Abstract
The development and use of antibacterial glycoconjugate vaccines have significantly reduced the occurrence of potentially fatal childhood and adult diseases such as bacteremia, bacterial meningitis, and pneumonia. In these vaccines, the covalent linkage of bacterial glycans to carrier proteins augments the immunogenicity of saccharide antigens by triggering T cell-dependent B cell responses, leading to high-affinity antibodies and durable protection. Licensed glycoconjugate vaccines either contain long-chain bacterial polysaccharides, medium-sized oligosaccharides, or short synthetic glycans. Here, we discuss factors that affect the glycan chain length in vaccines and review the available literature discussing the impact of glycan chain length on vaccine efficacy. Furthermore, we evaluate the available clinical data on licensed glycoconjugate vaccine preparations with varying chain lengths against two bacterial pathogens, Haemophilus influenzae type b and Neisseria meningitidis group C, regarding a possible correlation of glycan chain length with their efficacy. We find that long-chain glycans cross-linked to carrier proteins and medium-sized oligosaccharides end-linked to carriers both achieve high immunogenicity and efficacy. However, end-linked glycoconjugates that contain long untethered stretches of native glycan chains may induce hyporesponsiveness by T cell-independent activation of B cells, while cross-linked medium-sized oligosaccharides may suffer from suboptimal saccharide epitope accessibility.
Similar content being viewed by others
Introduction
Bacterial polysaccharide (PS) molecules are versatile compounds that include lipopolysaccharides with their O antigens, capsular PSs, and exopolysaccharides. They play an important role as virulence factors of bacterial pathogens1 and protect the bacterium against host immune responses in at least three ways. First, surface PSs shield bacterial structures from the destructive activity of the alternative complement pathway of the innate immune system2,3. Second, capsular PSs also obstruct the classical complement pathway since antibodies cannot easily reach subcapsular bacterial surface proteins, thwarting subsequent complement deposition. Third, adaptive immunity to the bacterial PSs remains inefficient since saccharide structures are poorly bound by the antigen-presenting cells’ major histocompatibility complex (MHC), which leads to minimal T-helper cell-dependent antibody responses4. The latter probably explains why many host-dependent bacteria have developed surface carbohydrate structures during their evolution.
Bacterial PSs also contribute to the ability of the microorganism to colonize specific ecological niches, resist antibiotics, and fight bacteriophages5. Their omnipresence in many bacterial pathogens has long generated interest in these compounds for vaccine development. The glycan chains of bacterial PSs form epitopes or antigenic determinants that can be recognized by B cell receptors (BCRs). These epitopes can be linear or conformational in nature and may consist of a minimum span of identical monosaccharides (in homopolymeric PS chains) or of various different monosaccharides including side chains (in heteropolymeric PS chains, Fig. 1). By the 1970s and 1980s, bacterial capsular PS vaccines became part of routine vaccinations, and licensed vaccines for adults based on purified PSs had been established for disease caused by Neisseria meningitidis, Haemophilus influenzae type b (Hib), Salmonella enterica serovar Typhi, and Streptococcus pneumoniae, among others6,7. Immunogenicity of those plain (i.e., unconjugated) PS vaccines is dependent on the capacity of long-chain PSs to cross-link surface immunoglobulin receptors on B cells8,9,10, leading to an antibody response with typical T cell-independent characteristics.
In homopolymeric polysaccharides, identical monosaccharide residues repeat as a polymer. In heteropolymeric (or complex) PS, the repeat unit (RU, in square brackets) consists of diverse monosaccharide residues and may include branching side chains. The minimal required unit for antigenicity in linear epitopes consists of about 6–7 contiguous monosaccharides121. Linear epitopes may include the terminal monosaccharide residues of glycan chains. Conformational epitopes are formed by residues that are in close spatial proximity but dispersed across their primary sequence122. These require a sequence of residues sufficiently long to build or mimic the spatial conformations necessary for interaction with the antibody. As an example, the homopolymeric conformational epitope of the Neisseria meningitidis group B capsular PS antigen contains ten residues, although only the inner six residues interact with its cognate antibody123,124. Glycan fragments that exceed the length of the identified antigenic epitope are usually used as immunogens in glycoconjugate vaccines125.
By themselves, bacterial PS generally induce only a relatively short-lived T cell-independent immune response and largely fail to activate maturation of memory B cells11 and long-lived plasma cells12. Consequently, they primarily induce low-affinity antibody responses and do not efficiently elicit long-term boostable immunological memory9,13,14. Because of their T cell-independent nature, plain PS vaccines such as those against Hib do not induce antibody responses and are therefore not effective in infants under 2 years of age15,16,17, with very few exceptions (such as the meningococcal serogroup A PS18). Fortuitously, conjugation of glycans to a carrier protein, a principle first investigated almost 100 years ago with pneumococcal PSs19, can convert these compounds to T cell-dependent antigens20 that induce high-affinity antibodies, antibody isotype switching, and a long-lasting memory immune response, making them efficacious in infants21,22. Coupling of a glycan to a carrier protein allows binding of the conjugate to saccharide-specific B cells, internalization of the conjugate, and presentation of carrier protein-derived peptide epitopes by MHC to T-helper cells. This eventually leads to clonal activation of the cognate B cells and their differentiation into plasma cells producing antibodies, with isotype switching to IgG and affinity maturation, and into memory B cells21,22.
All currently licensed glycoconjugate vaccines contain one of five carrier proteins. These are tetanus toxoid (TT), diphtheria toxoid (DT), a genetically modified cross-reacting material of the diphtheria toxin termed CRM197, meningococcal outer membrane protein complex OMPC, or H. influenzae protein D HiD23. However, a variety of alternative carrier proteins are also actively explored, such as modified versions of exotoxin A from Pseudomonas aeruginosa24,25, and the S. pneumoniae ABC transporter protein PiuA26.
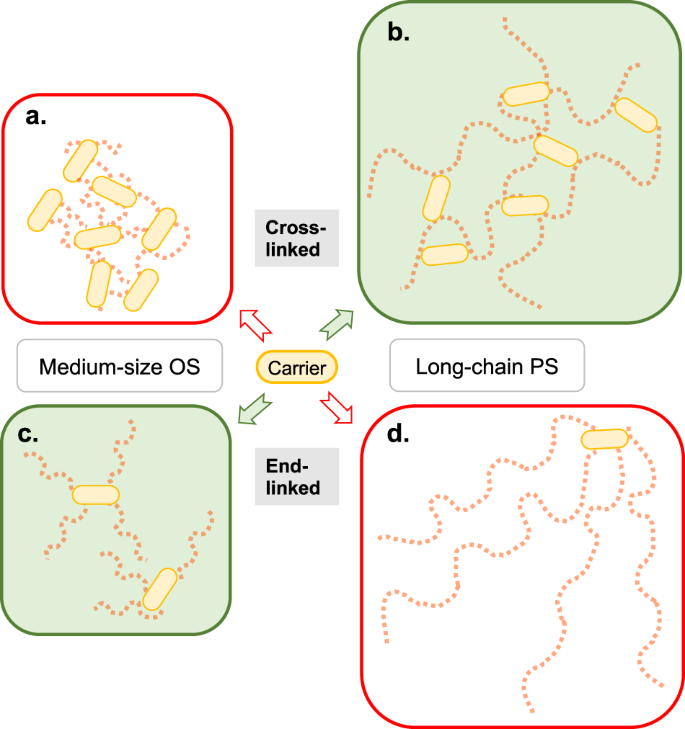
Among the five carriers used in licensed vaccines, CRM197 and TT are by far the most used carriers. DT is often less potent,27 probably due to epitope inactivation during the chemical detoxification process, and both OMPC and HiD are rarely employed. The carriers are covalently bound to glycan molecules, either in end-linked configurations after activation of sugar moieties at glycan ends or as cross-linked substances following random activation of PS chains (Fig. 2).
The basic structures of resultant glycoconjugates are shown. Glycan chains are depicted as strings of red squares, where each square indicates an epitope (see Fig. 1). Random activation of glycans results in cross-linked medium-sized oligosaccharide (OS) glycoconjugates (a) or cross-linked long-chain polysaccharide (PS) conjugates (b). Activation of glycan termini yields end-linked medium-sized OS glycoconjugates (c) or end-linked long-chain PS conjugates (d). Rarely performed activation of both ends of glycan chains may yield vaccines with a low-to-intermediate level of cross-linking. In current practice, b and c result in better-optimized glycoconjugate vaccines (see text for details).
Specific physicochemical properties of the glycoconjugate affect vaccine efficacy, and key parameters, such as the molecular size of the PS used for conjugation and the extent of the cross-linking, can have a profound impact on vaccine immunogenicity28. The number of attachment sites per carrier protein, e.g., needs to be sufficiently high to ensure efficient conjugation, limiting the level of residual free PS molecules. However, the degree of derivatization of carrier proteins requires careful optimization to avoid unwanted destruction of critical MHC epitopes within the protein sequence, which would abolish its T cell-stimulating activity. Selection of these key parameters as well as the choice of carrier protein and the activation and conjugation chemistries are influenced by the practicalities of the purification processes during vaccine production, where viscosity and molecular mass ratios of bound to unbound glycan may impact purity and yield of the final product6. As a consequence, different process chemistries and conjugation methods have been employed to prepare those conjugates. These chemistries include activation procedures to guide protein–glycan linkage sites and their numbers per molecule, as well as the introduction of spacer molecules between the two glycoconjugate components.
One of the primary quality attributes of microbial PSs obtained in vaccine production is the number of glycan repeating units per PS chain termed the degree of polymerization or chain length. This chain length is heavily affected by the production process. Often, the PSs are subjected to a size reduction from very large molecules (>500 kDa) to a heterogeneous mix of PSs of about 100–300 kDa prior to activation and chemical linkage to the specific carrier protein29,30. Size reduction of PS molecules improves subsequent conjugation efficiency in vaccine manufacturing.
The level of immunogenicity of plain bacterial PSs is correlated to their size and, therefore, their chain length31,32. Consequently, large PS size determination became a critical quality measurement to guarantee the immunogenicity of plain PS vaccines, probably explained by the need to cross-link BCRs to elicit T cell-independent antibody responses. But is this association also true for glycoconjugate vaccines? Unlike plain PS vaccines, induction of antibody mechanisms by glycoconjugates does not depend on cross-linking of BCRs. Instead, glycoconjugates are internalized by antigen-presenting cells, leading to T-helper cell responses triggered by carrier protein-derived peptides. Does a dependency on glycan chain length persist despite this difference? The following survey of the existing body of literature regarding the effect of chain length disparities on glycoconjugate vaccine efficacy was undertaken to clarify this question.
Factors determining the glycan chain length of vaccine preparations
The size of the glycan chain in vaccine preparations is one important aspect of its structure. It is determined in various ways, utilizing terminology outlined in Table 1.
Two major aspects govern the length of the glycan chain in glycoprotein vaccine preparations—the underlying microbial genetics of the bacterial strain used to produce the glycan and the vaccine production process. These aspects are briefly discussed below.
Microbial genetics
Depending on their genetic repertoire, bacterial strains can produce PSs of different chain lengths. To date, several of the bacterial regulators that govern the size of microbial glycans are known. Importantly, these can be genetically altered and swapped across species boundaries to generate glycans of altered lengths33,34,35.
Bacterial capsular PSs are, by and large, assembled either by Wzy-dependent polymerization or via the ABC transporter pathway. In Wzy-dependent polymerization, the PS is assembled in a stepwise manner in the bacterial periplasm from individual repeating units that are attached to a specific lipid carrier, undecaprenol diphosphate36,37,38,39. The so-called PS copolymerase proteins regulate the length of the polymer40. The best-studied of these enzymes, Wzz, has been known for a decade to affect the chain length of the O-antigen polymer in Escherichia coli lipopolysaccharide (LPS)41. In the ABC transporter pathway, however, the full-length PS is manufactured in the cytoplasm on a reducing terminal phosphatidylglycerol lipid bound to an oligosaccharide (OS) of β-linked KDO residues36,39,42. The chain length of PSs manufactured by this pathway is thought to be regulated in a geometry model by two interacting proteins, an extension enzyme called WbdA and a termination enzyme named WbdD. Overexpression of WbdD or diminished WbdA expression reduces chain length43,44. However, variations of this system have also been identified. As an example, polymerization, termination, and chain length regulation are performed by domains of a single protein, WbbB, in Klebsiella pneumoniae O1245. Similarly, S. pneumoniae serotype 3 uses a single membrane-bound synthase to produce its long-chain capsular PS. During polymerization, the nascent glycan remains tightly associated with the synthase until its release46, and enzyme catalytic phases may be affected by temperature and concentrations of the saccharide units47.
The vaccine production process
Standard glycoconjugate vaccine production
Traditionally, glycoconjugate vaccines are produced by extraction of PSs from bacterial fermentation, followed by a multitude of purification and fragmentation steps, chemical activation, and subsequent conjugation to the carrier protein48. The carrier protein itself is similarly harvested from bacteria and subsequently purified. After chemical activation, it can be conjugated to the glycan via its naturally existing functional groups or on chemically introduced linker molecules.
There are a number of critical junctures in a production process that may affect the final size of the glycan in a vaccine formulation (Fig. 3). The chain length in a glycoconjugate vaccine manufactured by the traditional production process is determined not only by the genetic makeup of the microorganism but also by the fermentation conditions employed, including the carbohydrate source and the carbon/nitrogen ratio in the growth medium, the pH, the temperature, oxygen tension, and the levels of amino acids, vitamins, phosphate, and minerals49. Moreover, glycan isolation and purification conditions have a profound impact on the glycan chain length. Consequently, the molecular weight of meningococcal and pneumococcal capsular PSs, e.g., after industrial fermentation can range from approximately 100 to roughly 1000 kDa.
a Standard glycoconjugate vaccine production process. b Bioconjugation process. Critical junctures are indicated by numbers. 1. Genetics of the bacterial strain employed. The bioconjugation process includes all genetic modifications introduced to the glycoconjugate production strain to facilitate in vivo coupling of the glycan to a carrier protein. 2. Growth conditions. 3. Strategies for isolation and purification of the glycan (or, in bioconjugation, of the conjugate). 4. Size-exclusion-based glycan or glycoconjugate purification procedures, such as tangential filtration (TFF) or size-exclusion chromatography. 5. Chemical or physical glycan size-reduction protocols, such as acid hydrolysis, ozonolysis, microfluidization, and/or periodate oxidation. 6. Activation chemistries of glycan and carrier protein. 7. Conditions applied for the chemical coupling of carrier and glycan. 8. Purification via combinations of chromatographic techniques, such as ion exchange and hydrophobic interaction.
Often, the harvested PSs is subsequently chemically or mechanically fragmented50 and size-fractionated to alleviate glycan heterogeneity in the vaccine product51,52, to improve yields and consistency during purification processes, and to optimize the conjugation process. PSs are typically fragmented to about 100–300 kDa in cross-linking chemistries (usually still referred to as PSs) and to 5–20 kDa (typically termed OSs) in end-linking chemistry approaches6. The polysaccharide or OS is then chemically activated prior to conjugation, either randomly at hydroxyl groups or localized at the glycan end(s). Cross-linking or end-linking conjugation methods are subsequently used to achieve either random linkage along the glycan chain, or a selective attachment at the terminal end(s) of the sugar moiety, with or without spacer molecules53 (Fig. 2).
Bioconjugation
In an alternative technology called in vivo production of glycoproteins (aka protein glycan-coupling technology, or bioconjugation), the glycoconjugate is produced in a single step inside a microbe, where the glycan and the carrier protein are expressed and coupled inside the bacterial cell54,55, most often E. coli. Bioconjugation omits several necessary chemical reaction and purification steps that may hamper the yield, purity, and homogeneity of vaccine preparations produced by the traditional process. However, bioconjugation requires deliberate and careful genetic manipulation (bioengineering) of the host bacterium to introduce production of the glycan of choice (as a lipid-linked OS), the selected carrier protein, and protein glycosylation capability, often via dedicated plasmids56.
Bioconjugates are basically OS-based end-linked chemical glycoconjugates. Bacterial protein glycosylation is either oligosaccharyltransferase (OTase)-dependent (which can be N-linked, where glycans get attached to the amide nitrogen of asparagine residues, or O-linked, where glycans bond with the hydroxyl oxygen of serine or threonine residues) or OTase-independent56. In the former, glycans are moved en bloc from preassembled lipid-bound precursors to proteins in the periplasm. In the latter, glycosyltransferases move monosaccharides from nucleotide-activated precursors to sequentially form glycoproteins in the cytoplasm56,57. OTase-dependent strategies are (currently) much more prevalently applied, often using the N-linked OTase PglB from Campylobacter jejuni with a remarkable capacity to transfer a number of different PSs. However, several O-linked OTases are also actively investigated for use in bioconjugation processes, including pilin-specific OTases PglL, and PglS, the latter of which can transfer glycans with glucose at the reducing end56,58. In modern bioconjugation techniques, the carrier protein is also often genetically modified to include glycosylation sites in specific sequence patterns.
Sophisticated strategies have been developed that can control the length of the glycan chain of the glycoconjugate produced in these bacterial strains. Genetic manipulation of the chain length controller Wzz is an established strategy to increase the chain length of PS residues. Its overexpression can increase the production of a defined, long-sized PS population and decrease the production of short and very long saccharides33.
Other technologies
The glycan of a conjugate vaccine can also be synthetically (chemically) produced, such as in the anti-Hib vaccine Quimi-Hib59,60, where the repeat unit (RU) octamer of ribosylribitol phosphate (RRP)61 is end-linked to the carrier protein, TT. Unfortunately, the cost-effective manufacture of the necessary quantities of more complex synthetic OSs for standard vaccine production has remained a challenge. Enzymatic production of glycans is another alternative52,62, but has not permeated vaccine production pipelines, to date. These technologies can also be merged, as reported for the production of immunogenic meningococcal group C polysialic acid–TT glycoconjugates63. Other interesting alternative technologies currently considered for glycan vaccine purposes use outer membrane vesicles from engineered bacteria, the so-called generalized modules for membrane antigens (GMMAs), or high-affinity noncovalently linked avidin–biotin PS/protein complexes, creating macromolecular multiple antigen-presenting systems48,64,65. Furthermore, liposomes and gold nanoparticles are currently investigated as novel delivery vehicles for glycoconjugates, with encouraging results52.
The impact of glycan chain length on vaccine immunogenicity
The minimal OS size to support immunogenicity
Efforts to determine the minimal glycan length to elicit immune responses are not only important from a cost perspective but are also useful in future research efforts to standardize and simplify vaccine production processes. OSs as short as tetramers have been shown to elicit effective immune responses, as components of an experimental N. meningitidis serogroup C (MenC) glycoconjugate66, as fragments of S. pneumoniae serotype 14 capsular PS conjugated to CRM19767, and as synthetic tetrameric RRP units conjugated to either TT or CRM197 for Hib59, the latter of which induced strong immune responses in nonhuman primates. For N. meningitidis serogroup A (MenA), randomly O-acetylated octamers were found to be sufficient to elicit protective antibodies in BALB/c mice68.
In tests using OSs representing the Shigella flexneri serotype 2a O antigen (OAg), the highest anti-LPS 2a IgG titer was generated by a glycoconjugate linked to TT that contained a chemically synthesized pentadecasaccharide that consisted of three RUs69. Subsequent studies showed that a minimum segment of nine sugar units is required to present the complete epitope to one of the protective antibodies70. Similarly, decasaccharide glycoconjugates elicited higher titers of anti-LPS IgG against the O-specific monomeric PS of Vibrio cholerae O1 serotype Ogawa than octamers or hexamers71. Consequently, the minimal oligo size capable of eliciting protective immune responses may depend, in part, on the RUs of the target PS and the steric characteristics that govern the size and structure of epitopes recognized by protective antibodies. A thorough understanding of these steric characteristics and their role in the interaction of the antigen with the host’s antibodies may help in future rational designs of synthetic glycans as components of novel glycoconjugate vaccines7.
Importantly, the minimal antigenic glycan epitope must be freely accessible to dock to the BCR’s immunoglobulin-binding cavity. In glycoconjugate vaccines, this can be achieved by the introduction of a spacer molecule between the antigenic epitope (glycan) and the carrier protein. These spacers enhance the exposure of antigenic epitopes on the surface of glycoconjugate vaccines and alleviate steric hindrances. They are (usually) small molecules with reactive groups on either end, which can be identical (such as in adipic acid, cystamine, or dithiobis(succinimidyl propionate)) or different (e.g., N-hydroxysuccinimidyl-3-maleinimidopropionate)72. However, many successful conjugates (both cross-linked long-chain PS and medium-sized end-linked OS formulations) do not employ spacers.
In addition, the immunogenicity profile of glycan epitopes that are composed of mostly homopolymers of monosaccharides with structural similarity to mammalian glycans is likely to differ from that of more complex glycan epitopes that contain unique pathogen-specific monosaccharides, many of which exist73. Therefore, the minimal glycan chain length to elicit a robust antibody response may depend on the exact serological characteristics of the PS.
The complex relationship between glycan length and immunogenicity
While glycan length undoubtedly impacts the immunogenicity of unconjugated PS vaccines, its impact on the performance of glycoconjugate vaccines seemed less clear, although early studies supported this viewpoint74,75. The complex relationship between these two parameters in glycoconjugates may be brought into better focus when separating end-linked constructs from cross-linked formulations.
End-linked constructs
An early clinical trial in the 1980s investigated the immunogenicity of two end-linked glycoconjugates against Hib, where one contained glycans of an average chain length of 8 RU and the other an average chain length of 20 RU15. While adults did not produce different titers of specific antibodies in response to vaccination with these different formulations, infants produced much more anti-polyribosylribitol phosphate (PRP) antibodies when the 20 RU formulation was used. Importantly, both of these conjugates were made without spacer molecules, which may have affected the accessibility of the glycan epitope for binding. Perhaps affecting interpretability even further, these constructs were end-linked on both ends of most glycan chains, presumably leading to a low-to-intermediate level of cross-linking in the final product.
The ostensible linear dependency of immunogenicity on glycan chain length was not corroborated in subsequent investigations on other end-linked formulations. Studies using glycoconjugate constructs of MenC PS fractions of different molecular weights (2-4, 4–10, and 10–50 kDa) conjugated to the carrier protein P64k did not identify differences in the immune response in BALB/c mice76. Moreover, a study using group B streptococcal (GBS) PSs conjugated with various end-linking chemistries to TT determined that immunogenicity of the preparations, as measured by IgG and opsonophagocytic titers in mice, increased with decreasing glycan size for GBS type II conjugates, whereas immunogenicity remained unaffected by glycan size for type III conjugates77.
A systematic investigation of the effect of chain lengths on the immunogenicity of primarily end-linked OS fragments of S. pneumoniae types 3, 6A, 18C, 19F, and 23F found little variation in antibody and opsonophagocytic titers generated by different conjugates that ranged in glycan molecular weights from 3 to 100 kDa78. However, a notable statistically relevant correlation was found for type 19F, where the shortest glycan fragments (about 8 kDa) generated by far the highest antibody titers in immunized rabbits, whereas native-sized conjugated glycan chains (100 kDa) displayed very poor immunogenicity, comparable with unconjugated native PS. The same study also confirmed that cross-linked native-length glycans (100 kDa) resulted in similar immunogenicity and opsonophagocytic killing as the end-linked construct produced with the shortest OS of S. pneumoniae type 6A (8 kDa).
Similarly, a study using end-linked conjugates of various sizes (10, 50, and 100 kDa) of capsular Hib PS to TT found that the shortest-length glycan achieved the highest immunogenicity of these preparations (as measured by IgG titers in rats), although statistical significance was not always achieved79. Finally, when testing several end-linked preparations of S. enterica serovar Typhimurium O-antigen (OAg)-CRM197 conjugates, those that were made with OAg of high molecular weight were considerably less immunogenic in mice than their counterparts of lower molecular weight, or those where the different HPLC-separated OAg preparations were mixed80.
A possible explanation for these observed phenomena was identified in a study that investigated end-linked glycoconjugate preparations of the S. enterica sv Typhi Vi antigen, coupled to CRM197, in mice. These constructs ranged in average glycan size from 9.5 kDa (about 37 RU) to 165 kDa (about 636 RU). The authors found that the conjugates with the longest glycan chains elicited a T cell-independent response (likely by cross-linking BCRs and, surprisingly, induced late apoptosis of Vi-specific B cells in spleen and early depletion of Vi-specific B cells in the bone marrow81. These detrimental effects were not observed in shorter-chain end-linked constructs, which induced a more prolonged proliferation of Vi-specific B cells in the spleen compared to their long-chain counterparts. The latter also generated a faster decline of Vi-specific IgG antibodies in mice than their smaller equivalents81.
Overall, these studies strongly suggest that medium-sized OS constructs result in better immunogenicity of end-linked glycoconjugates than long-chain PS formulations.
Cross-linked constructs
What about the effects of different glycan sizes on the performance of formulations that had been obtained using cross-linking chemistry? For group B type III streptococci, cross-linked glycoconjugate vaccine preparations of differing molecular glycan mass (approximate molecular weights of 349, 105, and 38 kDa) conjugated to TT produced different levels of specific IgG antibodies in mice. Titers largely correlated with saccharide length, although complete protection against a subsequent bacterial challenge was afforded by the two bigger saccharide fractions equally well28. A comparison of pentavalent pneumococcal cross-linked conjugate PS or OS vaccine preparations conjugated to CRM197 in infants showed that the PS formulations consistently produced higher anticapsular antibody titers, although not always in a statistically significant manner82. When comparing antibody titers generated by repeated vaccination of mice with pneumococcal type 4 PS- and OS-based cross-linked TT conjugates, the PS formulation resulted in significantly higher IgM and IgG titers compared with their OS counterparts83. Infants aged between 18 and 30 months generated higher antibody titers against S. pneumoniae types 6A and 23F when immunized with cross-linked glycoconjugates of higher chain length (3–5 RU versus 10–20 RU versus native PS)84.
When investigating full-length or fragmented Vi antigen cross-linked glycoconjugates bound to different carrier proteins (CRM197, DT, and TT), full-size fragments elicited higher amounts of anti-Vi IgG antibodies in mice after the first immunization—however, this advantage became statistically insignificant after the second immunization85. A study on glycoconjugates of the Francisella tularensis OAg of different sizes coupled to TT showed that cross-linked vaccine preparations from a genetically modified bacterium resulting in higher molecular weight (around 220 kDa) elicited a more protective immune response than low and native molecular weight preparations (25 and 80 kDa, respectively). Remarkably, the higher efficacy of the 220 kDa preparation was not due to superior antibody titers, but likely because of the antibodies’ improved affinity strength35.
These results all suggest that, unlike in end-linked glycoconjugates, in vaccines obtained with cross-linking conjugation chemistry, glycans of higher molecular weight may elicit a higher antibody response and/or improved protection. In cross-linked glycoconjugates, long-chain PS molecules may ensure optimal epitope density, with long glycan stretches that activate PS-specific BCRs. A carefully designed cross-linked long-chain conjugate vaccine avoids overly long glycan stretches. Such overly long chains may promote T cell-independent activation of B cells and subsequent hyporesponsiveness; the inability to mount an immune response after booster vaccination of at least the same magnitude as the response induced after primary vaccination86.
The most important parameter of a successful vaccine preparation remains its efficacy, i.e., whether it is able to prevent disease. Therefore, the next two chapters will briefly focus on the chain lengths of licensed glycoconjugate vaccines and investigate whether their efficacy correlates with glycan size.
Licensed H. influenzae vaccine preparations
A capsular plain PS vaccine against Hib (b-CAPSA), introduced and licensed in the United States in 1985, lacked efficacy in infants younger than 18 months, the primary target population17. Subsequently, the first glycoconjugate vaccine against this bacterium was licensed in the United States in December 1987. The product ProHIBit consisted of PRP cross-linked to DT (PRP-D)87. Three other Hib conjugate vaccine constructs soon obtained licensure—PRP cross-linked to OMPC (PRP-OMP, PedVaxHIB), PRP cross-linked to TT (PRP-T, OmniHIB, Hiberix), and RRP OSs of about 20 RU linked to CRM197 after activation of both glycan ends (HbOC aka HibTITER). A second glycoconjugate of RRP OS molecules linked to CRM197 was later produced with alternative chemistries, Vaxem-Hib. Finally, a vaccine consisting of chemically synthesized 8 RU of RRP end-linked to TT was also licensed, Quimi-Hib60 (Table 2). Consequently, licensed monovalent glycoconjugate vaccines against H. influenzae include long-chain PSs, medium-sized OSs, and short-chain octamers.
Initial studies of the four primary licensed Hib vaccine constructs (PRP-D, PRP-T, and PRP-OMP, and HbOC) showed that all constructs but PRP-D generated mean serum antibody levels against PRP above 1 µg/mL88,89. The failure of PRP-D was likely related to its weaker carrier protein DT27. Notably, in a study of almost 500 American children, there were no statistically significant differences in the geometric mean antibody concentration after three doses of PRP-T or HbOC, with 95 and 91% of infants producing >1.0 µg/mL of antibody90. In addition to antibody measurements, efficacy data, the ultimate parameter for vaccine success, had been determined for the four constructs. Post dose 3, PRP-D was found to reach 90–100% efficacy when tested in thousands of Finnish children91,92, but only about 35% in Alaskan native infants93. For the OS-based formulation HbOC, efficacies had been determined to be 100% in two large studies with thousands of children91,94, while a smaller case–control trial in the United States determined 94.4% efficacy of HbOC after dose 3. For PRP-OMP, the efficacy of about 93% after dose 2 had been determined in a large trial involving Navajo and Apache native children95.
Subsequent studies investigated serum bactericidal antibody (SBA) titers of newer vaccine formulations and found that the end-linked OS-CRM197 formulation Vaxem-Hib usually outperforms the cross-linked-TT PS-containing glycoconjugate ActHIB, although both generated high titers suggesting long-term seroprotection96,97,98. In addition, the reported antibody concentrations raised by Quimi-Hib, the synthetic 8-mer vaccine, matched that of Vaxem-Hib 12 months after the first immunization series60.
In conclusion, efficacious Hib glycoconjugate vaccines were obtained by both medium-sized OS-based end-linked constructs and cross-linked long-chain PS-based formulations. Moreover, even short-sized end-linked synthetic glycans can yield a successful Hib vaccine.
Licensed N. meningitidis vaccines with group C glycoconjugates
Worldwide, several glycoconjugate vaccine preparations that include glycans directed against MenC have been licensed (Table 3). These are generally of superior immunogenicity compared to their unconjugated counterparts, with longer-lasting effects (e.g., refs. 99,100,101), and are licensed for use at an earlier age. The different conjugate vaccines are built using various chemistries, using different carrier proteins, and are used either as monovalent vaccines or as part of a combination formula targeting several serotypes of the same or various bacterial pathogens.
At the turn of the century, three licensed OS-based MenC glycoconjugate vaccines existed—Menjugate (MenC-CRM197, end-linked, 14–19 RU), Meningitec (MenC-CRM197, primarily end-linked with a moderate level of cross-linking, 20–47 RU)102, and NeisVac-C (MenC-TT, primarily end-linked with a moderate level of cross-linking, 20–47 RU)103. When directly comparing the performance of these three vaccine formulations, both MenC-CRM197 vaccines were inferior to NeisVac-C in many studies104,105,106,107, and a hierarchy of NeisVac-C > Menjugate > Meningitec was usually observed. However, instead of glycan chain length, these differences in performance are perhaps associated with the fact that NeisVac-C used de-O-acetylated MenC OSs and TT as a carrier protein. Nevertheless, even Meningitec performed relatively well in absolute terms—a study evaluating its immunogenicity in over 100 British infants found that 98% of all participants achieved protective rabbit SBA titers of ≥1:8 post dose 3, with all of them achieving IgG titers of ≥2 µg/mL108.
Menactra, a meningococcal tetravalent ACWY conjugate vaccine, contains MenC glycans of roughly 20 kDa109, approximately 50 RU, end-linked to DT110. In trials encompassing hundreds of US-based infants, Menactra generated protective human SBA titers of ≥1:8 against MenC 30 days post dose 2 for 98.9% of participants111. The tetravalent ACWY end-linked mid-sized OS-based vaccine Menveo achieved the same level of human SBA protective titers ≥1:8 (82%) after a single dose as Menactra in a phase III study involving over 1000 adolescents112. In addition, phase III trials of the most recent addition to the arsenal of end-linked multivalent size-reduced MenC glycoconjugate vaccines, MenQuadFi, confirmed its non-inferiority compared to Menactra and Nimenrix and suggested a superior seroresponse against MenC, with protective human SBA titers 30 days after immunization in >99% of naive or preimmunized toddlers 12–23 months of age113, and >88% in adolescents and adults114.
There is only one MenC glycoconjugate construct that uses native PS, skipping a targeted glycan sizing step entirely. This construct is used in several cross-linked polyvalent vaccine formulations (MenHibrix, Nimenrix, and Menitorix). Immunogenicity experiments comparing MenHibrix with the OS-based end-linked MenC-CRM197 monovalent vaccine (Menjugate) in hundreds of healthy infants suggested no significant difference in rabbit SBA titers after the third dose115. Similarly, Nimenrix showed favorable immunogenicity profiles after single and double injections, with 100% of more than 200 participating infants obtaining rabbit SBA levels ≥1:8 against MenC 1 month after the dose, and 90.8% showing that level 3 years post dose116.
In summary, end-linking technologies with medium-sized glycans as well as cross-linking chemistries with long-chain glycans lead to efficacious MenC conjugates in licensed vaccines with no obvious correlating differences in vaccine immunogenicity. This observation mirrors the data collected for Hib glycoconjugates, above.
Conclusions
A thorough review of the literature suggested that cross-linked glycoconjugates may require long-chain PSs for optimal immunogenicity, whereas end-linked constructs may benefit from medium-sized OSs. Either strategy can achieve an optimal density of native saccharide epitopes, allowing strong immune responses. Short chains of too few saccharide molecules may not contain an adequate density of accessible epitopes, while saccharide chains that are too long and formulations containing an excess of unconjugated PS molecules may carry the risk of promoting T cell-independent activation of B cells81 by BCR cross-linking (Fig. 4). The model relies on the stimulation of either carrier-based peptide T-helper responses (the classical paradigm for conjugate vaccines) or carbohydrate-specific CD4+ T cell clones (Tcarbs) to produce the necessary cytokines for B cell activation117, a concept we eagerly await to be consolidated by future research.
The necessary steps for glycoconjugate vaccines to engender B cell maturation and production of glycan-specific antibodies are enumerated in the central blue segment. Glycoconjugate construct (a) contains medium-sized glycan chains that are excessively cross-linked and therefore not sufficiently accessible to be optimally recognized by the B cell receptors (BCRs). Instead, functional vaccines can be achieved by rational design of linkage sites and chain lengths in the vaccine molecule. Appropriate levels of cross-linking of long-chain polysaccharides (construct b) or end-linked chemistries for medium-sized oligosaccharides (construct c) can lead to protective glycoconjugate vaccines. In those settings, T-helper cells recognize the MHCII-bound peptide complexes and become activated. With T-helper cell support, cognate B cells mature to memory B cells and produce glycan-specific antibodies. However, excessively long glycan stretches (construct d) may act similar to unconjugated PS and cross-link BCRs, generating mainly T cell-independent immune responses resulting in hyporesponsiveness. Without T-helper cell support, B cells become temporarily activated but proceed to undergo apoptosis, impeding the production of long-lasting memory B cells and long-lived plasma cells81.
Other factors determining vaccine efficacy may lie in the specific conformational features of both the carrier and the glycan components, and in specific features of the host, such as their metabolic state and the degree of senescence or maturity of their immune response system. Chain density per carrier protein molecule has also long been suspected to be of vital importance15,78,118,119 and is heavily influenced by the process chemistries applied during vaccine preparation. Peak glycoconjugate performance may require the anchoring of minimal stretches of contiguous intact glycan epitopes to carrier proteins in a ratio that optimizes epitope loading for each protein molecule. At the optimal ratio, the carrier/glycan hybrid molecules maximally activate T helper cells.
In our survey, the clinical data on licensed glycoprotein vaccines of various glycan chain lengths do not indicate a relevant difference in protection, the ultimate goal of vaccination. We conclude that saccharide stretches in glycoconjugate vaccines need to be sufficient but not overly long for optimal vaccine performance. This crystallized paradigm can be delivered either via end-linked OS conjugates or via cross-linked PSs. This implies that successful glycoconjugate vaccines do not require long-chain lengths—an important realization that supports the future development of vaccines based on synthetic OSs that are linked to carriers via spacer molecules. Other technologies, such as GMMA and affinity non-covalent coupling, may also flourish65,120. The search for optimal glycan chain lengths (or, more precisely, the optimal number of contiguous epitopes between carrier proteins of glycoconjugates) will likely continue and be driven by efforts to minimize the size of each molecule without a loss (and possibly with gain) of function. In short term, though, bioconjugation using medium-sized OSs and direct enzymatic end-linking may offer the best balance between efficiency, simplicity, and optimal immunogenicity of glycoconjugate vaccines.
References
Willis, L. M. & Whitfield, C. Structure, biosynthesis, and function of bacterial capsular polysaccharides synthesized by ABC transporter-dependent pathways. Carbohydr. Res. 378, 35–44 (2013).
Abreu, A. G. & Barbosa, A. S. How Escherichia coli circumvent complement-mediated killing. Front. Immunol. 8, 452 (2017).
Roberts, I. S. The biochemistry and genetics of capsular polysaccharide production in bacteria. Annu Rev. Microbiol. 50, 285–315 (1996).
Watts, C. & Powis, S. Pathways of antigen processing and presentation. Rev. Immunogenet. 1, 60–74 (1999).
Patro, L. P. P. & Rathinavelan, T. Targeting the sugary armor of Klebsiella species. Front. Cell Infect. Microbiol. 9, 367 (2019).
Hennessey, J. P. et al. In Carbohydrate-Based Vaccines: From Concept to Clinic (ed. Prasad, A. K.) Vol. 1290, ACS Symposium Series, Ch. 13, 323–385 (American Chemical Society, 2018).
Anish, C., Schumann, B., Pereira, C. L. & Seeberger, P. H. Chemical biology approaches to designing defined carbohydrate vaccines. Chem. Biol. 21, 38–50 (2014).
Jones, C. Vaccines based on the cell surface carbohydrates of pathogenic bacteria. Acad. Bras. Cienc. 77, 293–324 (2005).
Mond, J. J., Lees, A. & Snapper, C. M. T cell-independent antigens type 2. Annu. Rev. Immunol. 13, 655–692 (1995).
Snapper, C. M. & Mond, J. J. A model for induction of T cell-independent humoral immunity in response to polysaccharide antigens. J. Immunol. 157, 2229–2233 (1996).
Mitchell, R., Kelly, D. F., Pollard, A. J. & Truck, J. Polysaccharide-specific B cell responses to vaccination in humans. Hum. Vaccin. Immunother. 10, 1661–1668 (2014).
Palm, A. E. & Henry, C. Remembrance of things past: long-term B cell memory after infection and vaccination. Front. Immunol. 10, 1787 (2019).
Mazmanian, S. K. & Kasper, D. L. The love-hate relationship between bacterial polysaccharides and the host immune system. Nat. Rev. Immunol. 6, 849–858 (2006).
Finco, O. & Rappuoli, R. Designing vaccines for the twenty-first century society. Front. Immunol. 5, 12 (2014).
Anderson, P. W. et al. Vaccines consisting of periodate-cleaved oligosaccharides from the capsule of Haemophilus influenzae type b coupled to a protein carrier: structural and temporal requirements for priming in the human infant. J. Immunol. 137, 1181–1186 (1986).
Käyhty, H., Peltola, H., Karanko, V. & Makela, P. H. The protective level of serum antibodies to the capsular polysaccharide of Haemophilus influenzae type b. J. Infect. Dis. 147, 1100 (1983).
Peltola, H., Kayhty, H., Sivonen, A. & Makela, H. Haemophilus influenzae type b capsular polysaccharide vaccine in children: a double-blind field study of 100,000 vaccinees 3 months to 5 years of age in Finland. Pediatrics 60, 730–737 (1977).
Al-Mazrou, Y. et al. Serologic responses to ACYW135 polysaccharide meningococcal vaccine in Saudi children under 5 years of age. Infect. Immun. 73, 2932–2939 (2005).
Avery, O. T. & Goebel, W. F. Chemo-immunological studies on conjugated carbohydrate-proteins: V. The immunological specifity of an antigen prepared by combining the capsular polysaccharide of type III Pneumococcus with foreign protein. J. Exp. Med.d 54, 437–447 (1931).
Rappuoli, R. & De Gregorio, E. A sweet T cell response. Nat. Med. 17, 1551–1552 (2011).
Micoli, F., Adamo, R. & Costantino, P. Protein carriers for glycoconjugate vaccines: History, selection criteria, characterization and new trends. Molecules 23, https://doi.org/10.3390/molecules23061451 (2018).
Pollard, A. J., Perrett, K. P. & Beverley, P. C. Maintaining protection against invasive bacteria with protein-polysaccharide conjugate vaccines. Nat. Rev. Immunol. 9, 213–220 (2009).
Pichichero, M. E. Protein carriers of conjugate vaccines: characteristics, development, and clinical trials. Hum. Vaccin. Immunother. 9, 2505–2523 (2013).
Ravenscroft, N. et al. Characterization and immunogenicity of a Shigella flexneri 2a O-antigen bioconjugate vaccine candidate. Glycobiology 29, 669–680 (2019).
Kowarik, M. et al. The development and characterization of an E. coli O25B bioconjugate vaccine. Glycoconj. J. https://doi.org/10.1007/s10719-021-09985-9 (2021).
Reglinski, M. et al. A recombinant conjugated pneumococcal vaccine that protects against murine infections with a similar efficacy to Prevnar-13. NPJ Vaccines 3, 53 (2018).
Dagan, R., Poolman, J. & Siegrist, C. A. Glycoconjugate vaccines and immune interference: a review. Vaccine 28, 5513–5523 (2010).
Wessels, M. R. et al. Structural properties of group B streptococcal type III polysaccharide conjugate vaccines that influence immunogenicity and efficacy. Infect. Immun. 66, 2186–2192 (1998).
Bardotti, A. et al. Physicochemical characterisation of glycoconjugate vaccines for prevention of meningococcal diseases. Vaccine 26, 2284–2296 (2008).
Lee, C. H. et al. Preparation and characterization of an immunogenic meningococcal group A conjugate vaccine for use in Africa. Vaccine 27, 726–732 (2009).
Kabat, E. A. & Bezer, A. E. The effect of variation in molecular weight on the antigenicity of dextran in man. Arch. Biochem. Biophys. 78, 306–318 (1958).
Martin, D. G., Jarvis, F. G. & Milner, K. C. Physicochemical and biological properties of sonically treated Vi antigen. J. Bacteriol. 94, 1411–1416 (1967).
Hegerle, N. et al. Overexpression of O-polysaccharide chain length regulators in Gram-negative bacteria using the Wzx-/Wzy-dependent pathway enhances production of defined modal length O-polysaccharide polymers for use as haptens in glycoconjugate vaccines. J. Appl. Microbiol. 125, 575–585 (2018).
Kalynych, S., Ruan, X., Valvano, M. A. & Cygler, M. Structure-guided investigation of lipopolysaccharide O-antigen chain length regulators reveals regions critical for modal length control. J. Bacteriol. 193, 3710–3721 (2011).
Stefanetti, G., Okan, N., Fink, A., Gardner, E. & Kasper, D. L. Glycoconjugate vaccine using a genetically modified O antigen induces protective antibodies to Francisella tularensis. Proc. Natl Acad. Sci. USA 116, 7062–7070 (2019).
Whitfield, C. Biosynthesis and assembly of capsular polysaccharides in Escherichia coli. Annu. Rev. Biochem. 75, 39–68 (2006).
Whitfield, C. & Larue, K. Stop and go: regulation of chain length in the biosynthesis of bacterial polysaccharides. Nat. Struct. Mol. Biol. 15, 121–123 (2008).
Drummelsmith, J. & Whitfield, C. Gene products required for surface expression of the capsular form of the group 1 K antigen in Escherichia coli (O9a:K30). Mol. Microbiol. 31, 1321–1332 (1999).
Raetz, C. R. & Whitfield, C. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 71, 635–700 (2002).
Batchelor, R. A., Haraguchi, G. E., Hull, R. A. & Hull, S. I. Regulation by a novel protein of the bimodal distribution of lipopolysaccharide in the outer membrane of Escherichia coli. J. Bacteriol. 173, 5699–5704 (1991).
Woodward, R. et al. In vitro bacterial polysaccharide biosynthesis: defining the functions of Wzy and Wzz. Nat. Chem. Biol. 6, 418–423 (2010).
Willis, L. M. et al. Conserved glycolipid termini in capsular polysaccharides synthesized by ATP-binding cassette transporter-dependent pathways in Gram-negative pathogens. Proc. Natl Acad. Sci. USA 110, 7868–7873 (2013).
Clarke, B. R., Cuthbertson, L. & Whitfield, C. Nonreducing terminal modifications determine the chain length of polymannose O antigens of Escherichia coli and couple chain termination to polymer export via an ATP-binding cassette transporter. J. Biol. Chem. 279, 35709–35718 (2004).
Greenfield, L. K. et al. Domain organization of the polymerizing mannosyltransferases involved in synthesis of the Escherichia coli O8 and O9a lipopolysaccharide O-antigens. J. Biol. Chem. 287, 38135–38149 (2012).
Williams, D. M. et al. Single polysaccharide assembly protein that integrates polymerization, termination, and chain-length quality control. Proc. Natl Acad. Sci. USA 114, E1215–E1223 (2017).
Cartee, R. T., Forsee, W. T., Schutzbach, J. S. & Yother, J. Mechanism of type 3 capsular polysaccharide synthesis in Streptococcus pneumoniae. J. Biol. Chem. 275, 3907–3914 (2000).
Forsee, W. T., Cartee, R. T. & Yother, J. Role of the carbohydrate binding site of the Streptococcus pneumoniae capsular polysaccharide type 3 synthase in the transition from oligosaccharide to polysaccharide synthesis. J. Biol. Chem. 281, 6283–6289 (2006).
Berti, F. & Micoli, F. Improving efficacy of glycoconjugate vaccines: from chemical conjugates to next generation constructs. Curr. Opin. Immunol. 65, 42–49 (2020).
Zeidan, A. A. et al. Polysaccharide production by lactic acid bacteria: from genes to industrial applications. FEMS Microbiol. Rev. 41, S168–S200 (2017).
Costantino, P. et al. Size fractionation of bacterial capsular polysaccharides for their use in conjugate vaccines. Vaccine 17, 1251–1263 (1999).
Broker, M., Berti, F. & Costantino, P. Factors contributing to the immunogenicity of meningococcal conjugate vaccines. Hum. Vaccin. Immunother. 12, 1808–1824 (2016).
Micoli, F. et al. Glycoconjugate vaccines: current approaches towards faster vaccine design. Expert Rev. Vaccines 18, 881–895 (2019).
Costantino, P., Rappuoli, R. & Berti, F. The design of semi-synthetic and synthetic glycoconjugate vaccines. Expert Opin. Drug Discov. 6, 1045–1066 (2011).
Kay, E., Cuccui, J. & Wren, B. W. Recent advances in the production of recombinant glycoconjugate vaccines. NPJ Vaccines 4, 16 (2019).
Wacker, M. et al. N-linked glycosylation in Campylobacter jejuni and its functional transfer into E. coli. Science 298, 1790–1793 (2002).
Harding, C. M. & Feldman, M. F. Glycoengineering bioconjugate vaccines, therapeutics, and diagnostics in E. coli. Glycobiology 29, 519–529 (2019).
Valguarnera, E., Kinsella, R. L. & Feldman, M. F. Sugar and spice make bacteria not nice: Protein glycosylation and its influence in pathogenesis. J. Mol. Biol. 428, 3206–3220 (2016).
Harding, C. M. et al. A platform for glycoengineering a polyvalent pneumococcal bioconjugate vaccine using E. coli as a host. Nat. Commun. 10, 891 (2019).
Peeters, C. C. et al. Synthetic trimer and tetramer of 3-beta-d-ribose-(1-1)-d-ribitol-5-phosphate conjugated to protein induce antibody responses to Haemophilus influenzae type b capsular polysaccharide in mice and monkeys. Infect. Immun. 60, 1826–1833 (1992).
Vérez-Bencomo, V. et al. A synthetic conjugate polysaccharide vaccine against Haemophilus influenzae type b. Science 305, 522–525 (2004).
Crisel, R. M., Baker, R. S. & Dorman, D. E. Capsular polymer of Haemophilus influenzae, type b. I. Structural characterization of the capsular polymer of strain Eagan. J. Biol. Chem. 250, 4926–4930 (1975).
Wen, L. et al. Toward automated enzymatic synthesis of oligosaccharides. Chem. Rev. 118, 8151–8187 (2018).
McCarthy, P. C. et al. Chemoenzymatic synthesis of immunogenic meningococcal group C polysialic acid-tetanus Hc fragment glycoconjugates. Glycoconj. J. 30, 857–870 (2013).
Stevenson, T. C. et al. Immunization with outer membrane vesicles displaying conserved surface polysaccharide antigen elicits broadly antimicrobial antibodies. Proc. Natl Acad. Sci. USA 115, E3106–E3115 (2018).
Zhang, F., Lu, Y. J. & Malley, R. Multiple antigen-presenting system (MAPS) to induce comprehensive B- and T-cell immunity. Proc. Natl Acad. Sci. USA 110, 13564–13569 (2013).
Dalal, J. et al. Development and pre-clinical evaluation of a synthetic oligosaccharide-protein conjugate vaccine against Neisseria meningitidis serogroup C. Vaccine 37, 5297–5306 (2019).
Safari, D. et al. Identification of the smallest structure capable of evoking opsonophagocytic antibodies against Streptococcus pneumoniae type 14. Infect. Immun. 76, 4615–4623 (2008).
Enotarpi, J. et al. A stabilized glycomimetic conjugate vaccine inducing protective antibodies against Neisseria meningitidis serogroup A. Nat. Commun. 11, 4434 (2020).
Phalipon, A. et al. Characterization of functional oligosaccharide mimics of the Shigella flexneri serotype 2a O-antigen: implications for the development of a chemically defined glycoconjugate vaccine. J. Immunol. 176, 1686–1694 (2006).
Vulliez-Le Normand, B. et al. Structures of synthetic O-antigen fragments from serotype 2a Shigella flexneri in complex with a protective monoclonal antibody. Proc. Natl Acad. Sci. USA 105, 9976–9981 (2008).
Ftacek, P., Nelson, V. & Szu, S. C. Immunochemical characterization of synthetic hexa-, octa- and decasaccharide conjugate vaccines for Vibrio cholerae O:1 serotype Ogawa with emphasis on antigenic density and chain length. Glycoconj. J. 30, 871–880 (2013).
Khatun, F., Stephenson, R. J. & Toth, I. An overview of structural features of antibacterial glycoconjugate vaccines that influence their immunogenicity. Chemistry 23, 4233–4254 (2017).
Herget, S. et al. Statistical analysis of the Bacterial Carbohydrate Structure Data Base (BCSDB): characteristics and diversity of bacterial carbohydrates in comparison with mammalian glycans. BMC Struct. Biol. 8, 35 (2008).
Szu, S. C., Zon, G., Schneerson, R. & Robbins, J. B. Ultrasonic irradiation of bacterial polysaccharides. Characterization of the depolymerized products and some applications of the process. Carbohydr. Res. 152, 7–20 (1986).
Fattom, A. et al. Synthesis and physicochemical and immunological characterization of pneumococcus type 12F polysaccharide-diphtheria toxoid conjugates. Infect. Immun. 56, 2292–2298 (1988).
Carmenate, T. et al. Effect of conjugation methodology on the immunogenicity and protective efficacy of meningococcal group C polysaccharide-P64k protein conjugates. FEMS Immunol. Med. Microbiol. 40, 193–199 (2004).
Michon, F. et al. Group B streptococcal type II and III conjugate vaccines: physicochemical properties that influence immunogenicity. Clin. Vaccin. Immunol. 13, 936–943 (2006).
Laferrière, C. A., Sood, R. K., de Muys, J. M., Michon, F. & Jennings, H. J. The synthesis of Streptococcus pneumoniae polysaccharide-tetanus toxoid conjugates and the effect of chain length on immunogenicity. Vaccine 15, 179–186 (1997).
Rana, R. et al. Development and characterization of Haemophilus influenzae type b conjugate vaccine prepared using different polysaccharide chain lengths. Vaccine 33, 2646–2654 (2015).
Rondini, S. et al. Design of glycoconjugate vaccines against invasive African Salmonella enterica serovar Typhimurium. Infect. Immun. 83, 996–1007 (2015).
Micoli, F. et al. Short Vi-polysaccharide abrogates T-independent immune response and hyporesponsiveness elicited by long Vi-CRM197 conjugate vaccine. Proc. Natl Acad. Sci. USA https://doi.org/10.1073/pnas.2005857117 (2020).
Daum, R. S. et al. Infant immunization with pneumococcal CRM197 vaccines: effect of saccharide size on immunogenicity and interactions with simultaneously administered vaccines. J. Infect. Dis. 176, 445–455 (1997).
Peeters, C. C. et al. A comparative study of the immunogenicity of pneumococcal type 4 polysaccharide and oligosaccharide tetanus toxoid conjugates in adult mice. J. Immunol. 146, 4308–4314 (1991).
Steinhoff, M. C. et al. A randomized comparison of three bivalent Streptococcus pneumoniae glycoprotein conjugate vaccines in young children: effect of polysaccharide size and linkage characteristics. Pediatr. Infect. Dis. J. 13, 368–372 (1994).
Arcuri, M. et al. The influence of conjugation variables on the design and immunogenicity of a glycoconjugate vaccine against Salmonella Typhi. PLoS ONE 12, e0189100 (2017).
Poolman, J. & Borrow, R. Hyporesponsiveness and its clinical implications after vaccination with polysaccharide or glycoconjugate vaccines. Expert Rev. Vaccines 10, 307–322 (2011).
American Academy of Pediatrics Committee on Infectious Diseases. Haemophilus influenzae type b conjugate vaccine. Pediatrics 81, 908–911 (1988).
Decker, M. D. & Edwards, K. M. Haemophilus influenzae type b vaccines: history, choice and comparisons. Pediatr. Infect. Dis. J. 17, S113–116 (1998).
Decker, M. D., Edwards, K. M., Bradley, R. & Palmer, P. Comparative trial in infants of four conjugate Haemophilus influenzae type b vaccines. J. Pediatr. 120, 184–189 (1992).
Holmes, S. J. et al. Immunogenicity of Haemophilus influenzae type b polysaccharide-tetanus toxoid conjugate vaccine in infants. Am. J. Dis. Child 147, 832–836 (1993).
Eskola, J. et al. A randomized, prospective field trial of a conjugate vaccine in the protection of infants and young children against invasive Haemophilus influenzae type b disease. N. Engl. J. Med. 323, 1381–1387 (1990).
Eskola, J. et al. Efficacy of Haemophilus influenzae type b polysaccharide-diphtheria toxoid conjugate vaccine in infancy. N. Engl. J. Med. 317, 717–722 (1987).
Ward, J., Brenneman, G., Letson, G. W. & Heyward, W. L. Limited efficacy of a Haemophilus influenzae type b conjugate vaccine in Alaska Native infants. The Alaska H. influenzae Vaccine Study Group. N. Engl. J. Med. 323, 1393–1401 (1990).
Black, S. B. et al. Efficacy in infancy of oligosaccharide conjugate Haemophilus influenzae type b (HbOC) vaccine in a United States population of 61,080 children. The Northern California Kaiser Permanente Vaccine Study Center Pediatrics Group. Pediatr. Infect. Dis. J. 10, 97–104 (1991).
Santosham, M. et al. The efficacy in Navajo infants of a conjugate vaccine consisting of Haemophilus influenzae type b polysaccharide and Neisseria meningitidis outer-membrane protein complex. N. Engl. J. Med. 324, 1767–1772 (1991).
Akeda, Y. et al. Comparison of serum bactericidal and antibody titers induced by two Haemophilus influenzae type b conjugate vaccines: a phase III randomized double-blind study. Vaccine 36, 1528–1532 (2018).
Togashi, T. et al. Immunogenicity and safety of a fully liquid aluminum phosphate adjuvanted Haemophilus influenzae type b PRP-CRM197-conjugate vaccine in healthy Japanese children: a phase III, randomized, observer-blind, multicenter, parallel-group study. Vaccine 34, 4635–4641 (2016).
Jun, L. et al. Assessment of immunogenicity and safety following primary and booster immunisation with a CRM197-conjugated Haemophilus influenzae type B vaccine in healthy Chinese infants. Int J. Clin. Pract. 67, 971–978 (2013).
Keyserling, H. et al. Safety, immunogenicity, and immune memory of a novel meningococcal (groups A, C, Y, and W-135) polysaccharide diphtheria toxoid conjugate vaccine (MCV-4) in healthy adolescents. Arch. Pediatr. Adolesc. Med. 159, 907–913 (2005).
Lagos, R. et al. Safety and immunogenicity of a meningococcal (Groups A, C, Y, W-135) polysaccharide diphtheria toxoid conjugate vaccine in healthy children aged 2 to 10 years in Chile. Hum. Vaccin. 1, 228–231 (2005).
Pichichero, M. et al. Comparative trial of the safety and immunogenicity of quadrivalent (A, C, Y, W-135) meningococcal polysaccharide-diphtheria conjugate vaccine versus quadrivalent polysaccharide vaccine in two- to ten-year-old children. Pediatr. Infect. Dis. J. 24, 57–62 (2005).
Ho, M. M., Bolgiano, B. & Corbel, M. J. Assessment of the stability and immunogenicity of meningococcal oligosaccharide C-CRM197 conjugate vaccines. Vaccine 19, 716–725 (2000).
Jumel, K., Ho, M. M. & Bolgiano, B. Evaluation of meningococcal C oligosaccharide conjugate vaccines by size-exclusion chromatography/multi-angle laser light scattering. Biotechnol. Appl. Biochem. 36, 219–226 (2002).
Badahdah, A. M., Rashid, H. & Khatami, A. Update on the use of meningococcal serogroup C CRM197-conjugate vaccine (Meningitec) against meningitis. Expert Rev. Vaccin. 15, 9–29 (2016).
Borrow, R. et al. Kinetics of antibody persistence following administration of a combination meningococcal serogroup C and haemophilus influenzae type b conjugate vaccine in healthy infants in the United Kingdom primed with a monovalent meningococcal serogroup C vaccine. Clin. Vaccin. Immunol. 17, 154–159 (2010).
Southern, J. et al. Immunogenicity of a reduced schedule of meningococcal group C conjugate vaccine given concomitantly with the Prevenar and Pediacel vaccines in healthy infants in the United Kingdom. Clin. Vaccin. Immunol. 16, 194–199 (2009).
Souza, A. R. et al. Antibody persistence after serogroup C meningococcal conjugate vaccine in children with sickle cell disease. Vaccine 34, 4327–4334 (2016).
Bramley, J. C. et al. Safety and immunogenicity of three lots of meningococcal serogroup C conjugate vaccine administered at 2, 3 and 4 months of age. Vaccine 19, 2924–2931 (2001).
Cai, X. et al. LC/MS characterization of meningococcal depolymerized polysaccharide group C reducing endgroup and internal repeating unit. Anal. Chem. 76, 7387–7390 (2004).
Berti, F. In Carbohydrate-Based Vaccines: From Concept to Clinic (ed. Prasad, A. K.) Vol. 1290, ACS Symposium Series, Ch. 6, 123–137 (American Chemical Society, 2018).
Pina, L. M., Bassily, E., Machmer, A., Hou, V. & Reinhardt, A. Safety and immunogenicity of a quadrivalent meningococcal polysaccharide diphtheria toxoid conjugate vaccine in infants and toddlers: three multicenter phase III studies. Pediatr. Infect. Dis. J. 31, 1173–1183 (2012).
Jackson, L. A. et al. Phase III comparison of an investigational quadrivalent meningococcal conjugate vaccine with the licensed meningococcal ACWY conjugate vaccine in adolescents. Clin. Infect. Dis. 49, e1–10 (2009).
van der Vliet, D. et al. Immunogenicity and safety of a quadrivalent meningococcal tetanus toxoid-conjugate vaccine (MenACYW-TT) vs. a licensed quadrivalent meningococcal tetanus toxoid-conjugate vaccine in meningococcal vaccine-naive and meningococcal C conjugate vaccine-primed toddlers: a phase III randomised study. Epidemiol. Infect. 149, e50 (2021).
Dhingra, M. S. et al. Immunogenicity, safety and inter-lot consistency of a meningococcal conjugate vaccine (MenACYW-TT) in adolescents and adults: a Phase III randomized study. Vaccine 38, 5194–5201 (2020).
Nolan, T. et al. A novel combined Haemophilus influenzae type b-Neisseria meningitidis serogroups C and Y-tetanus-toxoid conjugate vaccine is immunogenic and induces immune memory when co-administered with DTPa-HBV-IPV and conjugate pneumococcal vaccines in infants. Vaccine 25, 8487–8499 (2007).
Vesikari, T. et al. Randomized trial to assess the immunogenicity, safety and antibody persistence up to three years after a single dose of a tetravalent meningococcal serogroups A, C, W-135 and Y tetanus toxoid conjugate vaccine in toddlers. Hum. Vaccin. Immunother. 8, 1892–1903 (2012).
Avci, F. Y., Li, X., Tsuji, M. & Kasper, D. L. A mechanism for glycoconjugate vaccine activation of the adaptive immune system and its implications for vaccine design. Nat. Med. 17, 1602–1609 (2011).
Anderson, P. W. et al. Effect of oligosaccharide chain length, exposed terminal group, and hapten loading on the antibody response of human adults and infants to vaccines consisting of Haemophilus influenzae type b capsular antigen unterminally coupled to the diphtheria protein CRM197. J. Immunol. 142, 2464–2468 (1989).
Micoli, F. et al. Vi-CRM 197 as a new conjugate vaccine against Salmonella Typhi. Vaccine 29, 712–720 (2011).
Launay, O. et al. Booster vaccination with GVGH Shigella sonnei 1790GAHB GMMA vaccine compared to single vaccination in unvaccinated healthy European adults: results from a phase 1 clinical trial. Front. Immunol. 10, 335 (2019).
Kabat, E. A. The upper limit for the size of the human antidextran combining site. J. Immunol. 84, 82–85 (1960).
Arnon, R. & Van Regenmortel, M. H. Structural basis of antigenic specificity and design of new vaccines. FASEB J. 6, 3265–3274 (1992).
Jennings, H. J., Roy, R. & Michon, F. Determinant specificities of the groups B and C8 polysaccharides of Neisseria meningitidis. J. Immunol. 134, 2651–2657 (1985).
Michon, F., Brisson, J. R. & Jennings, H. J. Conformational differences between linear alpha (2–8)-linked homosialooligosaccharides and the epitope of the group B meningococcal polysaccharide. Biochemistry 26, 8399–8405 (1987).
Oldrini, D. et al. Structure-guided design of a group B Streptococcus type III synthetic glycan-conjugate vaccine. Chemistry 26, 7018–7025 (2020).
Beurret, M., Hamidi, A. & Kreeftenberg, H. Development and technology transfer of Haemophilus influenzae type b conjugate vaccines for developing countries. Vaccine 30, 4897–4906 (2012).
Ravenscroft, N. et al. Size determination of bacterial capsular oligosaccharides used to prepare conjugate vaccines. Vaccine 17, 2802–2816 (1999).
Kensinger, R. D. & Hauser, S. L. Neisseria meningitidis vaccine. US patent application 2019/0175718 A1 (2019).
Acknowledgements
All costs associated with the development of this manuscript were funded by the authors’ employer, Janssen Vaccines & Prevention, part of the Janssen Pharmaceutical Companies of Johnson and Johnson, Department of Bacterial Vaccines Discovery and Early Development, in Leiden, The Netherlands. Writing and editorial support was provided by Steffen Porwollik and Maria Thompson (APEX Think Corporation, Sheridan, WY, USA).
Author information
Authors and Affiliations
Contributions
M.B. conceived the review, A.C. wrote the first draft, and J.P. provided supervision and expertise in review design. All authors contributed equally to the critical revisions of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors are employees of Janssen Vaccines & Prevention B.V., Leiden, The Netherlands. They have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in this review.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Anish, C., Beurret, M. & Poolman, J. Combined effects of glycan chain length and linkage type on the immunogenicity of glycoconjugate vaccines. npj Vaccines 6, 150 (2021). https://doi.org/10.1038/s41541-021-00409-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41541-021-00409-1
This article is cited by
-
Engineering Escherichia coli for increased Und-P availability leads to material improvements in glycan expression technology
Microbial Cell Factories (2024)
-
The effect of O-antigen length determinant wzz on the immunogenicity of Salmonella Typhimurium for Escherichia coli O2 O-polysaccharides delivery
Veterinary Research (2023)
-
Antibody enhanced HPLC for serotype-specific quantitation of polysaccharides in pneumococcal conjugate vaccine
npj Vaccines (2023)
-
Current progress in the development of prophylactic and therapeutic vaccines
Science China Life Sciences (2023)