Abstract
Gamma-aminobutyric acid (GABA) plays a crucial role in the central nervous system as an inhibitory neurotransmitter. Imbalances of this neurotransmitter are associated with neurological diseases, such as Alzheimer’s and Parkinson’s disease, and psychological disorders, including anxiety, depression, and stress. Since GABA has long been believed to not cross the blood–brain barrier, the effects of circulating GABA on the brain are neglected. However, emerging evidence has demonstrated that changes in both circulating and brain levels of GABA are associated with changes in gut microbiota composition and that changes in GABA levels and microbiota composition play a role in modulating mental health. This recent research has raised the possibility that GABA may be a potent mediator of the gut–brain axis. This review article will cover up-to-date information about GABA-producing microorganisms isolated from human gut and food sources, explanation why those microorganisms produce GABA, food factors inducing gut–GABA production, evidence suggesting GABA as a mediator linking between gut microbiota and mental health, including anxiety, depression, stress, epilepsy, autism spectrum disorder, and attention deficit hyperactivity disorder, and novel information regarding homocarnosine-a predominant brain peptide that is a putative downstream mediator of GABA in regulating brain functions. This review will help us to understand how the gut microbiota and GABA-homocarnosine metabolism play a significant role in brain functions. Nonetheless, it could support further research on the use of GABA production-inducing microorganisms and food factors as agents to treat neurological and psychological disorders.
Similar content being viewed by others
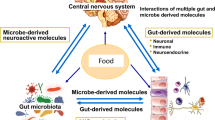
In the gut, trillions of microbes form a complex community, commonly known as the gut microbiota1. Gut microbiota produces thousands of unique small molecules or metabolites that can potentially affect host health2. Commonly identified metabolites include short-chain fatty acids (SCFAs), bile acids, choline metabolites3,4, vitamins5, amino acids6, and neurotransmitters7. The bidirectional communication pathway between the gut microbiota and the gut and their interaction with the central nervous system is termed the brain–gut–microbiome axis8. The gut microbiota and its metabolites affect host health through the brain8 and peripheral systems9. Metabolites travel by sending signals to the brain via the vagus nerve10 or blood–brain barrier (BBB) after crossing the intestinal barrier11. These metabolites are considered postbiotics because they can improve disease phenotypes and regulate the gut microbiota and metabolic pathways12 (Fig. 1). In contrast, dyshomeostasis of the gut microbiota and postbiotics leads to a variety of diseases in the host, such as metabolic, cardiovascular, and neurological diseases13,14.
These dietary factors, including probiotics, prebiotics, fermented foods, and microbial enzymes, positively affect gut microbiota composition that stimulates the release of GABA and other microbial metabolites. As microbial GABA passes through the intestinal barrier, it influences brain compound levels via blood-brain barrier or vagus nerve and improves brain function. This figure was created using Biorender.com.
Among recently developed postbiotics, GABA has gained much attention. Liu et al. (2017) showed changes in bacteria with the glutamic acid decarboxylase (GAD, K01580) enzyme gene, which is responsible for converting glutamate to GABA between control and obese individuals15. Furthermore, Kootte et al. (2017) demonstrated that GABA and GABA-producing bacteria were the most altered plasma metabolites and bacteria in fecal microbiota transplantation from lean individuals to people with metabolic syndrome16. Moreover, the intake of probiotics, such as Lactobacillus and Bifidobacterium, promotes an increase in GABA in both the gut and the brain11,17,18. These findings indicate that GABA is a possible postbiotic mediator of the gut–brain axis, which in turn regulates host health.
This manuscript reviews the development of GABA-producing microorganisms isolated from the human gut and fermented food products, as well as their potential to mediate the gut–brain axis based on available scientific evidence.
GABA metabolism
GABA was first discovered in the brain in 195019; years later, it was recognized as a key inhibitory neurotransmitter20. The functional importance of GABA is not limited to the brain; evidence suggests that it also has significance in peripheral tissues such as the gut, urinary bladder, heart, lung, ovary, and pancreas21. In terms of GABA concentration, the brain contains a high concentration with an average value of 2–3 mmol/g wet weight (20–30 mmol/g protein) and a regional distribution of 10–30 mmol/g protein, whereas most peripheral tissues have low GABA content, which is approximately 1% of that in the brain21. Among peripheral organs, GABA is abundant in the pancreas, and recent research suggests that the pancreatic GABA system plays an important role in protecting the pancreas and regulating insulin metabolism22.
GABA synthesis
GABA is synthesized by various organisms, including humans, plants, and bacteria23. In the synthesizing process, GABA is produced from glutamate by the glutamic acid decarboxylase (GAD) enzyme that requires pyridoxal-5′-phosphate (PLP) as a cofactor24. GAD enzymes exist in two forms, GAD65 and GAD67, which are regulated by GAD1 and GAD2, respectively25. In humans, GAD genes play a crucial role in the brain, where they are involved in the release of the inhibitory neurotransmitter GABA. GAD65 and GAD67 are present in the axon terminals and cell bodies, respectively26. GAD65 operates at a small fraction of its maximal catalytic capacity because its activity is very sensitive to changes in the energy state (inorganic phosphate, phosphocreatine, pH, magnesium, adenosine diphosphate (ADP), and adenosine triphosphate (ADP)) and the availability of PLP (an active form of vitamin B6)27, which is an allosteric cofactor of GAD enzymes. GAD expression is regulated at the transcriptional and post-translational levels, and it plays a key role in maintaining the balance between glutamate and GABA28. In peripheral organs, GAD is highly expressed in the pancreas as both the GAD65 and GAD67 isoforms, similar to its expression in the brain22. In plants, GAD is activated by abiotic stress (hypoxia, heat, cold, drought, and mechanical wounding) or biotic stress (predation and infection-induced wounding) to accumulate GABA29. In bacteria, GAD expression is induced during stationary or log phase growth under osmotic stress30.
GABA degradation
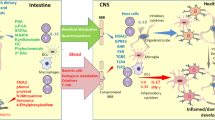
In the presence of the GABA-transaminase (GABA-T) enzyme, GABA is catabolized to succinic semialdehyde (SSA) by transamination with the co-substrate of α-ketoglutarate. Subsequently, SSA is oxidized by SSA dehydrogenase (SSADH) to succinate, a constituent of the tricarboxylic acid (TCA) cycle31. GABA-T is highly expressed in human glial cells and is responsible for clearing released GABA from the synapses to convert GABA into glutamate, which is then fed into the glutamine pool (Fig. 2)32. In addition to its expression in glial cells, GABA-T is also expressed in brain capillary endothelial cells, where it is believed to act as a neurotransmitter-metabolizing enzyme that possibly hydrolyzes circulating GABA and protects it from entering the brain33. In peripheral organs, GABA-T is highly expressed in the liver and, to some extent, in the pancreas and kidneys34. However, why GABA-T is highly expressed in the liver remains unclear. One hypothesis is that GABA-T hydrolyzes dietary or exogenous GABA and prevents the entry of peripheral GABA into the brain. To support this hypothesis, a previous study demonstrated that 2% GABA mixed in the diet (20 g GABA/kg diet) did not increase blood GABA levels, and even at a high dose (5% GABA), it could increase blood GABA levels to only +2% of the control group35. Although it seems likely that peripheral GABA is highly hydrolyzed in the liver, many studies have demonstrated the beneficial effects of low doses of GABA derived from diet or gut bacteria. It would be interesting to investigate this paradox further.
The highly expressed GABA transaminase (GABA-T) from glial cells is responsible for the clearing of released GABA from the synapses to convert GABA into glutamate, which is fed into the glutamine pool. Then, glutamine is transported from the glial cells to the presynaptic element, where it is converted back to glutamate. Then, glutamate is converted to GABA by glutamic acid decarboxylase (GAD). This figure was created using Biorender.com.
GABA-producing microorganisms
GABA-producing microorganisms isolated from the human gut
Several gastrointestinal (GI) bacteria contain the gene encoding GAD36,37, which is responsible for GABA production (Table 1). Among the human microbiota, Bifidobacterium, Lactobacillus, and Bacteroides are the most well-known GABA producers36,37. GABA production by Bifidobacterium and Lactobacillus has been extensively studied because of their probiotic functions and the need for probiotic and fermented food development. Emerging evidence has revealed that Bacteroides may be the primary genus in gut microbiota influencing mental health through the regulation of GABA production. Compared to Bifidobacterium and Lactobacillus, Bacteroides is one of the most abundant and prevalent genera in the human gut microbiota37,38. Recent findings from animal and human studies have shown a strong relationship between mental health disorders and dysregulation of the gut microbiota linked to glutamate–GABA metabolism, in which changes in the composition of Bacteroides were pronounced in the mental health disorder group37,39,40. In this section of the review, we focus only on those that have been reported to produce GABA in humans.
A recent Integrated Microbial Genomes/Human Microbiome Project database showed that Bacteroides (31.7%) was the most abundant genus in human gut microbiota processing GAD orthologs (specifically gadB), followed by Escherichia (22.5%) and Fusobacterium (9.9%); and both Bifidobacterium and Lactobacillus processed only 2.2%38. A recent study identifying uncultured bacteria in the human microbiome revealed that GABA is a previously undescribed growth factor in uncultured bacteria, and the main GABA producer is Bacteroides fragilis37. In addition to Bacteroides, Parabacteroides, Eubacterium, and Bifidobacterium have been identified as GABA producers in human stool samples37. However, only Bacteroides can produce GABA under a physiological pH for the human large intestine (pH 5.7–7.4), in which generally acid-tolerant pathogens such as E. coli produce GABA at a lower pH (<5.5)37,38. Transcriptome analysis of stool samples from healthy individuals confirmed that Bacteroides, Parabacteroides, and E. coli are GABA producers in the human gut38. Subsequently, information on GABA production by Bacteroides strains isolated from the human intestine was provided. Eleven species of human-intestinal Bacteroides, B. caccae, B. dorei, B. faecis, B. fragilis, B. intestinalis, B. ovatus, B. plebeius, B. thetaiotaomicron, B. uniformis, B. vulgatus, and B. xylanisolvensi, were found to produce GABA within the range from 0.1 to 61 mM36, comparable to GABA levels produced by high GABA-producing Lactobacillus and Bifidobacterium strains isolated from infant feces (12–300 mM)41. Using B. thetaiotaomicron as a culture model, it was found that both glutamate and glutamine are substrates for GABA production, and Bacteroides can produce GABA at pH values ranging pH from 3.1 to 6.3, with the highest production ability at pH 3.136. In silico analysis using a total of 961 Bacteroides genomes revealed that 96% of human–gut isolated Bacteroides genomes uniquely harbor four genes, a GAD (gadB ortholog; IPR010107), a glutaminase (glsA ortholog; IPR015868), a glutamate/GABA antiporter (gadC ortholog, IPR022520), and a K+ channel (IPR028325). These genes are involved in the GAD enzyme system36 and may exert a protective mechanism against acid stress in Bacteroides. Bacteroides can adapt to human gut conditions during the host lifespan with the flexibility to use various energy sources from both diet- and host-derived nutrients. This makes them resilient and robust against colonization of the human gastrointestinal tract36.
Lactobacillus and Bifidobacterium are well known for their probiotic effects. Several studies have reported the isolation of Lactobacillus and Bifidobacterium from the human gut37,38,41,42,43,44. A previous study demonstrated that GABA can be produced from human fecal fermentation, with GABA concentrations ranging from 5.4 to 56.4 µM41. By screening 91 strains from seven species of Lactobacillus and 13 species of Bifidobacterium isolated from infants and adults, it was found that only one Lactobacillus and three Bifidobacterium strains could produce GABA; these four GABA-producing strains were isolated from infant feces, dental carriers, and ileocecal junction areas41. With 10 mg/mL MSG supplementation, L. brevis DPC6108, B. adolescentis DPC6044, B. dentium DPC6333, B. dentium NFBC2243, and B. infantis UCC35624, exhibited GABA production at 106.7, 21.3, 50.9, 59.1, and 33.6 mM, respectively41. A later study showed that among 135 strains from 13 species of Lactobacillus and three species of Bifidobacterium isolated from healthy adults, only two species of Lactobacillus, L. plantarum, and L. brevis, exhibited GABA production, while all three species of Bifidobacterium (B. adolescentis, B. angulatum, and B. dentium), displayed GABA production36. L. plantarum (30 strains) and L. brevis (three strains) produced GABA at 0.5–2.9 mM and 0.5–6.5 mM, respectively, while B. adolescentis (21 strains), B. angulatum (three strains), and B. dentium (one strain) produced GABA at 4.7–58.2, 25.4–33.6, and 23.9 mM, respectively42. The addition of exogenous PLP, a cofactor of GAD, to the culture medium was found to increase GABA production in L. plantarum but not in L. brevis, B. angulatum, or B. adolescentis42. Recent research suggests that the Bifidobacteriaceae family, together with Streptococcaceae, is associated with a higher abundance of fecal GABA in healthy individuals with no systemic or psychiatric illnesses45. This high abundance of Bifidobacterium is beneficial, especially when alpha diversity in the gut is low (associated with specific diseases) because it restores microbial diversity46. The key player responsible for the high abundance of GABA in feces is Bifidobacterium adolescentis, which was recently recognized as a gut microorganism involved in GABA production44,45. B. adolescentis 150, B. adolescentis PRL2019, and B. adolescentis HD17T2H were bifidobacterial strains that are efficient GABA producers43,44. A recent study suggested that some Bifidobacterium adolescentis may represent a GABA producer model due to their performance in vitro and in vivo44. These B. adolescentis strains were identified as B. adolescentis PRL2019 and B. adolescentis HD17T2H, which can produce 7.1 mM and 9.4 mM of GABA, respectively44. By de novo genome assembly, B. angulatum GT102 and B. adolescentis 150 strains contain gadB gene encoding glutamate decarboxylase, gadС gene encoding putative glutamate/gamma-aminobutyrate antiporter, and gene encoding monoamine oxidase involved in the catabolism of monoamines47. In addition to their GABA-producing ability, both L. plantarum 90sk and B. adolescentis 150 exhibit antibiotic resistance and antioxidant properties43.
In addition to Bacteroides, Lactobacillus, and Bifidobacterium, a recent study demonstrated that Lactococcus, a genus of lactic acid bacteria, can produce GABA48. Lactococcus garvieae MJF010 was found to be the most efficient GABA producer among 23 lactic acid bacteria strains isolated from healthy human feces48. The GAD enzyme of L. garvieae MJF010 showed the highest GABA-producing activity at 35 °C and pH 5, whereas exogenous PLP addition had no effect48.
These studies provide compelling evidence that human gut microbiota is capable of producing GABA and may play a role in mediating gut and host health. Research focusing on other gut microorganisms is of great importance to further understand their critical roles in the gastrointestinal tract. Moreover, commensal probiotic strains in the human gut can be considered delivery vehicles for GABA in specific regions of the gut49.
GABA-producing microorganisms isolated from foods
Extensive studies have been conducted to develop GABA-rich food supplements50 and fermented foods51 that leverage many health benefits of GABA52. Recently, GABA production has been focused on seeking highly productive GABA strains and optimizing the growth conditions of these bacteria53. In Japan, the food industry is mainly interested in GABA production because it is considered a bioactive compound that promotes health and can be leveraged in the development of foods for specific health use (FOSHU)54.
Fermenting vegetables, meat, and fruits using lactic acid bacteria (LAB) is a standard method for preserving and improving the dietary and sensory characteristics of food commodities55. The complex nutritional substances of food commodities are a rich source of vitamins and minerals necessary for the growth of LAB strains, which facilitate the microbial production of enzymes and other metabolites56. LAB efficiently and rapidly converts sugars to lactic acid as a primary metabolic product, contributing to the preservation of fermented foods. Many of these raw materials contain significant amounts of glutamate, which can be utilized by LAB to convert glutamate to GABA using the GAD enzyme to increase tolerance to acidic conditions57. Several GABA-producing LAB have been isolated from a wide range of fermented foods (Table 2).
The predominant species of GABA-producing microorganisms described in Table 2 are Lactobacillus spp., including L. brevis58, L. plantarum50, L. paracasei59, L. buchneri60, and L. helveticus61. Among these, Lactobacillus paracasei NFRI 7415, isolated from fermented fish, produces high levels of GABA (302 mM) under appropriate conditions59. GABA-producing microorganisms were isolated from a wide range of fermented foods including cheese55, yogurt62, tea63, ground pork64, and sourdough65 as well as various Asian fermented products such as kimchi66, jeotgal (Korean fermented fish)67, nham (fermented Thai sausage)68, paocai (Chinese fermented vegetables)69, kung-som (Thai fermented shrimp)70, and ika-koujizuke (Japanese squid fermented with malted rice) and ika-kurozukuri (Japanese squid fermented with squid ink)71.
Recently, microorganisms belonging to the genera Lactococcus, Lactobacillus, Leuconostoc, and Kluyveromyces were identified and isolated from Mexican milk kefir grains and showed good probiotic properties through aggregation abilities, antimicrobial activity, antibiotic susceptibility, and resistance to in vitro gastrointestinal digestion, comparable to commercial probiotics72. Specific isolates of Kluyveromyces (BIOTEC009 and BIOTEC010), Leuconostoc (BIOTEC011 and BIOTEC012), and Lactobacillus (BIOTEC014 and BIOTEC15) exhibited high fermentability in media supplemented with commercial prebiotics72. The capacity to produce GABA was classified as a medium-level GABA producer for L. lactis BIOTEC006, BIOTEC007, BIOTEC008, K. lactis BIOTEC009, L. pseudomesenteroides BIOTEC012, and L. kefiri BIOTEC014 and was comparable to that obtained for commercial probiotics72. The classification system for GABA production by microorganisms was adapted from Tsukatani et al. (2005): less than 0.5 mM was considered a low-level GABA-producer, 0.5–2.1 mM was a medium-level GABA-producer, and more than 2.1 mM was a high-level GABA-producer73. Moreover, Saccharomyces cerevisiae SC125 and Lactobacillus plantarum BC114, both isolated and identified from traditional Chinese paocai, yielded 23.5 mM GABA as a co-culture that promotes the production of flavor compounds and GABA in mulberry beverage brewing74.
Despite the great diversity of fermented food products available worldwide as products of various cultures and traditions, little is known about the microorganisms involved in the fermentation process. There may be undiscovered microorganisms in traditional fermented products that are more efficient producers of GABA and other compounds than those previously identified and documented. Moreover, commercially available fermented products, such as kimchi, provide more data and information on the microorganisms involved in the fermentation process. Hence, a focus on other traditional fermented products is necessary to help diversify the information on fermentation and the potential of fermenting microorganisms to produce GABA and other beneficial metabolites.
Why do microorganisms produce GABA?
It is common knowledge in this research field that bacteria, especially those with probiotic properties, can produce GABA because of their ability to express GAD genes. However, the reason by which these bacteria produce GABA remains unclear. It has been hypothesized that GABA is produced under anaerobic and acidic conditions, allowing bacteria to survive in extreme environments. As shown in Fig. 3, glycolysis takes place in the cytosol under anaerobic conditions, where two molecules each of nicotinamide adenine dinucleotide (NAD+) and ADP are required to convert a glucose molecule into two molecules each of pyruvate nicotinamide adenine dinucleotide + hydrogen (NADH), and ATP75. Next, pyruvate is converted into fermentation products such as lactate, ethanol, and organic compounds, including acetate, butyrate, and propionate, in which two NAD+ molecules are generated, fed back, and reutilized in the glycolysis process75. The production of lactate and other acids by bacteria during fermentation lowers the pH, which leads to bacteria utilizing the GAD gene system76. The GAD gene system, consisting of gadB, glutaminase, gadC (glutamate/GABA antiporter), and K+ channels, helps bacteria cope with changes in intracellular pH36. Activation of the GAD gene system owing to a decline in pH then triggers the production of GABA76,77. As shown in Fig. 3, free glutamate in the environment is transported into the cell by a specific transporter (glutamate/GABA antiporter), which leads to the decarboxylation of glutamate to GABA by GAD, in which intracellular H+ ions are consumed36,76,78. GABA is then exported from the cell via the antiporter, which results in an increase in the pH of the cytoplasm due to the removal of H+ ions and a slight increase in the extracellular pH due to the exchange of extracellular glutamate for more alkaline GABA76,78. Hence, the release of GABA helps bacteria cope with acid stress, which is crucial for the colonization of the GI tract and survival in acidic fermentation environments.
Under anaerobic and acidic conditions in the human gut and fermentation, it appears that bacteria produce GABA for their own survival purposes under these extreme environments. Under anaerobic conditions, glycolysis takes place in the cytosol, where NAD+ and ADP are required to convert glucose into pyruvate, in which NADH and ATP are produced from the process75. Pyruvate is then converted into lactate or other organic compounds, such as acetate, butyrate, and propionate, where NADH is utilized, and NAD+ is generated in the process. Then, NAD+ is fed back and reutilized in the glycolysis process75. The acidic fermentation by-products, lactate, and other organic compounds lower the pH, which leads bacteria to utilize the GAD gene system and triggers the production of GABA76,77. To produce GABA, exogenous glutamate is transported into the cell by a glutamate/GABA antiporter, then glutamate is converted into GABA by glutamic acid decarboxylase (GAD)36,76,78. Then, GABA is exported from the cell via the antiporter, resulting in an increase in the pH of the cytoplasm due to the removal of H+ ions and a slight increase in the extracellular pH due to the exchange of extracellular glutamate for more alkaline GABA76,78. This figure was created using Biorender.com.
Gut–GABA-production inducing food factors
In addition to fermented food products that promote GABA production owing to the presence of GABA-producing bacteria, several researchers have explored the potential of other food factors that can induce GABA production in the gut. As mentioned above, probiotics, Bifidobacterium and Lactobacillus, and the predominant gut bacteria Bacteroides are the main GABA producers in the human gut, and food factors that can enhance the abundance of these gut bacteria are candidates for GABA production-inducing food factors. In addition to typical well-known prebiotics, such as fructooligosaccharides (FOS), emerging research suggests that microorganism-derived enzymes, such as proteases, lipases, amylases, and cellulases, have the potential to act as prebiotics to increase probiotics in the gut79,80,81,82. Recent studies have shown that dietary Aspergillus oryzae-derived protease markedly increases the abundance of both Bifidobacterium and Lactobacillus in the rat cecum and induces the production of various postbiotics, including GABA, which was not detected in the cecum of rats receiving no dietary protease80,81. Taken together with the fact that GABA is a non-proteinogenic amino acid, these findings suggest that GABA was possibly produced from elevated levels of the probiotics Bifidobacterium and Lactobacillus. More recent studies have revealed that other dietary factors, such as lipase from Penicillium camemberti, which is generally used in cheese production, also induce an increased abundance of Bifidobacterium and Lactobacillus in the rat cecum83. These studies imply that the digestive enzymes produced by Aspergillus and Penicillium exert prebiotic-like effects by increasing the abundance of the GABA-producing probiotics Bifidobacterium and Lactobacillus, in the gut, possibly making them efficient in GABA production. The same is true for inulin, which stimulates GABA production in the gut84. More work is needed to identify and investigate other food factors that have the potential to induce GABA production in the gut or brain, regardless of whether they are microorganism-derived or naturally derived.
Gaba as a mediator of the gut–brain axis
Association of gut microbiota and GABA in mental health and brain function
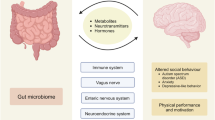
It has been well-accepted that dysbiosis of the gut microbiota is strongly linked to human health, including mental health. Gut microbiota and probiotics impact host health through various mechanisms, including the production of metabolites, recently defined as postbiotics, such as short-chain fatty acids, peptides, and amino acids. Among these postbiotics, GABA has received much attention from researchers owing to its essential role in the nervous system and its strong correlation with the gut microbiota. Studies have suggested that peripheral or circulating GABA is mainly attributed to the gut microbiota40,85,86. In germ-free mice, blood (3.7 times) and fecal (1.3 times) GABA levels were lower than those in fecal-oral-inoculated germ-free mice85. Another study showed that cecal GABA levels were markedly decreased in mice treated with vancomycin86. Oral administration of GABA-producing B. dentium ATCC 27678, but not non-GABA-producing B. breve NCIMB8807, increased cecal GABA levels and reduced colon-specific sensory neuron excitability, which are the general causes of abdominal pain38. Taken together, these studies indicate the potential role of GABA as a moderator in the gut–brain axis. The following section presents recent information regarding the involvement of the gut microbiota and GABA in mental health and brain diseases. A summary of this interaction is shown in Table 3.
Gut microbiota and GABA in neurological disorders
Neurological disorders such as schizophrenia (SCZ), Alzheimer’s disease (AD), and Parkinson’s disease (PD) have been linked to dysbiosis because of the strong connection between the gut and brain87. A recent study revealed that treated and non-treated SCZ patients had a decreased microbiome diversity index compared to healthy controls, where an increased abundance of Veillonellaceae, Prevotellaceae, Bacteroidaceae, Coriobacteriaceae and a decreased abundance of Lachnospiraceae, Ruminococceae, and Norank were found in SCZ patients88. Additionally, a lower abundance of Bacteroides and Streptococcus in the gut microbiota is a feature of SCZ, and these bacteria are associated with glutamate and GABA metabolism89. Furthermore, germ-free mice receiving the SCZ microbiome showed decreased glutamate but increased GABA levels in the hippocampus, displaying SCZ-relevant behaviors similar to other mouse models of SCZ involving glutamatergic hypofunction88. In AD, the fecal microbial composition and metabolic output were evident. Patients with AD had an increased abundance of Lachnospiraceae, Ruminococcaceae, Prevotellaceae, Atopobiaceae, Clostridiales, Synergistaceae, Erysipelotrichaceae, and Pseudomonadaceae and a decreased abundance of Lachnospiraceae (genus Tyzzerella) and Erysipelotrichaceae (genus Erysipelatoclostridium) compared to normal controls, and these microorganisms were significantly associated with a decreased abundance of N-docosahexaenoyl GABA, 19-oxoandrost-4-ene-3,17-dione, trigofoenoside F, and 22-angeloylbarringtogenol C metabolites90. Bidirectional Mendelian randomization analysis has revealed a causal relationship between the relative abundance of Blautia, a new functional genus with potential probiotic properties91, and AD92. Elevated levels of GABA, a downstream product of Blautia-dependent arginine metabolism, in the cerebrospinal fluid (CSF) are related to a reduced risk of AD92,93. Patients with PD had a significant increase in Akkermansia and a decrease in Lactobacillus compared to healthy controls94. The differences in postural instability gait difficulty (PIGD) and tremor-dominant (TD) PD motor subtypes in basal ganglia GABA levels could be lower in TD than in PIGD, which may indicate a difference in the pathophysiological mechanisms of TD and PIGD95. In addition, treatment with Pediococcus pentosaceus improved the gut microbial dysbiosis and increased GABA levels in methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced PD96.
Gut microbiota and GABA in anxiety, depression, and stress
Recently, altered gut microbiota and reduced function of the GABA system in the prefrontal cortex following chronic ethanol exposure led to anxiety-like behaviors97. Administration of Lactobacillus rhamnosus JB-1 improved stress-induced anxiety- and depression-like behaviors in mice by increasing GABA mRNA expression in the hippocampus10. Increased small intestine GABA level (0.03–0.04 mM) of metabolic syndrome mice model fed with diet incorporated with Lactobacillus brevis DPC6108 and DSM32386 strains improved depression-like behavior in the forced swim test and resting stress hormone corticosterone level compared to high-fat control diet18. Metagenomics-based analyses involving datasets collected from children with subclinical symptoms of depression and anxiety revealed high metagenomic reads of gad in groups with a high abundance of Bifidobacterium adolescentis44. Furthermore, the depressed phenotype had a greater prevalence of GABA-consuming microorganisms in the selected strains of Flavonifractor plautii, Pseudomonas spp., and Acinetobacter spp. then the healthy phenotype, thereby favoring GABA degradation98. Moreover, a decreased abundance of Bacteroides eggerthii was found to be associated with a decrease in GABA synthesis in subjects with stress and anxiety, and gut microbiota modulation through probiotic supplementation enriched GABA-synthesizing Bifidobacterium adolescentis and Bifidobacterium longum that alleviated stress- and anxiety-related symptoms99.
Gut microbiota and GABA in epilepsy
An imbalance in neuroactive compounds, including GABA, and intestinal dysbiosis are two important considerations in epilepsy100,101 and are commonly observed in humans and dogs102. In humans, it was found that patients with four or fewer seizures per year had higher fecal Bifidobacteria and Lactobacilli than those who had more than four seizures103. These flora promote GABA synthesis36,37. In dogs, the epileptic group had a significantly reduced abundance of fecal GABA-producing (Pseudomonadales, Pseudomonadaceae, Pseudomonas, and Pseudomona_graminis) and SCFA-producing bacteria (Peptococcaceae, Ruminococcaceae and Anaerotruncus), as well as bacteria associated with a reduced risk of brain disease (Prevotellaceae) compared to the control group102. Despite difficulties with implementation, dietary compliance, and adverse side effects, a ketogenic diet (or low-carbohydrate, high-fat diet; KD)104 is an effective dietary intervention to treat epilepsy. KD positively altered the gut microbiota by increasing the abundance of Akkermansia muciniphila and Parabacteriodes from 4 to 14 days of treatment, demonstrating an anti-seizure effect in a wide-range anti-epileptic drug-resistant seizure model105. Administration of the KD paired with Akkermansia muciniphila and Parabacteriodes significantly increased hippocampal GABA/glutamate ratios105. Probiotic administration (several Lactobacillus, Bifidobacterium, and Streptococcus strains) to drug-resistant epileptic (DRE) patients decreased the number of seizure occurrences and increased the serum GABA concentration after a 12-week treatment106.
Gut microbiota and GABA in autism spectrum disorder (ASD) and attention deficit hyperactivity disorder (ADHD)
Occurrence of high gut Clostridium spp. is associated with ASD in patients with gastrointestinal disease107. Specifically, 76–87% of beta2-toxin-producing Clostridium perfringens were significantly higher in children with ASD compared to control children, indicating that these opportunistic pathogens thrive in immature or compromised immune systems107. A recent study has shown that infants with increased-likelihood of ASD have a decreased abundance of Bifidobacterium but an increased abundance of Clostridium and Klebsiella compared to those with lower likelihood of ASD108. Moreover, fecal GABA levels of infants with increased likelihood of ASD were lower than those with lower likelihood of ASD, in which fecal GABA levels are positively correlated with Bifidobacterium, but negatively correlated with Clostridium108. A lower abundance of Prevotella copri, Feacalibacterium prausnitzii, and Haemophilus parainfluenzae and decreased concentrations of fecal GABA were found in children with ADS when compared to healthy children109. In contrast, an increased ratio of fecal GABA/glutamate with a higher abundance in Escherichia/Shigella and a lower abundance of Bacteroides was found in mild ADS children than in healthy children110. Dialister, Escherichia/Shigella, and Bifidobacterium were more abundant in ASD children, while Prevotella 9, Megamonas, and Ruminococcus 2 were more abundant in healthy children, in which GABA precursors, such as N-carboxyethyl-g-aminobutyric acid, glutamylproline, pyroglutamic acid, and gamma-glutamylglycine, were higher in ASD children111.
In ADHD, magnetic resonance spectroscopy revealed a significant reduction in brain GABA concentration in children with ADHD112. In contrast, increased cortical GABA concentration was observed in adults with ADHD, which suggests that GABA levels may be correlated with the age of patients with ADHD113. A recent study has shown that the top five depleted bacteria families in infants (6 months of age) with ADHD are Lachnospiraceae, Ruminococcus, Bacteroides, Lachnospiraceae, and Enterococcus, while the top five enriched bacteria families are Bacteroides, Dorea, Erysipelotrichaceae, Ruminococcaceae, and Dialister114. Interestingly, 50% of the depleted families belong to the Lactobacillales order, or lactic acid bacteria114. Due to the fact that lactic acid bacteria are strong GABA producers, this can suggest that the depletion of lactic acid bacteria in the gut of infants with ADHD might be related to a decrease in GABA. On the other hand, in a case study, an adult with ADHD was found to have a high abundance of Bifidobacterium adolescentis, Bifidobacterium animalis, Bifidobacterium breve, Bifidobacterium longum, Bacteroides ovatus, Bacteroides uniformis, Fusobacterium ulcerans, Enterocococcus avium, and Enterococcus gallinarumi, but fecal microbiota transplant significantly reduced the abundance of those bacteria with the relief of ADHD symptoms115. Since most of those bacteria (Bifidobacterium, Bacteroides, and Enterocococcus) are well-known GABA producers37, this may support a positive correlation of GABA with ADHD in adults as previously reported113. It seems likely that GABA may play a role in the pathogenesis of ADHD in children and adults, but possibly in different ways.
Brain-specific GABA-containing peptide
Homocarnosine
Homocarnosine (GABA-l-histidine) is a GABA-containing dipeptide that is predominantly found in the brain116. It is an analog of the predominant muscle dipeptide carnosine (β-alanine-l-histidine). Homocarnosine is synthesized from GABA and histidine by carnosine synthase in neurons and is degraded by carnosinase117,118. The occipital cortex, basal ganglia, and cervical cord have the highest human homocarnosine synthetase, currently known as carnosine synthase, activity while the cerebellar cortex has the lowest117. It is present in greater amounts in the human brain than in the brains of other mammals119. Homocarnosine concentration of the autopsied brain ranges from 0.4 mmol/kg in the corpus callosum and temporal cortex to 1.0 mmol/kg in the thalamus and basal ganglia and varies independently of GABA concentrations120. The homocarnosine concentration is threefold to sixfold higher in adults than in infants121. Recently, areas of the human central nervous system, particularly the olfactory bulb, spinal cord, medulla oblongata, thalamus, cerebellum, white matter, and frontal cortex, had a considerable amount of homocarnosine while the human CSF abundantly contains homocarnosine122. Although its concentration in the brain is high, the function of homocarnosine in the brain remains underexplored, which has led to a limited understanding of its high maintained concentrations in the brain. However, several biochemical properties of homocarnosine have been reported.
For instance, homocarnosine acts as a protective agent against a wide range of disease conditions, including the protection of brain endothelial cells from amyloid peptide-induced toxicity123 and anti-inflammatory action in brain ischemic injuries124. Homocarnosine demonstrates similar properties to carnosine in protecting Cu and Zn superoxide dismutase from oxidative damage through a combination of copper chelation and peroxyl radical scavenging125. Moreover, homocarnosine, in combination with carnosine and anserine, reduces oxidative damage by decreasing lipid peroxidation and increasing antioxidant levels in the brain126. Many studies have explored the biological role of homocarnosine in the brain and other neurological diseases. Hence, a thorough investigation is required to better understand the role of homocarnosine.
Because homocarnosine is a GABA-containing peptide, changes in GABA levels may contribute to changes in homocarnosine homeostasis. This hypothesis is supported by the notion that homocarnosine is a possible GABA reservoir, as approximately 40% of GABA measured in human CSF is homocarnosine127. In addition, it has been hypothesized that the release of homocarnosine contributes to glutamate–GABA cycling and reflects an adaptive response to excess extracellular glutamate128, wherein a strong linear correlation between GABA and homocarnosine concentrations has been observed in healthy CSF (GABA concentration is higher than homocarnosine concentration)129.
Possible link of gut–brain axis and GABA-homocarnosine in brain-related diseases
Homocarnosine homeostasis in the brain plays a critical role in clinical studies of neurological diseases, such as Alzheimer’s disease and epilepsy130,131. Low homocarnosine levels may reflect decreased fractional volumes of homocarnosine-containing neurons, and homocarnosine deficits may indicate either the loss or dysfunction of GABAergic neurons128,130. Drugs may be administered to improve homocarnosine levels in the brain. Vigabatrin and gabapentin, known antiepileptic drugs, improve seizures by increasing levels of brain GABA and homocarnosine131,132. Topiramate, another anti-seizure drug, improves brain homocarnosine and GABA levels, contributing to its potent anti-epileptic action in patients with complex partial seizures127. Moreover, isoniazid supplementation in healthy patients elevates homocarnosine and GABA concentrations133. As mentioned above, homocarnosine could possibly be a good reservoir of GABA in the brain, and other neurological disorders, such as AD, ASD, and SCZ, can be associated with homocarnosine as they are characterized by low GABA levels, and GABA can induce homocarnosine production35,88,92,134.
It is worth mentioning that the above-mentioned neurological disorders alter gut GABA-producing microorganisms that affect GABA homeostasis in the gut and brain. An increase in the abundance of the well-known probiotics Lactobacillus and Bifidobacterium induces gut GABA production79,80. However, to date, the direct interaction and correlation between homocarnosine and the gut microbiota as affected by diet remain unknown. Recently (unpublished data), our group discovered the ability of Aspergillus-derived enzymes together with FOS to exhibit a tendency to increase cecal and brain GABA levels. Moreover, the dietary intake of these prebiotic-linked enzymes and FOS increases homocarnosine levels in the brain. These findings indicate that dietary factors may act as one of the modulators of GABA and homocarnosine levels in the gut and brain.
To summarize, GABA has long been the subject of rigorous research, and its health benefits have been proven through in vitro and in vivo experiments. Although circulating GABA has long been believed to not cross the BBB, GABA’s permeability through the BBB remains contested due to conflicting evidence. Recent research has demonstrated that GABA can be a potent mediator of the gut–brain axis, as it is circulating and brain levels are regulated by the microbiota, and that changes in GABA levels and microbiota composition play a role in modulating mental health. Generally, GABA is present at trace concentrations in the bloodstream. Recent studies have suggested that circulating GABA is mainly attributed to gut microbiota. Several studies have isolated GABA-producing bacteria from the human gut, such as Lactobacillus, Bifidobacterium, and Bacteroides, and from fermented foods, such as Lactobacillus, Streptococcus, Leuconostoc, and Weisella. In addition to probiotics, non-typical prebiotic food factors, such as Aspergillus- and Penicillium-derived enzymes, have been demonstrated to stimulate an increased abundance of probiotics and gut GABA production. Supplementation with probiotics and probiotic-rich products improves the cognitive function of patients with neurological disorders; eases anxiety, depression, and stress; and increases circulating and brain GABA availability. In addition to GABA, a predominant GABA-containing brain peptide, homocarnosine, has recently been demonstrated to be a possible downstream mediator of GABA in the gut–brain axis. Currently, there is limited information regarding the connections between homocarnosine, gut microbiota, and brain function. Thus, it is of great importance to further investigate this issue because this information may help clarify how the gut microbiota and GABA-homocarnosine metabolism play a role in brain function. This information will contribute to the development of functional foods and mental health interventions.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
References
Zhang, T. et al. Stronger gut microbiome modulatory effects by postbiotics than probiotics in a mouse colitis model. npj Sci. Food 6, 53 (2022).
Furness, J. B. The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 9, 286–294 (2012).
Visconti, A. et al. Interplay between the human gut microbiome and host metabolism. Nat. Commun. 10, 4505 (2019).
Smith, P. M. et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341, 569–573 (2013).
Milshteyn, A., Colosimo, D. A. & Brady, S. F. Accessing bioactive natural products from the human microbiome. Cell Host Microbe 23, 725–736 (2018).
Shapiro, H., Thaiss, C. A., Levy, M. & Elinav, E. The cross talk between microbiota and the immune system: metabolites take center stage. Curr. Opin. Immunol. 30, 54–62 (2014).
Nicholson, J. K. et al. Host-gut microbiota metabolic interactions. Science 336, 1262–1267 (2012).
Bravo, J. A. et al. Communication between gastrointestinal bacteria and the nervous system. Curr. Opin. Pharmacol. 12, 667–672 (2012).
Marcobal, A. et al. A metabolomic view of how the human gut microbiota impacts the host metabolome using humanized and gnotobiotic mice. ISME J. 7, 1933–1943 (2013).
Bravo, J. A. et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl Acad. Sci. USA 108, 16050–16055 (2011).
Yano, J. M. et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161, 264–276 (2015).
Rudzki, L. et al. Gut microbiota-derived vitamins—underrated powers of a multipotent ally in psychiatric health and disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 107, 110240 (2021).
Karczewski, J. et al. Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 298, G851–G859 (2010).
De Roos, N. M. et al. The effects of the multispecies probiotic mixture Ecologic® Barrier on migraine: results of an open-label pilot study. Benef. Microbes 6, 641–646 (2015).
Liu, R. et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat. Med. 23, 859–868 (2017).
Kootte, R. S. et al. Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metab. 26, 611–619 (2017).
Janik, R. et al. Magnetic resonance spectroscopy reveals oral Lactobacillus promotion of increases in brain GABA, N-acetyl aspartate and glutamate. Neuroimage 125, 988–995 (2016).
Patterson, E. et al. Gamma-aminobutyric acid-producing Lactobacilli positively affect metabolism and depressive-like behaviour in a mouse model of metabolic syndrome. Sci. Rep. 9, 16323 (2019).
Roberts, E. & Frankel, S. γ-Aminobutyric acid in brain. Its formation from glutamic acid. J. Biol. Chem. 187, 55–63 (1950).
Krnjevic, K. & Phillis, J. W. Iontophoretic studies of neurons in the mammalian cerebral cortex. J. Physiol. 165, 274–304 (1963).
Erdo, S. L. & Kiss, B. Presence of GABA, glutamate decarboxylase, and GABA transaminase in peripheral tissues: a collection of quantitative data in GABAergic Mechanisms in the Mammalian Periphery (eds Erdo, S. L. & Bowery, N. G.) 5–18 (Raven Press, 1986).
Wan, Y., Wang, Q. & Prud’homme, G. J. GABAergic system in the endocrine pancreas: a new target for diabetes treatment. Diabetes Metab. Syndr. Obes. 8, 79 (2015).
Dhakal, R., Bajpai, V. K. & Baek, K. H. Production of GABA (gamma-aminobutyric acid) by microorganisms: a review. Braz. J. Microbiol. 43, 1230–1241 (2012).
Yogeswara, I. B. A., Maneerat, S. & Haltrich, D. Glutamate decarboxylase from lactic acid bacteria—a key enzyme in GABA synthesis. Microorganisms 8, 1923 (2020).
Bu, D. F. et al. Two human glutamate decarboxylases, 65-kDa GAD and 67-kDa GAD, are each encoded by a single gene. Proc. Natl Acad. Sci. USA 89, 2115–2119 (1992).
Müller, I., Çalışkan, G. & Stork, O. The GAD65 knock out mouse—a model for GABAergic processes in fear‐and stress‐induced psychopathology. Genes Brain Behav. 14, 37–45 (2015).
Martin, D. L. & Rimvall, K. Regulation of gamma-aminobutyric acid synthesis in the brain. J. Neurochem. 60, 395–407 (1993).
Chen, Y., Dong, E. & Grayson, D. R. Analysis of the GAD1 promoter: trans-acting factors and DNA methylation converge on the 5’ untranslated region. Neuropharmacology 60, 1075–1087 (2011).
Shelp, B. J. et al. Strategies and tools for studying the metabolism and function of γ-aminobutyrate in plants. II. Integrated analysis. Botany 90, 781–793 (2012).
Lim, H. S. et al. Expression and characterization of glutamate decarboxylase from Lactobacillus brevis HYE1 isolated from kimchi. World J. Microbiol. Biotechnol. 34, 44 (2018).
Kaupmann, K. et al. Specific gamma-hydroxybutyrate-binding sites but loss of pharmacological effects of gamma-hydroxybutyrate in GABA(B)(1)- deficient mice. Eur. J. Neurosci. 18, 2722–2730 (2003).
Deutch, A. Y. Neurotransmitter in Fundamental Neuroscience (ed Squire, L. R. et al.) 117–138 (Academic Press, 2013).
Dash, P. Chapter 11: Blood Brain Barrier and Cerebral Metabolism. Neurosci. Online https://nba.uth.tmc.edu/neuroscience/s4/chapter11.html (2020).
Tillakaratne, N. J., Medina-Kauwe, L. & Gibson, K. M. Gamma-aminobutyric acid (GABA) metabolism in mammalian neural and nonneural tissues. Comp. Biochem. Physiol. Part A 112, 247–263 (1995).
Kumrungsee, T. et al. Dietary GABA induces endogenous synthesis of a novel imidazole peptide homocarnosine in mouse skeletal muscles. Amino Acids 52, 743–753 (2020).
Otaru, N. et al. GABA production by human intestinal Bacteroides spp.: prevalence, regulation, and role in acid stress tolerance. Front. Microbiol. 12, 656895 (2021).
Strandwitz, P. et al. GABA-modulating bacteria of the human gut microbiota. Nat. Microbiol. 4, 396–403 (2019).
Pokusaeva, K. et al. GABA-producing Bifidobacterium dentium modulates visceral sensitivity in the intestine. Neurogastroenterol. Motil. 29, e12904 (2017).
Zheng, P. et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 21, 786–796 (2016).
Valles-Colomer, M. et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 4, 623–632 (2019).
Barrett, E., Ross, R., O’Toole, P., Fitzgerald, G. & Stanton, C. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J. Appl. Microbiol. 113, 411–417 (2012).
Yunes, R. et al. GABA production and structure of gadB/gadC genes in Lactobacillus and Bifidobacterium strains from human microbiota. Anaerobe 42, 197–204 (2016).
Yunes, R. et al. A Multi-strain potential probiotic formulation of GABA-producing Lactobacillus plantarum 90sk and Bifidobacterium adolescentis 150 with antidepressant effects. Probiotics Antimicrob. Proteins 12, 973–979 (2020).
Duranti, S. et al. Bifidobacterium adolescentis as a key member of the human gut microbiota in the production of GABA. Sci. Rep. 10, 14112 (2020).
Altaib, H. et al. Differences in the concentration of the fecal neurotransmitters GABA and glutamate are associated with microbial composition among healthy human subjects. Microorganisms 9, 378 (2021).
Lozupone, C. et al. Diversity, stability and resilience of the human gut microbiota. Nature 489, 220–230 (2012).
Dyachkova, M. S. et al. Draft genome sequences of Bifidobacterium angulatum GT102 and Bifidobacterium adolescentis 150: focusing on the genes potentially involved in the gut-brain axis. Genome Announc 3, e00709–e00715 (2015).
Lim, H. J., Jung, D. H., Cho, E. S. & Seo, M. J. Expression, purification, and characterization of glutamate decarboxylase from human gut-originated Lactococcus garvieae MJF010. World J. Microbiol. Biotechnol. 38, 69 (2022).
Ghatge, M. S. et al. Pyridoxal 5′-phosphate is a slow tight binding inhibitor of E. coli pyridoxal kinase. PLoS ONE 7, e41680 (2012).
Boonstra, E. et al. Neurotransmitters as food supplements: the effects of GABA on brain and behavior. Front. Psychol. 6, 1520 (2015).
Selhub, E. M., Logan, A. C. & Bested, A. C. Fermented foods, microbiota, and mental health: ancient practice meets nutritional psychiatry. J. Physiol. Anthropol. 33, 2 (2014).
Sharon, G. et al. Specialized metabolites from the microbiome in health and disease. Cell Metab. 20, 719–730 (2014).
Diana, M., Tres, A., Quílez, J., Llombart, M. & Rafecas, M. Spanish cheese screening and selection of lactic acid bacteria with high gamma-aminobutyric acid production. LWT–Food Sci. Technol. 56, 351–355 (2014).
Martirosyan, D. M. & Singh, J. A new definition of functional food by FFC: what makes a new definition unique? Funct. Foods Health Dis. 5, 209–223 (2015).
Di Cagno, R., Coda, R., de Angelis, M. & Gobbetti, M. Exploitation of vegetables and fruits through lactic acid fermentation. Food Microbiol. 33, 1–10 (2013).
Swain, M., Anandharaj, M., Ray, R. & Rani, R. Fermented fruits and vegetables of Asia: a potential source of probiotics. Biotechnol. Res. Int. 2014, 2504241 (2014).
Cotter, P. & Hill, C. Surviving the acid test: responses of Gram-positive bacteria to low pH. Microbiol. Mol. Biol. Rev. 67, 429–453 (2003).
Lim, H., Cha, I., Roh, S., Shin, H. & Seo, M. Enhanced production of γ-aminobutyric acid by optimizing culture conditions of Lactobacillus brevis HYE1 isolated from kimchi, a Korean fermented food. J. Microbiol. Biotechnol. 27, 450–459 (2017).
Komatsuzaki, N., Shima, J., Kawamoto, S., Momose, H. & Kimura, T. Production of γ- aminobutyric acid (GABA) by Lactobacillus paracasei isolated from traditional fermented foods. Food Microbiol. 22, 497–504 (2005).
Zhao, A., Hu, X., Pan, L. & Wang, X. Isolation and characterization of a γ-aminobutyric acid producing strain Lactobacillus buchneri WPZ001 that could efficiently utilize xylose and corncob hydrolysate. Appl. Microbiol. Biotechnol. 99, 3191–3200 (2015).
Sun, T. et al. ACE-inhibitory activity and γ-aminobutyric acid content of fermented skim milk by Lactobacillus helveticus isolated from Xinjiang koumiss in China. Eur. Food Res. Technol. 228, 607–612 (2009).
Lu, X., Xie, C. & Gu, Z. Isolation of γ-aminobutyric acid-producing bacteria and optimization of fermentative medium. Biochem. Eng. J. 41, 48–52 (2008).
Jeng, K., Chen, C., Fang, Y., Hou, R. & Chen, Y. Effect of microbial fermentation on content of statin, GABA, and polyphenols in Pu-Erh tea. J. Agric. Food Chem. 55, 8787–8792 (2007).
Jitpakdee, J., Kantachote, D., Kanzaki, H. & Nitoda, T. Selected probiotic lactic acid bacteria isolated from fermented foods for functional milk production: Lower cholesterol with more beneficial compounds. LWT–Food Sci. Technol. 135, 110061 (2020).
Demirbaş, F., İspirli, H., Kurnaz, A. A., Yilmaz, M. T. & Dertli, E. Antimicrobial and functional properties of lactic acid bacteria isolated from sourdoughs. LWT–Food Sci. Technol. 79, 361–366 (2017).
Han, S., Hong, K. & Suh, H. Biotransformation of monosodium glutamate to γ-aminobutyric acid by isolated strain Lactobacillus brevis L-32 for potentiation of pentobarbital-induced sleep in mice. Food Biotechnol. 31, 80–93 (2017).
Lee, B. et al. Antioxidant activity and γ-aminobutyric acid (GABA) content in sea tangle fermented by Lactobacillus brevis BJ20 isolated from traditional fermented foods. Food Chem. 122, 271–276 (2010).
Ratanaburee, A., Kantachote, D., Charernjiratrakul, W. & Sukhoom, A. Selection of γ- aminobutyric acid-producing lactic acid bacteria and their potential as probiotics for use as starter cultures in Thai fermented sausages (Nham). Int. J. Food Sci. Technol. 48, 1371–1382 (2013).
Li, H., Gao, D. & Cao, Y. A high γ-aminobutyric acid-producing Lactobacillus brevis isolated from Chinese traditional paocai. Ann. Microbiol. 58, 649–653 (2008).
Sanchart, C., Rattanaporn, O., Haltrich, D., Phukpattaranont, P. & Maneerat, S. Lactobacillus futsaii CS3, a new GABA-producing strain isolated from Thai fermented shrimp (Kung-som). Indian J. Microbiol. 57, 211–217 (2017).
Barla, F. et al. The γ-aminobutyric acid-producing ability under low pH conditions of lactic acid bacteria isolated from traditional fermented foods of Ishikawa Prefecture, Japan, with a strong ability to produce ACE-inhibitory peptides. Biotechnol. Rep. 10, 105–110 (2016).
Hurtado-Romero, A., Del Toro-Barbosa, M., Gradilla-Hernández, M., Garcia-Amezquita, L. & García-Cayuela, T. Probiotic properties, prebiotic fermentability, and GABA-producing capacity of microorganisms isolated from Mexican milk kefir grains: a clustering evaluation for functional dairy food applications. Foods 10, 2275 (2021).
Tsukatani, T., Higuchi, T. & Matsumoto, K. Enzyme-based microtiter plate assay for γ-aminobutyric acid: Application to the screening of γ-aminobutyric acid-producing lactic acid bacteria. Anal. Chim. Acta 540, 293–297 (2005).
Zhang, Q. et al. Characterization of g-aminobutyric acid (GABA)-producing Saccharomyces cerevisiae and coculture with Lactobacillus plantarum for mulberry beverage brewing. J. Biosci. Bioeng. 129, 447–453 (2020).
Wang, Y. et al. Metabolism characteristics of lactic acid bacteria and the expanding applications in food industry. Front. Bioeng. Biotechnol. 9, 612285 (2021).
Feehily, C. & Karatzas, K. A. G. Role of glutamate metabolism in bacterial responses towards acid and other stresses. J. Appl. Microbiol. 114, 11–24 (2013).
Jydegaard-Axelsen, A. M., Høiby, P. E., Holmstrøm, K., Russell, N. & Knøchel, S. CO2-and anaerobiosis-induced changes in physiology and gene expression of different Listeria monocytogenes strains. Appl. Environ. Microbiol. 70, 4111–4117 (2004).
Sarasa, S. B. et al. A brief review on the non-protein amino acid, gamma-amino butyric acid (GABA): its production and role in microbes. Curr. Microbiol. 77, 534–544 (2020).
Yang, Y. et al. Consumption of an acid protease derived from Aspergillus oryzae causes bifidogenic effect in rats. Nutr. Res. 44, 60–66 (2017).
Yang, Y., Kumrungsee, T., Kuroda, M., Yamaguchi, S. & Kato, N. Feeding Aspergillus protease preparation combined with adequate protein diet to rats increases levels of cecum gut-protective amino acids, partially linked to Bifidobacterium and Lactobacillus. Biosci. Biotechnol. Biochem. 83, 1901–1911 (2019).
Yang, Y. et al. Supplemental Aspergillus lipase and protease preparations display powerful bifidogenic effects and modulate the gut microbiota community of rats. Fermentation 7, 294 (2021).
Yang, Y. et al. Aspergillus-derived cellulase preparation exhibits prebiotic-like effects on gut microbiota in rats. Fermentation 8, 71 (2022).
Yang, Y. et al. Exogenous Penicillium camemberti lipase preparation exerts prebiotic-like effects by increasing cecal Bifidobacterium and Lactobacillus abundance in rats. Fermentation 9, 227 (2023).
Carafa, I. et al. Production of conjugated linoleic acid (CLA): effect of inulin on microbial composition and CLA concentration in a human intestinal model. Proc. Nutr. Soc. 79, E628 (2020).
Matsumoto, M. et al. Colonic absorption of low-molecular-weight metabolites influenced by the intestinal microbiome: a pilot study. PloS ONE 12, e0169207 (2017).
Fujisaka, S. et al. Diet, genetics, and the gut microbiome drive dynamic changes in plasma metabolites. Cell Rep. 22, 3072–3086 (2018).
Diez-Gutiérrez, L., San Vicente, L., Barrón, L. J. R., del Carmen Villarán, M. & Chávarri, M. Gamma-aminobutyric acid and probiotics: multiple health benefits and their future in the global functional food and nutraceuticals market. J. Funct. Foods 64, 103669 (2020).
Zheng, P. et al. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci. Adv. 5, eaau8317 (2019).
McGuinness, A. J. et al. A systematic review of gut microbiota composition in observational studies of major depressive disorder, bipolar disorder and schizophrenia. Mol. Psychiatry 27, 1920–1935 (2022).
Xi, J. et al. Disturbed microbial ecology in Alzheimer’s disease: evidence from the gut microbiota and fecal metabolome. BMC Microbiol 21, 226 (2021).
Liu, X. et al. Blautia—a new functional genus with potential probiotic properties? Gut Microbes 13, 1875796 (2021).
Zhuang, Z., Yang, R., Wang, W., Qi, L. & Huang, T. Associations between gut microbiota and Alzheimer’s disease, major depressive disorder, and schizophrenia. J. Neuroinflamm. 17, 288 (2020).
Manyevitch, R. et al. Evaluation of metabolic and synaptic dysfunction hypotheses of Alzheimer’s disease (AD): A meta-analysis of CSF markers. Curr. Alzheimer Res. 15, 164–181 (2018).
Li, C. et al. Gut microbiota differs between Parkinson’s disease patients and healthy controls in Northeast China. Front. Mol. Neurosci. 12, 171 (2019).
Gong, T. et al. Inhibitory motor dysfunction in Parkinson’s disease subtypes. J. Magn. Reson. Imaging 47, 1610–1615 (2018).
Pan, S. et al. Probiotic Pediococcus pentosaceus ameliorates MPTP-induced oxidative stress via regulating the gut microbiota–gut–brain axis. Front. Cell. Infect. Microbiol. 12, 1022879 (2022).
Yao, H. et al. Chronic ethanol exposure induced anxiety‐like behaviour by altering gut microbiota and GABA system. Addict. Biol. 27, e13203 (2022).
Stevens, B. R. et al. Depression phenotype identified by using single nucleotide exact amplicon sequence variants of the human gut microbiome. Mol. Psychiatry 26, 4277–4287 (2021).
Ma, T. et al. Probiotic consumption relieved human stress and anxiety symptoms possibly via modulating the neuroactive potential of the gut microbiota. Neurobiol. Stress 14, 100294 (2021).
Barker-Haliski, M. & White, H. S. Glutamatergic mechanisms associated with seizures and epilepsy. Cold Spring Harb. Perspect. Med. 5, a022863 (2015).
Wu, J. et al. Intestinal microbiota as an alternative therapeutic target for epilepsy. Can. J. Infect. Dis. Med. Microbiol 2016, 9032809 (2016).
García-Belenguer, S. et al. Gut microbiota in canine idiopathic epilepsy: effects of disease and treatment. Animals 11, 3121 (2021).
Peng, A. et al. Altered composition of the gut microbiome in patients with drug-resistant epilepsy. Epilepsy Res. 147, 102–107 (2018).
Freeman, J. M. & Kossoff, E. H. Ketosis and the ketogenic diet, 2010: advances in treating epilepsy and other disorders. Adv. Pediatr. 57, 315–329 (2010).
Olson, C. A. et al. The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell 173, 1728–1741 (2018).
Gómez-Eguílaz, M., Ramón-Trapero, J. L., Pérez-Martínez, L. & Blanco, J. R. The beneficial effect of probiotics as a supplementary treatment in drug-resistant epilepsy: a pilot study. Benef. Microbes 9, 875–881 (2018).
Finegold, S. M., Summanen, P. H., Downes, J., Corbett, K. & Komoriya, T. Detection of Clostridium perfringens toxin genes in the gut microbiota of autistic children. Anaerobe 45, 133–137 (2017).
Zuffa, S. et al. Early-life differences in the gut microbiota composition and functionality of infants at elevated likelihood of developing autism spectrum disorder. Transl. Psychiatry 13, 257 (2023).
Kang, D. W. et al. Differences in fecal microbial metabolites and microbiota of children with autism spectrum disorders. Anaerobe 49, 121–131 (2018).
Wang D. et al. Excess Escherichia elicits mild autism spectrum disorder in young subjects via disturbing the balance of gut microbial GABA metabolism. Res. Sq. https://www.researchsquare.com/article/rs-1505710/v1 (2022).
Dan, Z. et al. Altered gut microbial profile is associated with abnormal metabolism activity of Autism Spectrum Disorder. Gut Microbes 11, 1246–1267 (2020).
Pärtty, A., Kalliomäki, M., Wacklin, P., Salminen, S. & Isolauri, E. A possible link between early probiotic intervention and the risk of neuropsychiatric disorders later in childhood: a randomized trial. Pediatr. Res. 77, 823–828 (2015).
Edden, R. A., Crocetti, D., Zhu, H., Gilbert, D. L. & Mostofsky, S. H. Reduced GABA concentration in attention-deficit/hyperactivity disorder. Arch. Gen. Psychiatry 69, 750–753 (2012).
Cassidy-Bushrow, A. E. et al. Early-life gut microbiota and attention deficit hyperactivity disorder in preadolescents. Pediatr. Res. 93, 2051–2060 (2023).
Hooi, S. L. et al. A case report of improvement on ADHD symptoms after fecal microbiota transplantation with gut microbiome profiling pre- and post-procedure. Curr. Med. Res. Opin. 38, 1977–1982 (2022).
Bauer, K. Carnosine and homocarnosine, the forgotten, enigmatic peptides of the brain. Neurochem. Res. 30, 1339–1345 (2005).
Jackson, M. C., Scollard, D. M., Mack, R. J. & Lenney, J. F. Localization of a novel pathway for the liberation of GABA in human CNS. Brain Res. Bull. 33, 379–385 (1994).
Boldyrev, A. A., Aldini, G. & Derave, W. Physiology and pathophysiology of carnosine. Physiol. Rev. 93, 1803–1845 (2013).
Kish, S. J., Perry, T. L. & Hansen, S. Regional distribution of homocarnosine, homocarnosine‐carnosine synthetase and homocarnosinase in human brain. J. Neurochem. 32, 1629–1636 (1979).
Perry, T. L., Berry, K., Hansen, S., Diamond, S. & Mok, C. Regional distribution of amino acids in human brain obtained at autopsy. J. Neurochem. 18, 513–519 (1971).
Perry T. Cerebral Amino Acid Pools in Chemical and Cellular Architecture (ed Lajtha, A.) 151–180 (Springer, 1982).
Van der Stede, T. et al. Extensive profiling of histidine-containing dipeptides reveals species-and tissue-specific distribution and metabolism in mice, rats and humans. Acta Physiol. 239, e14020 (2023).
Preston, J. E., Hipkiss, A. R., Himsworth, D. T., Romero, I. A. & Abbott, J. N. Toxic effects of β-amyloid(25–35) on immortalised rat brain endothelial cell: protection by carnosine, homocarnosine and β-alanine. Neurosci. Lett. 242, 105–108 (1998).
Huang, J. et al. L-Homocarnosine attenuates inflammation in cerebral ischemia–reperfusion injury through inhibition of nod-like receptor protein 3 inflammasome. Int. J. Biol. Macromol. 118, 357–364 (2018).
Kang, J. H. et al. Protective effects of carnosine, homocarnosine and anserine against peroxyl radical-mediated Cu, Zn-superoxide dismutase modification. Biochim. Biophys. Acta 1570, 89–96 (2002).
Qi, Z. et al. L-Homocarnosine, L-carnosine, and anserine attenuate brain oxidative damage in a pentylenetetrazole-induced epilepsy model of ovariectomized rats. 3 Biotech 8, 363 (2018).
Petroff, O. A., Hyder, F., Mattson, R. H. & Rothman, D. L. Topiramate increases brain GABA, homocarnosine, and pyrrolidinone in patients with epilepsy. Neurology 52, 473–478 (1999).
Petroff, O. A. Book review: GABA and glutamate in the human brain. Neuroscientist 8, 562–573 (2002).
Jansen, E. E., Gibson, K. M., Shigematsu, Y., Jakobs, C. & Verhoeven, N. M. A novel, quantitative assay for homocarnosine in cerebrospinal fluid using stable-isotope dilution liquid chromatography–tandem mass spectrometry. J. Chromatogr. B 830, 196–200 (2006).
Bai, X. et al. Decreased γ-aminobutyric acid levels in the parietal region of patients with Alzheimer’s disease. J. Magn. Reson. Imaging 41, 1326–1331 (2015).
Petroff, O. A., Mattson, R. H., Behar, K. L., Hyder, F. & Rothman, D. L. Vigabatrin increases human brain homocarnosine and improves seizure control. Ann. Neurol. 44, 948–952 (1998).
Petroff, O. A., Hyder, F., Rothman, D. L. & Mattson, R. H. Effects of gabapentin on brain GABA, homocarnosine, and pyrrolidinone in epilepsy patients. Epilepsia 41, 675–680 (2000).
Landheer, K., Prinsen, H., Petroff, O. A., Rothman, D. L. & Juchem, C. Elevated homocarnosine and GABA in subject on isoniazid as assessed through 1H MRS at 7T. Anal. Biochem. 599, 113738 (2020).
Mendez, M. A. et al. The brain GABA-benzodiazepine receptor alpha-5 subtype in autism spectrum disorder: A pilot [11C] Ro15-4513 positron emission tomography study. Neuropharmacology 68, 195–201 (2013).
Binh, T., Ju, W., Jung, W. & Park, R. Optimization of γ-amino butyric acid production in a newly isolated Lactobacillus brevis. Biotechnol. Lett. 36, 93–98 (2014).
Seo, M. et al. γ-Aminobutyric acid production in skim milk co-fermented with Lactobacillus brevis 877G and Lactobacillus sakei 795. Food Sci. Biotechnol. 22, 751–755 (2013).
Das, D. & Goyal, A. Antioxidant activity and γ-aminobutyric acid (GABA) producing ability of probiotic Lactobacillus plantarum DM5 isolated from Marcha of Sikkim. LWT–Food Sci. Technol. 61, 263–268 (2015).
Di Cagno, R. et al. Synthesis of γ-aminobutyric acid (GABA) by Lactobacillus plantarum DSM19463: functional grape must beverage and dermatological applications. Appl. Microbiol. Biotechnol. 86, 731–741 (2010).
Siragusa, S. et al. Synthesis of γ-aminobutyric acid by lactic acid bacteria isolated from a variety of Italian cheeses. Appl. Environ. Microbiol. 73, 7283–7290 (2007).
Franciosi, E. et al. Biodiversity and γ-aminobutyric acid production by lactic acid bacteria isolated from traditional alpine raw cow’s milk cheeses. Biomed. Res. Int. 2015, 625740 (2015).
Cho, Y., Chang, J. & Chang, H. Production of γ-aminobutyric acid (GABA) by Lactobacillus buchneri isolated from kimchi and its neuroprotective effect on neuronal cells. J. Microbiol. Biotechnol. 17, 104–109 (2007).
Lim, H., Cha, I., Lee, H. & Seo, M. Optimization of γ-aminobutyric acid production by Enterococcus faecium JK29 isolated from a traditional fermented foods. Korean J. Microbiol. Biotechnol. 44, 26–33 (2016).
Yogeswara, A. I., Kusumawati, I. G. A. W., Sumadewi, N. L. U., Rahayu, E. S. & Indrati, R. Isolation and identification of lactic acid bacteria from Indonesian fermented foods as γ-aminobutyric acid-producing bacteria. Int. Food Res. J. 25, 1753–1757 (2018).
Kanklai, J., Somwong, T., Rungsirivanich, P. & Thongwai, N. Screening of GABA-producing lactic acid bacteria from Thai fermented foods and probiotic potential of Levilactobacillus brevis F064A for GABA-fermented mulberry juice production. Microorganisms 9, 33 (2021).
Mancini, A. et al. In vitro probiotic characterization of high GABA producing strain Lactobacillus brevis DSM32386 isolated from traditional “wild” Alpine cheese. Ann. Microbiol. 69, 1435–1443 (2019).
Author information
Authors and Affiliations
Contributions
J.D.B. and T.K.—Conceptualization; J.D.B.—Literature search, visualization, and writing—drafted the original paper; T.K.—supervised and edited the paper; M.T.—provided academic comments. All the authors read and approved the final draft for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Braga, J.D., Thongngam, M. & Kumrungsee, T. Gamma-aminobutyric acid as a potential postbiotic mediator in the gut–brain axis. npj Sci Food 8, 16 (2024). https://doi.org/10.1038/s41538-024-00253-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41538-024-00253-2