–46 (2023).
Ganesan, P., Ramalingam, P., Karthivashan, G., Ko, Y. T. & Choi, D. K. Recent developments in solid lipid nanoparticle and surface-modified solid lipid nanoparticle delivery systems for oral delivery of phyto-bioactive compounds in various chronic diseases. Int. J. Nanomed. 13, 1569–1583 (2018).
Ferraresso, F. et al. Comparison of DLin-MC3-DMA and ALC-0315 for siRNA delivery to hepatocytes and hepatic stellate cells. Mol. Pharm. 19, 2175–2182 (2022).
Kim, J., Eygeris, Y., Gupta, M. & Sahay, G. Self-assembled mRNA vaccines. Adv. Drug Deliv. Rev. 170, 83–112 (2021).
Halamoda-Kenzaoui, B. & Bremer-Hoffmann, S. Main trends of immune effects triggered by nanomedicines in preclinical studies. Int. J. Nanomed. 13, 5419–5431 (2018).
Milla, P., Dosio, F. & Cattel, L. PEGylation of proteins and liposomes: a powerful and flexible strategy to improve the drug delivery. Curr. Drug Metab. 13, 105–119 (2012).
Richter, A. W. & Akerblom, E. Antibodies against polyethylene glycol produced in animals by immunization with monomethoxy polyethylene glycol modified proteins. Int. Arch. Allergy Appl. Immunol. 70, 124–131 (1983).
Ju, Y. et al. Anti-PEG antibodies boosted in humans by SARS-CoV-2 lipid nanoparticle mRNA vaccine. ACS Nano 16, 11769–11780 (2022).
Kiaie, S. H. et al. Recent advances in mRNA-LNP therapeutics: immunological and pharmacological aspects. J. Nanobiotechnol. 20, 276 (2022).
Szebeni, J. et al. Hemodynamic changes induced by liposomes and liposome-encapsulated hemoglobin in pigs: a model for pseudoallergic cardiopulmonary reactions to liposomes. Role of complement and inhibition by soluble CR1 and anti-C5a antibody. Circulation 99, 2302–2309 (1999).
Hoang Thi, T. T. et al. The Importance of poly(ethylene glycol) alternatives for overcoming peg immunogenicity in drug delivery and bioconjugation. Polym. (Basel) 12, 298 (2020).
Kariko, K., Buckstein, M., Ni, H. & Weissman, D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity 23, 165–175 (2005).
Sahin, U., Kariko, K. & Tureci, O. mRNA-based therapeutics—developing a new class of drugs. Nat. Rev. Drug Discov. 13, 759–780 (2014).
Kariko, K., Muramatsu, H., Ludwig, J. & Weissman, D. Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA. Nucleic Acids Res. 39, e142 (2011).
Schlake, T., Thess, A., Thran, M. & Jordan, I. mRNA as novel technology for passive immunotherapy. Cell Mol. Life Sci. 76, 301–328 (2019).
Thess, A. et al. Sequence-engineered mRNA without chemical nucleoside modifications enables an effective protein therapy in large animals. Mol. Ther. 23, 1456–1464 (2015).
Kon, E., Elia, U. & Peer, D. Principles for designing an optimal mRNA lipid nanoparticle vaccine. Curr. Opin. Biotechnol. 73, 329–336 (2022).
Rosa, S. S., Prazeres, D. M. F., Azevedo, A. M. & Marques, M. P. C. mRNA vaccines manufacturing: Challenges and bottlenecks. Vaccine 39, 2190–2200 (2021).
Wouters, O. J. et al. Challenges in ensuring global access to COVID-19 vaccines: production, affordability, allocation, and deployment. Lancet 397, 1023–1034 (2021).
Acknowledgements
This work was supported by grants from the Ministry of Food and Drug Safety (grant number 22213MFDS421 to J.H.N and 22203MFDS402 to E.H.K), the National Research Foundation (NRF) funded by the Korean government, the Ministry of Science and ICT (grant number NRF-2021M3E5E3080558 to J.H.N and NRF-2023M3A9G6057281 to E.H.K), and partially supported by the Brain Korea 21 Plus Program.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, J., Woodruff, M.C., Kim, E.H. et al. Knife’s edge: Balancing immunogenicity and reactogenicity in mRNA vaccines. Exp Mol Med 55, 1305–1313 (2023). https://doi.org/10.1038/s12276-023-00999-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s12276-023-00999-x
This article is cited by
-
Breaking the mold with RNA—a “RNAissance” of life science
npj Genomic Medicine (2024)
-
Generating prophylactic immunity against arboviruses in vertebrates and invertebrates
Nature Reviews Immunology (2024)
-
Immunogenicity and protective efficacy of a co-formulated two-in-one inactivated whole virus particle COVID-19/influenza vaccine
Scientific Reports (2024)
-
RNA therapy
Experimental & Molecular Medicine (2023)