Abstract
Cell-based scaffold-free therapies seek to develop in vitro organotypic three-dimensional (3D) tissue-like surrogates, capitalising upon the inherent capacity of cells to create tissues with efficiency and sophistication that is still unparalleled by human-made devices. Although automation systems have been realised and (some) success stories have been witnessed over the years in clinical and commercial arenas, in vitro organogenesis is far from becoming a standard way of care. This limited technology transfer is largely attributed to scalability-associated costs, considering that the development of a borderline 3D implantable device requires very high number of functional cells and prolonged ex vivo culture periods. Herein, we critically discuss advancements and shortfalls of scaffold-free cell-based tissue engineering strategies, along with pioneering concepts that have the potential to transform regenerative and reparative medicine.
Similar content being viewed by others
Introduction
Cell-based therapy has gained tremendous interest in the past decades and holds promise for transforming treatments for a wide range of injuries and diseases. The market for cell therapy products is set to expand based on increasing investment from the industry and the implementation of advanced manufacturing technologies. In fact, the global cell therapy market is forecast to reach €7.24 billion by 2025 with compound annual growth rate of 14.9% from 2017, which would make it the fastest growing sector in the regenerative medicine industry1.
Cells have enormous therapeutic potential, as they provide sophisticated tissue-specific mechanisms of actions that chemical compounds cannot imitate. Mesenchymal stem cells (MSCs), permanently differentiated cells and, more recently, induced pluripotent stem cells (iPSCs) have been used in preclinical and clinical trials with successful outcomes2,3,4,5,6,7. One critical aspect for therapeutic efficacy after cell transplantation is the delivery method (Fig. 1). The optimal cell delivery format should ensure high cell retention and survival rate, good tissue integration and zero to low side effects for the patient. Intra-venous/intra-arterial infusion or direct intra-tissue injection are the most common routes of cell transplantation. However, these approaches have shown limited success, mainly due to the poor cell localisation, retention and survival at the site of injury post-transplantation. In fact, numerous studies have shown that <5% of the injected cells persist at the site of injection in the first day(s) after transplantation, indicating a survival rate of as low as 1% (refs. 8,9,10,11,12).
The figure was created with BioRender.com.
Scaffold-based tissue engineering was pioneered to overcome the limitations of direct cell suspensions, aiming not only to develop efficient cell delivery strategies, but also to produce elegant three-dimensional (3D) tissue analogues. Traditional scaffold-based tissue engineering strategies employ a cytocompatible, biodegradable and mechanically stable natural or synthetic in origin polymeric scaffold with a fully interconnected porous network for efficient transport and exchange of oxygen, nutrients and metabolites13,14. Although very many scaffold conformations (e.g., hydrogels15,16, sponges17,18, fibres19,20, films21,22) have been developed, and have demonstrated safety and efficacy in preclinical setting and phase I clinical trials as cell delivery vehicles, only a handful of them constitute a Food and Drug Administration (FDA)/European Medicines Agency (EMA) approved device (Table 1). This limited technology transfer from laboratory benchtop to clinical applicability has been attributed to component (e.g., limited understanding of the mechanism of action of the various device components; device components do not comply with regulatory frameworks; toxicity issues) and process (e.g., too complex to allow for large-scale efficient and reproducible manufacturing; too long to be profitable) limitations.
Considering that tissues are formed by cells and their secreted components with precision, efficiency, order and sophistication that is still unmatched by human-made devices, it made sense to develop means to exploit this inherent capacity of cells for the development of tissue analogues. In this case, the cell-secreted extracellular matrix (ECM) acts as carrier and protector of the transplanted cells. Further, as no artificial scaffold is used, the produced constructs are of superior biocompatibility and with less chances of foreign body response than any other technology that has been assessed to-date. Although the scaffold-free tissue engineering concept is far from new (the first scaffold-free device was developed in 1975 (ref. 23), assessed in preclinical models in 1980 (ref. 24) and assessed in humans in 1981 (ref. 25)), only a handful of products have been commercialised (Table 2). Herein, we critically discuss recent advancements and limitations that prohibit wide acceptance, clinical translation and commercialisation of scaffold-free cell-based tissue engineering strategies.
Cell sheet tissue engineering
Tissues and organs are comprised of different cell types that are surrounded by their secreted, tissue-specific ECM. This densely populated microenvironment allows for efficient cell–cell and cell–ECM communications, which determine cell fate and function26,27. Cell sheet tissue engineering takes advantage of the close cell–cell and cel–ECM interactions to autonomously engineer microtissues, utilising the temperature-responsive cell culture technology. The temperature-responsive polymer used [usually poly(N-isopropylacrylamide) (pNIPAM), although a variety of polymers with diverse properties have been developed and assessed over the years (Table 3)] undergoes a transition from hydrophobic to hydrophilic across its lower critical solution temperature (LCST) of 32 °C. At temperatures >32 °C, the surfaces are hydrophobic and allow for the culture of adherent cells as on normal tissue culture polystyrene at 37 °C. As the cells grow, they deposit ECM proteins that assemble into interconnected tissue-like structures. When the temperature is reduced <32 °C, pNIPAm molecules become highly hydrated, thus the pNIPAM grafted surfaces become hydrophilic. After this thermal transition, cultured cells almost spontaneously detach from the pNIPAm surface as a contiguous cell sheet with preserved cell–cell junctions and deposited ECM28,29. Since ECM proteins remain on the surface of the cell sheets, they are adhesive to biological surfaces and therefore can be transplanted to injured tissues without the need of sutures or external fixation30. The deposited ECM also acts as a depot of numerous bioactive and trophic molecules, and also protects and localises the transplanted cells at the site of implantation31,32,33. This unique approach, with or without the use of a temperature-responsive polymer, has been used to develop implantable devices out of numerous human cells, and their safety and efficacy has been demonstrated in preclinical and clinical setting for a diverse range of clinical indications (Table 4).
Over the years, significant strides have been achieved in developing tissue analogues, with high levels of architectural biomimicry34. For example, recapitulation of native anisotropic tissue topographies has been realised via the use of bi-directionally aligned temperature-responsive electrospun scaffolds35, microcontact printing of aligned fibronectin patterns36,37, photolithography on non-cell adhesive anisotropic patterns38,39,40, grafting of temperature-responsive polymers onto micropatterned poly(dimethysiloxane) substrates41,42 or unidirectional mechanical stimulation43. With respect to the development of 3D tissue-like assemblies, multi-layered cell sheet stacking has been proposed, which has resulted in the formation of sophisticated microtissues in vitro (e.g., skeletal muscle-like tissue out of myoblasts44,45, myocardial-like tissue out of cardiomyocytes46, annulus fibrosus-like tissue out of bone marrow MSCs47, tubular neural-like tissue out of astrocytes and iPSC-derived neurons48).
To further de-risk the technology and increase its scalability, automated systems have also been developed. For example, automated technologies have been utilised for the production of multi-layered tissue constructs, using robotic systems. Specifically, five layers of human skeletal muscle myoblast sheets were successfully stacked by a robotic apparatus within 100 minu, representing a cost-effective manufacturing system for the manipulation of cell sheets49. Automated modular platforms have also been assembled for the sequential seeding, expansion and cell (e.g., skeletal myoblasts, articular chondrocytes and iPSCs) sheet preparation that have been shown to maintain aseptic conditions and to produce high quality cellular constructs, comparable to those produced in manual operations50. Automated systems have been further advanced for the production of high number of cell sheet batches. For instance, 10 human oral mucosa epithelial cell sheets were simultaneously cultured into 5 separate fully closed culture vessels, automated with a circuit system, that yielded up to 50 cell sheets and satisfied the quality standards of manual procedures51.
Considering that the thickness limitation of 3D constructs without vascular networks is ~40–80 μm (ref. 52), co-culture cell sheet approaches have been proposed for the development of pre-vascularised networks53,54. For example, human umbilical vein endothelial cells co-cultured within human myoblasts sheets formed capillary-like structures within the construct and increased neovascularisation and graft survival after transplantation into the subcutaneous tissues of nude rats55. However, in order to support the long-term culture of thick 3D tissue equivalents, the formation of functional mature blood vessels is required. To this end, bioreactor systems have been utilised in combination with engineered vascular beds, based on resected femoral muscles or synthetic collagen gels, which allowed continuous perfusion of culture media, formation of a functional vasculature and survival of 12-layer cell sheets56,57.
For the development of tubular tissues, such as blood vessels58, tendons59 and neurons48, cell and deposited ECM layers are rolled into tubular structures. Automated systems have also been designed, which utilise a wrapping device module composed of a fibrin tube holder and a sliding dish, capable of rolling three layers of cardiomyocyte sheets without any deformation60. A pioneering study reported the fabrication of human biological tissue-engineered blood vessel composed of smooth muscle cells and skin fibroblast sheets, which were mechanically peeled off from the culture dishes and rolled onto a supportive tubular mandrel (polytetrafluoroethylene)58. After maturation (8 weeks under dynamic conditions) of the construct into a cohesive vascular structure, the supportive mandrel was removed and endothelial cells were seeded in the lumen, ultimately creating a well‐defined, three‐layered device with abundant ECM. The major limitation of this technology was the time required to produce functional grafts (~28 weeks61), which resulted in the company closing down. Advanced anisotropic tubular assemblies have also been realised, using surface patterning to culture human astrocytes and human iPSC‐derived neurons, to create neural microtissues48.
Limitations and way forward
Despite the significant advances that have been achieved in the field of scaffold-free tissue engineering, the technology is far for optimal, as evidenced by the limited number of clinically and commercially available products. Several interconnected and interdependent limitations must be addressed for this technology to become clinical standard.
Transitioning from 2D to 3D systems
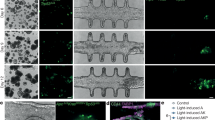
The limiting factor in clinical translation and commercialisation of scaffold-free concepts is the high number of cells required to produce in a commercially relevant timeframe an ECM-rich and truly 3D tissue equivalent (Fig. 2A). Indeed, temperature-responsive surface derived single-layer cell sheets, as well as more elegant micro-stereolithography and electrochemical desorption for cell transfer systems62,63, require a substantial cell number and/or culture time to produce a barely 3D scaffold-free construct (Table 5). For example 104,000 cells/cm2 human endometrial gland-derived MSCs produced a 50 µm thick cell sheet after 5 days64, 300,000/cm2 iPSCs-derived cardiomyocytes produced a 10 µm thick cell sheet after 27 days65, 612,000/cm2 human corneal endothelial cells produced a 15 µm thick cell sheet after 28 days66. To overcome these limitations, multi-layer cell sheet stacking has been proposed. Regrettably, even this approach has been found wanting, as again very high cell numbers and relatively long culture times are required to develop borderline 3D implantable devices (Table 6). For instance, five layers of 1,000,000/cm2 human skeletal muscle myoblasts grown on a temperature-responsive dish for 5 days produced a 50 μm thick device49, three layers of 300,000/cm2 human adipose-derived MSCs grown on a temperature-responsive dish for 5 days produced a 20 μm thick device67, nine layers of 200,000/cm2 human iPSCs-derived cardiomyocytes per layer grown on a temperature-responsive dish for 7–10 days produced a 359 μm thick device68. Unfortunately, due to the absence of sufficient ECM, these high-density cultures are associated with poor nutrient and oxygen diffusion and waste accumulation in the middle layers that ultimately lead to cell necrosis69,70 and delamination71 (Fig. 2B). To address these issues, multiple operations of up to three layers have been proposed52; however, these multiple operations are associated with prolonged patient distress and high healthcare expenditure. To overcome the dimensionality limitation of scaffold-free systems, cell sheets (whole or in fragments) have been combined with various scaffold conformations (e.g., hydrogels72, particles73, tissue grafts74)/fabrication systems (e.g., electrospinning75,76, 3D printing77,78), which have been summarised elsewhere recently79.
Traditional single-layer scaffold-free systems require high cell numbers and prolonged culture periods to produce barely 3D tissue equivalents (A). Due to the absence of sufficient ECM, cell necrosis and delamination frequently occur in multi-layer scaffold-free systems (B). The figure was created with BioRender.com.
Hypoxia
Considering the importance of ECM in regulating cell survival and tissue homoeostasis80,81, it is imperative to develop means to accelerate ECM synthesis and deposition. Physiological hypoxia (<10% O2) poses a biochemical cue crucial for the regulation of ECM synthesis and deposition. Indeed, hypoxia has been shown to increase mRNA levels of procollagen α1(I) in fibroblasts isolated from different tissues82,83,84,85. Hypoxia also regulates ECM homoeostasis through the activation of hypoxia-inducible transcription factor 1 (HIF-1)86. HIF-1 regulates collagen secretion and deposition by driving the transcription of prolyl 4-hydroxylase, which catalyses intracellularly the hydroxylation of proline and lysine residues and lysyl oxidase, which catalyses extracellularly collagen crosslinking87,88,89,90. Hypoxia therefore can be an ally in the fabrication of biomimetic tissue-engineered constructs.
Considerable efforts have been conducted to optimise oxygen levels of cultured cells, in order to control (stem) cell fate91 and promote ECM synthesis and deposition for the desired tissue engineering application (Table 7), such as skin92, cartilage93,94, bone95,96, tendon97,98 and heart89. For example, low oxygen tension (5% O2) has been shown to retain undifferentiated and multipotent status of MSC cultures99,100. In addition, hypoxia modulates the paracrine activity of MSCs and enhances the secretion of soluble growth factors, especially pro-angiogenic factors, such as vascular endothelial growth factor (VEGF)101. In scaffold-free tissue engineering, only few studies have utilised low oxygen tension for the production of implantable cell constructs. For example, multi-layered human chondrocyte sheets fabricated in a co‐culture system with synoviocytes and cultured at 2% oxygen tension showed greater cell metabolic activity and proliferation compared to cells cultured at 21% oxygen tension. Furthermore, hypoxic conditions accelerated and enhanced the deposition of cartilage-specific ECM, mainly composed of proteoglycans and collagen type II (ref. 102). Preconditioning of rabbit BMSCs or mouse cardiosphere-derived cell sheets under 2% oxygen tension, remarkably increased the expression of VEGF and significantly improved left ventricular function in myocardial infarction models in comparison to cells cultured under normoxia condition103,104.
Despite the importance of hypoxia in eukaryotic cell culture and in the development of functional cell therapies, routinely cell culture studies are performed under hyperoxic conditions (21% O2) that do not match physiological oxygen levels (e.g., 5–13% in blood, 2–9% in most tissues)105. Further, hyperoxic cell cultures lead to poor and slow ECM synthesis, cellular senescence and activation of stress pathways106,107,108. Although hypoxia chambers and incubators are utilised to perform hypoxic experiments, implementation of physioxia in industrial scale is expensive to purchase and maintain, thus of limited applicability despite the fact that studies have argued that hypoxia precondition should be a prerequisite for clinical translation of cell therapies109,110.
Mechanical stimulation
Another microenvironmental cue critical for optimal ECM synthesis and deposition is mechanical loading111. Considering that uniaxial or multiaxial tensile, compressive or shear mechanical loads regulate ECM composition and function and tissue homoeostasis112, mechanical stimulation of tissue-engineered constructs, with the use of bioreactors, is attracting growing interest in order to recapitulate the in vivo microenvironment of native (primarily musculoskeletal) tissues in in vitro setting (Table 8). For example, mechanical stimulation, in the form of shear force, hydrostatic pressure or compression, has been shown to promote ECM synthesis in human chondrocyte and MSC cultures, and to produce tissue‐engineered cartilaginous substitutes113,114,115. Similarly, in tendon engineering, mechanical loading has been shown to maintain tenocyte phenotype and to increase their proliferation and ECM synthesis116,117, and to direct MSCs towards tenogenic lineage118,119,120. In bone engineering, mechanical loading has been employed extensively to enhance mineralised matrix synthesis and deposition121,122.
With regards to scaffold-free tissue engineering, unidirectional stretching has been applied to induce the alignment of human iPSCs-derived cardiomyocyte sheets after detachment from temperature-responsive dishes43. Two weeks after transplantation into the superficial gluteal muscle of athymic rats, the stretched cell sheets retained the unidirectionality of their myocardial fibres, holding great potential for heart engineering. While the application of mechanical loading has not been applied extensively in temperature-responsive systems, it has been successfully implemented in other scaffold-free tissue-engineered models. For example, cartilage constructs have been produced using high-density porcine chondrocytes, centrifugation and non-adhesive agarose substrates. Simultaneous application of cyclic unconfined compression and perfusion to the cartilage constructs increased the deposition of glycosaminoglycans and collagen type II in comparison to static control groups123. Another study reported the fabrication of scaffold-free cartilage by applying high-amplitude compressive strain, using porcine chondrocyte seeded onto a hydroxyapatite carrier. Compression amplitude of 20% had the highest positive effect by inducing the synthesis of cartilage-specific ECM and enhancing the mechanical properties of the constructs124.
An overwhelming amount of literature has demonstrated the positive effects of mechanical stimulation and/or conditioning on advanced tissue-engineered constructs. Nevertheless, the limited fundamental understanding of the molecular and cellular mechanisms, the lack of standardised protocols for mechanical stimulation and the very high costs of bioreactor systems, limit their scalability and use in commercial space.
Macromolecular crowding
Although various in vitro microenvironment modulators have been assessed over the years to control cell fate during in vitro culture, only marginally enhance and accelerate ECM synthesis and deposition. For example, mechanical stimulation and oxygen tension have been shown to increase by 2–5-fold ECM synthesis, and deposition both in permanently differentiated and stem cell cultures125,126,127,128, which although mathematically may be considered as an improvement, commercially, the associated expenditure does not justify the change in the process.
In recent years, macromolecular crowding (MMC) has emerged as a means to substantially increase and accelerate ECM deposition in vitro (e.g., up to 120-fold increase in collagen and associated ECM deposition within 4–6 days in differentiated129,130,131,132,133 and stem134,135,136,137,138,139,140,141 cell cultures). In tissues, the presence of numerous macromolecules, such as carbohydrates, proteins, lipids and nucleic acids, creates a crowded or confined microenvironment that affects the rate of biological and biochemical reactions142,143. The MMC’s mechanism of action is based on the theory of mutual excluded volume effect, which refers to the volume that is inaccessible in the system to new molecules as a result of pre-existing molecules144; two molecules cannot be at the same place at the same time. Although the effect of MMC on protein folding and assembly145,146,147, DNA condensation and replication148,149,150,151 and biochemical reactions152,153 has been well established, it is still under investigation in cell culture context. Nonetheless, it is accepted now that in eukaryotic cell culture scenario, MMC accelerates the enzymatic processing of procollagen to collagen, resulting in enhanced collagen, and bound ECM, deposition (Fig. 3). Indeed, in standard cell culture setting, the conversion of water-soluble procollagen to insoluble collagen is relatively slow, since the proteinases required for the enzymatic cleavage of procollagen are dispersed in the dilute culture media. The addition of macromolecules to the culture media results in a more efficient volume occupancy, preventing their dispersion154. MMC has been shown to drive the molecular assembly of collagen fibrils in vitro and to stabilise the formed matrix through enzymatic crosslinking155,156.
In the dilute cell culture context (−MMC), the N- and C-proteinases and the water-soluble procollagen are diffused, and respectively deactivated and dissolved, resulting in very low amounts of deposited ECM (A). Under macromolecular crowding conditions (+MMC), the diffusion of procollagen and N- and C-proteinases is restricted, resulting in enhanced and accelerated ECM deposition (B). The figure was created with BioRender.com.
Several macromolecules, alone or in cocktail form, have been utilised as crowders to enhance and accelerate ECM deposition (Table 9). It has been demonstrated that negative charge and polydispersity are key regulators for ECM deposition129. Indeed, negatively charged crowders cause a stronger volume-excluding effect due to electrostatic repulsion157 and polydispersity, indicative of the heterogeneity of sizes and/or shapes of molecules in a mixture, maximises the excluded volume effect through reduction in diffusion158. These prompted the use mixed crowding molecules systems to achieve higher volume exclusion effect, reduced procollagen/proteinases diffusion and ultimately enhanced and accelerated ECM deposition. For instance, a cocktail of Ficoll™ 70 kDa/Ficoll™ 400 kDa/Ficoll™ 1000 kDa has been shown to lead to higher ECM deposition in dermal fibroblast cultures than the traditionally used Ficoll™ 70 kDa/Ficoll™ 400 kDa cocktail129. Carrageenan, a naturally polydisperse and negatively sulfated polysaccharide has been shown to induce the highest volume exclusion effect, as judged by the highest and fastest ECM deposition in vitro136,159,160. Although in traditional protein assembly investigations, for simplicity purposes, crowders are considered as inert macromolecules, eukaryotic cell culture experiments indicate that the chemistry of the crowder affects cell phenotype. For example, in corneal fibroblasts, dextran sulfate-induced myofibroblast trans-differentiation, while the Ficoll™ 70 kDa/400 kDa cocktail161 and carrageenan160 maintained their phenotype. Further, in stem cell cultures, non-sulfated polysaccharides have been shown to induce adipogenesis135,137,162, while sulfated polysaccharides have been shown to induce chondrogenesis and osteogenesis134,136,140.
In the field of scaffold-free tissue engineering, MMC has advanced the production of ECM-rich cell sheets, showing the possibility to dramatically speed up the production of implantable tissue equivalents130,163,164. Interestingly, these studies demonstrated that the commercially available pNIPAM-based culture dishes were not able to induce detachment of intact cell sheets, due to the presence of abundant ECM produced under MMC conditions. Co-polymerisation of pNIPAM with the hydrophobic N-tert-butylacrylamide (NTBA) monomer, at an optimal ratio of 35%, allowed for first time the production of dense and cohesive cell sheets with intact cell–cell and cell–ECM junctions. The efficiency of the pNIPAM-NTBA copolymer to produce such ECM-rich construct was attributed to additional steric hindrance induced by the NTBA group, which decreased hydrogen bonding and consequently decreased protein adsorption, ultimately facilitating cell detachment. In addition, MMC has been used for the development of scaffold-free cell-derived matrices133,141,165 that have been used successfully for in vitro cell propagation166,167,168 and for the generation of skin equivalents with complete stratification and a mature dermal–epidermal junction for either regenerative medicine169,170 or drug discovery purposes171,172,173,174. To further boost the development of scaffold-free tissue equivalents, multifactorial approaches combining MMC and different in vitro microenvironment modulators have been explored. For example, the use of MMC in combination with low oxygen tension synergistically contributed to the development of ECM-rich tissue equivalents136,159,160,175. Similarly, the use of MMC simultaneously to mechanical stimulation facilitated the fabrication of tendon-like tissue constructs in vitro176.
Despite the significant contribution and potential of MMC in tissue engineering, the optimal (with respect to maximum ECM deposition in the shortest period of time, while precisely controlling cell fate) crowding agent/cocktail remains elusive.
Roadmap to commercialisation
Although significant strides that have been achieved in the production of ECM-rich tissue equivalents within commercially relevant timeframes, a major roadblock in the commercialisation of cell-based strategies is the high costs of manufacturing solutions, which need to comply with current good manufacturing practices. A recent study analysed eight case studies in Europe using both autologous and allogeneic therapies (academic and other small-scale enterprises scale) and estimated manufacturing costs (i.e., materials, equipment, personnel and facility running costs) to be in the range of € 23,033 and € 190,799 per batch, with batch yield varying between 1 and 88 doses177. With regards to scaffold-based strategies, another study reported the costs of stem cell-engineered airway transplants to range from US$ 174,420 to US$ 740,500 in three UK patients178. It is worth noting that such regenerative medicine strategies are also associated with several risks (e.g., human errors, batch-to-batch variability, high risk of contamination), which further limit their availability179. In order to overcome these limitations, automated platforms could significantly reduce the cost of goods up to 30% (ref. 180), ensuring reliability and reproducibility across the life cycle of the product, from cell isolation and expansion to in‐line product quality assurance181,182. To further standardise the manufacturing process, the development of xeno-free, chemically defined media has been advocated to reduce the risk of pathogen sources and simplify the regulatory approval183,184.
Besides reducing the costs associated to manufacturing systems, it is also imperative to consider from outset both regulatory hurdles and reimbursement concepts to successfully translate cell-based concepts into blockbuster therapies. In fact, one should consider that no Advanced Therapy Medicinal Products (ATMPs) has yet achieved widespread reimbursement and access across the five biggest European countries (Germany, France, UK, Italy, Spain), and many have been restricted beyond the regulatory label185. Both public and private healthcare systems are still under-equipped to absorb the high financial implications associated with cell therapies, also considering additional costs related to expensive hospital stays, procedures and rehabilitation, which come on top of the product price. Another factor which limits cellular therapies to secure reimbursement following market authorisation is the lack of comparative effectiveness data. Manufacturers must demonstrate that the product has incremental benefit against existing standard of care, not only from an economical perspective, but, most importantly, from a clinical one, ensuring long-term safety and efficacy186. For example, ChondroCelect®, an autologous chondrocyte therapy licensed for the treatment of single symptomatic cartilage defects of the femoral condyle of the knee, despite being the first cell-based regenerative therapy to obtain a centralised marketing authorisation in Europe, has been withdrawn from the market, failing to established robust clinical efficacy and consequently not fulfilling reimbursement criteria187.
Considering the unpredictability around the long-term efficacy and safety of regenerative medicines, new models for financing and reimbursement have been proposed to ensure patient access to such therapies. For instance, annuity or instalment payment models minimise high up-front single payment, allowing healthcare providers to amortise the cost of therapies over multiple years, and recognises the potential of single-administered cell therapies based on evidence that the treatment continues to be effective over a specified period of time188. Another example are pay-for-performance models, where the reimbursement for a treatment depends on whether a specified clinical outcome is achieved. These models aim to partially shift financial risks from payers to manufacturers, which may prompt the interest of healthcare providers189.
Overall, cell manufacturers need to address many challenges during product development, in order to gain approval from regulatory authorities and ensure that the proposed therapy will not be held back by reimbursement policies.
Conclusions
In vitro scaffold-free organogenesis has come long way since early 1980’s that was first appeared in the literature. Despite though the significant strides in in vitro, preclinical and clinical setting, only a handful of concepts have become clinical and commercial reality. Issues associated with required number of functional cells, dimensionality, production timeframe, automation, scalability (that affect reimbursement) and regulatory requirements/classification must be addressed for wide acceptance of this transformative and disruptive concept.
References
Frost & Sullivan. Growth Opportunities in the Global Cell Therapy Market, Forecast to 2025. Report No. 739355 (2018).
Brown, C. et al. Mesenchymal stem cells: cell therapy and regeneration potential. J. Tissue Eng. Regen. Med. 13, 1738–1755 (2019).
Pittenger, M. F. et al. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen. Med. 4, 22 (2019).
Galipeau, J. & Sensébé, L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell 22, 824–833 (2018).
Ichim, T. E., O’Heeron, P. & Kesari, S. Fibroblasts as a practical alternative to mesenchymal stem cells. J. Transl. Med. 16, 212 (2018).
Robinton, D. A. & Daley, G. Q. The promise of induced pluripotent stem cells in research and therapy. Nature 481, 295–305 (2012).
Bragança, J., Lopes, J. A., Mendes-Silva, L. & Almeida Santos, J. M. Induced pluripotent stem cells, a giant leap for mankind therapeutic applications. World J. Stem Cells 11, 421–430 (2019).
Salvadori, M. et al. Dissecting the pharmacodynamics and pharmacokinetics of MSCs to overcome limitations in their clinical translation. Mol. Ther. Methods Clin. Dev. 14, 1–15 (2019).
Gao, J., Dennis, J. E., Muzic, R. F., Lundberg, M. & Caplan, A. I. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs 169, 12–20 (2001).
De Becker, A. & Riet, I. V. Homing and migration of mesenchymal stromal cells: how to improve the efficacy of cell therapy? World J. Stem Cells 8, 73–87 (2016).
Amer, M. H., Rose, F. R. A. J., Shakesheff, K. M., Modo, M. & White, L. J. Translational considerations in injectable cell-based therapeutics for neurological applications: concepts, progress and challenges. NPJ Regen. Med. 2, 23–23 (2017).
Abdelwahid, E. et al. Stem cell death and survival in heart regeneration and repair. Apoptosis 21, 252–268 (2016).
Hollister, S. J. Porous scaffold design for tissue engineering. Nat. Mater. 4, 518–524 (2005).
Nichol, J. W. & Khademhosseini, A. Modular tissue engineering: engineering biological tissues from the bottom up. Soft Matter 5, 1312–1319 (2009).
Thomas, D. et al. Temporal changes guided by mesenchymal stem cells on a 3D microgel platform enhance angiogenesis in vivo at a low-cell dose. Proc. Natl Acad. Sci. USA 117, 19033–19044 (2020).
Hasani-Sadrabadi, M. M. et al. An engineered cell-laden adhesive hydrogel promotes craniofacial bone tissue regeneration in rats. Sci. Transl. Med. 12, eaay6853 (2020).
Cunniffe, G. M. et al. Tissue-specific extracellular matrix scaffolds for the regeneration of spatially complex musculoskeletal tissues. Biomaterials 188, 63–73 (2019).
Markowicz, M. et al. Human bone marrow mesenchymal stem cells seeded on modified collagen improved dermal regeneration in vivo. Cell Transpl. 15, 723–732 (2006).
Schüttler, K. F. et al. Direct incorporation of mesenchymal stem cells into a nanofiber scaffold - in vitro and in vivo analysis. Sci. Rep. 10, 9557 (2020).
Hashi, C. K. et al. Antithrombogenic property of bone marrow mesenchymal stem cells in nanofibrous vascular grafts. Proc. Natl Acad. Sci. USA 104, 11915–11920 (2007).
Kobayashi, K. et al. On-site fabrication of bi-layered adhesive mesenchymal stromal cell-dressings for the treatment of heart failure. Biomaterials 209, 41–53 (2019).
Fu, N. et al. PCL-PEG-PCL film promotes cartilage regeneration in vivo. Cell Prolif. 49, 729–739 (2016).
Rheinwald, J. G. & Green, H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell 6, 331–343 (1975).
Banks-Schlegel, S. & Green, H. Formation of epidermis by serially cultivated human epidermal cells transplanted as an epithelium to athymic mice. Transplantation 29, 308–313 (1980).
O’Connor, N., Mulliken, J., Banks-Schlegel, S., Kehinde, O. & Green, H. Grafting of burns with cultured epithelium prepared from autologous epidermal cells. Lancet 1, 75–78 (1981).
Weaver, V. M. & Roskelley, C. D. Extracellular matrix: the central regulator of cell and tissue homeostasis. Trends Cell Biol. 7, 40–42 (1997).
Muncie, J. M. & Weaver, V. M. The physical and biochemical properties of the extracellular matrix regulate cell fate. Curr. Top. Dev. Biol. 130, 1–37 (2018).
Yamato, M. & Okano, T. Cell sheet engineering. Mater. Today 7, 42–47 (2004).
Matsuda, N., Shimizu, T., Yamato, M. & Okano, T. Tissue engineering based on cell sheet technology. Adv. Mater. 19, 3089–3099 (2007).
Haraguchi, Y. et al. Fabrication of functional three-dimensional tissues by stacking cell sheets in vitro. Nat. Protoc. 7, 850–858 (2012).
Wilgus, T. A. Growth factor-extracellular matrix interactions regulate wound repair. Adv. Wound Care 1, 249–254 (2012).
Schultz, G. S. & Wysocki, A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 17, 153–162 (2009).
Yang, J. et al. Cell sheet engineering: recreating tissues without biodegradable scaffolds. Biomaterials 26, 6415–6422 (2005).
Patel, N. G. & Zhang, G. Stacked stem cell sheets enhance cell-matrix interactions. Organogenesis 10, 170–176 (2014).
Dang, J. M. & Leong, K. W. Myogenic induction of aligned mesenchymal stem cell sheets by culture on thermally responsive electrospun nanofibers. Adv. Mater. 19, 2775–2779 (2007).
Elloumi Hannachi, I. et al. Fabrication of transferable micropatterned-co-cultured cell sheets with microcontact printing. Biomaterials 30, 5427–5432 (2009).
Williams, C. et al. Aligned cell sheets grown on thermo-responsive substrates with microcontact printed protein patterns. Adv. Mater. 21, 2161–2164 (2009).
Takahashi, H., Nakayama, M., Itoga, K., Yamato, M. & Okano, T. Micropatterned thermoresponsive polymer brush surfaces for fabricating cell sheets with well-controlled orientational structures. Biomacromolecules 12, 1414–1418 (2011).
Takahashi, H., Shimizu, T., Nakayama, M., Yamato, M. & Okano, T. Anisotropic cellular network formation in engineered muscle tissue through the self-organization of neurons and endothelial cells. Adv. Health. Mater. 4, 356–360 (2015).
Kumashiro, Y. et al. Rate control of cell sheet recovery by incorporating hydrophilic pattern in thermoresponsive cell culture dish. J. Biomed. Mater. Res A 102, 2849–2856 (2014).
Backman, D. E., LeSavage, B. L., Shah, S. B. & Wong, J. Y. A Robust Method to Generate Mechanically Anisotropic Vascular Smooth Muscle Cell Sheets for Vascular Tissue Engineering. 17 (2017). https://doi.org/10.1002/mabi.201600434.
Lin, J. B. et al. Thermo-responsive poly(N-isopropylacrylamide) grafted onto microtextured poly(dimethylsiloxane) for aligned cell sheet engineering. Colloids Surf. B Biointerfaces 99, 108–115 (2012).
Homma, J., Shimizu, S., Sekine, H., Matsuura, K. & Shimizu, T. A novel method to align cells in a cardiac tissue-like construct fabricated by cell sheet-based tissue engineering. J. Tissue Eng. Regen. Med.– 14, 944–954 (2020).
Takahashi, H., Shimizu, T., Nakayama, M., Yamato, M. & Okano, T. The use of anisotropic cell sheets to control orientation during the self-organization of 3D muscle tissue. Biomaterials 34, 7372–7380 (2013).
Jiao, A. et al. Regulation of skeletal myotube formation and alignment by nanotopographically controlled cell-secreted extracellular matrix. J. Biomed. Mater. Res A 106, 1543–1551 (2018).
Haraguchi, Y., Shimizu, T., Yamato, M., Kikuchi, A. & Okano, T. Electrical coupling of cardiomyocyte sheets occurs rapidly via functional gap junction formation. Biomaterials 27, 4765–4774 (2006).
Chuah, Y. J. et al. Scaffold-free tissue engineering with aligned bone marrow stromal cell sheets to recapitulate the microstructural and biochemical composition of annulus fibrosus. Acta Biomater. 107, 129–137 (2020).
Takahashi, H., Itoga, K., Shimizu, T., Yamato, M. & Okano, T. Human neural tissue construct fabrication based on scaffold-free tissue engineering. Adv. Health. Mater. 5, 1931–1938 (2016).
Kikuchi, T., Shimizu, T., Wada, M., Yamato, M. & Okano, T. Automatic fabrication of 3-dimensional tissues using cell sheet manipulator technique. Biomaterials 35, 2428–2435 (2014).
Kikuchi, T. et al. A novel, flexible and automated manufacturing facility for cell-based health care products: tissue. Fact. Regen. Ther. 9, 89–99 (2018).
Nishimura, A. et al. Fabrication of tissue-engineered cell sheets by automated cell culture equipment. J. Tissue Eng. Regen. Med. 13, 2246–2255 (2019).
Shimizu, T. et al. Polysurgery of cell sheet grafts overcomes diffusion limits to produce thick, vascularized myocardial tissues. FASEB J. 20, 708–710 (2006).
Novosel, E. C., Kleinhans, C. & Kluger, P. J. Vascularization is the key challenge in tissue engineering. Adv. Drug Deliv. Rev. 63, 300–311 (2011).
Shimizu, T. Cell sheet-based tissue engineering for fabricating 3-dimensional heart tissues. Circ. J. 78, 2594–2603 (2014).
Sasagawa, T. et al. Design of prevascularized three-dimensional cell-dense tissues using a cell sheet stacking manipulation technology. Biomaterials 31, 1646–1654 (2010).
Sekine, H. et al. In vitro fabrication of functional three-dimensional tissues with perfusable blood vessels. Nat. Commun. 4, 1399 (2013).
Sakaguchi, K. et al. In vitro engineering of vascularized tissue surrogates. Sci. Rep. 3, 1316 (2013).
L’Heureux, N., Pâquet, S., Labbé, R., Germain, L. & Auger, F. A. A completely biological tissue-engineered human blood vessel. FASEB J. 12, 47–56 (1998).
Ni, M. et al. Engineered scaffold-free tendon tissue produced by tendon-derived stem cells. Biomaterials 34, 2024–2037 (2013).
Kubo, H., Shimizu, T., Yamato, M., Fujimoto, T. & Okano, T. Creation of myocardial tubes using cardiomyocyte sheets and an in vitro cell sheet-wrapping device. Biomaterials 28, 3508–3516 (2007).
L’Heureux, N. et al. Human tissue-engineered blood vessels for adult arterial revascularization. Nat. Med. 12, 361–365 (2006).
Enomoto, J. et al. Engineering thick cell sheets by electrochemical desorption of oligopeptides on membrane substrates. Regen. Ther. 3, 24–31 (2016).
Kobayashi, Y. et al. Tailored cell sheet engineering using microstereolithography and electrochemical cell transfer. Sci. Rep. 9, 10415 (2019).
Sekine, W. et al. Chondrocyte differentiation of human endometrial gland-derived MSCs in layered cell sheets. ScientificWorldJournal 2013, 359109 (2013).
Ishigami, M. et al. Human iPS cell-derived cardiac tissue sheets for functional restoration of infarcted porcine hearts. PLoS ONE 13, e0201650 (2018).
Sumide, T. et al. Functional human corneal endothelial cell sheets harvested from temperature-responsive culture surfaces. FASEB J. 20, 392–394 (2006).
Cerqueira, M. T. et al. Human adipose stem cells cell sheet constructs impact epidermal morphogenesis in full-thickness excisional wounds. Biomacromolecules 14, 3997–4008 (2013).
Komae, H. et al. Three-dimensional functional human myocardial tissues fabricated from induced pluripotent stem cells. J. Tissue Eng. Regen. Med. 11, 926–935 (2017).
Takahashi, H., Nakayama, M., Shimizu, T., Yamato, M. & Okano, T. Anisotropic cell sheets for constructing three-dimensional tissue with well-organized cell orientation. Biomaterials 32, 8830–8838 (2011).
Sekine, W., Haraguchi, Y., Shimizu, T., Umezawa, A. & Okano, T. Thickness limitation and cell viability of multi-layered cell sheets and overcoming the diffusion limit by a porous-membrane culture insert. J. Biochip Tissue Chip S1, 007 (2011).
Haraguchi, Y. et al. Thicker three-dimensional tissue from a “symbiotic recycling system” combining mammalian cells and algae. Sci. Rep. 7, 41594 (2017).
Kasai, Y., Takeda, N., Kobayashi, S., Takagi, R. & Yamato, M. Cellular events and behaviors after grafting of stratified squamous epithelial cell sheet onto a hydrated collagen gel. FEBS Open Bio 7, 691–704 (2017).
Ma, G. et al. Scaffold-based delivery of bone marrow mesenchymal stem cell sheet fragments enhances new bone formation in vivo. J. Oral. Maxillofac. Surg. 75, 92–104 (2017).
Shang, X. et al. Human mesenchymal stromal cell sheet enhances allograft repair in a mouse model. Sci. Rep. 7, 7982 (2017).
Vaquette, C., Saifzadeh, S., Farag, A., Hutmacher, D. & Ivanovski, S. Periodontal tissue engineering with a multiphasic construct and cell sheets. J. Dent. Res. 98, 673–681 (2019).
Zhao, B., Chen, J., Zhao, L., Deng, J. & Li, Q. A simvastatin-releasing scaffold with periodontal ligament stem cell sheets for periodontal regeneration. J. Appl. Biomater. Funct. Mater. 18, 2280800019900094 (2020).
Bakirci, E., Toprakhisar, B., Zeybek, M., Ince, G. & Koc, B. Cell sheet based bioink for 3D bioprinting applications. Biofabrication 9, 024105 (2017).
Cochis, A. et al. 3D printing of thermo-responsive methylcellulose hydrogels for cell-sheet engineering. Materials 11, 579 (2018).
Zurina, I. et al. Tissue engineering using a combined cell sheet technology and scaffolding approach. Acta Biomater. 113, 63–83 (2020).
Cox, T. & Erler, J. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis. Model Mech. 4, 165–178 (2011).
Buchheit, C., Weigel, K. & Schafer, Z. Cancer cell survival during detachment from the ECM: multiple barriers to tumour progression. Nat. Rev. Cancer 14, 632–641 (2014).
Falanga, V. et al. Low oxygen tension increases mRNA levels of alpha 1 (I) procollagen in human dermal fibroblasts. J. Cell Physiol. 157, 408–412 (1993).
Falanga, V. et al. Hypoxia upregulates the synthesis of TGF-β1 by human dermal fibroblasts. J. Investig. Dermatol. 97, 634–637 (1991).
Norman, J. T., Clark, I. M. & Garcia, P. L. Hypoxia promotes fibrogenesis in human renal fibroblasts. Kidney Int. 58, 2351–2366 (2000).
Tamamori, M., Ito, H., Hiroe, M., Marumo, F. & Hata, R. I. Stimulation of collagen synthesis in rat cardiac fibroblasts by exposure to hypoxic culture conditions and suppression of the effect by natriuretic peptides. Cell Biol. Int. 21, 175–180 (1997).
Weidemann, A. & Johnson, R. S. Biology of HIF-1α. Cell Death Differ. 15, 621–627 (2008).
Bentovim, L., Amarilio, R. & Zelzer, E. HIF1α is a central regulator of collagen hydroxylation and secretion under hypoxia during bone development. Development 139, 4473–4483 (2012).
Gilkes, D. M., Bajpai, S., Chaturvedi, P., Wirtz, D. & Semenza, G. L. Hypoxia-inducible factor 1 (HIF-1) promotes extracellular matrix remodeling under hypoxic conditions by inducing P4HA1, P4HA2, and PLOD2 expression in fibroblasts. J. Biol. Chem. 288, 10819–10829 (2013).
van Vlimmeren, M. A., Driessen-Mol, A., van den Broek, M., Bouten, C. V. & Baaijens, F. P. Controlling matrix formation and cross-linking by hypoxia in cardiovascular tissue engineering. J. Appl Physiol. (1985) 109, 1483–1491 (2010).
Erler, J. T. et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell 15, 35–44 (2009).
Mohyeldin, A., Garzón-Muvdi, T. & Quiñones-Hinojosa, A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell 7, 150–161 (2010).
Mieremet, A. et al. Human skin equivalents cultured under hypoxia display enhanced epidermal morphogenesis and lipid barrier formation. Sci. Rep. 9, 7811 (2019).
Duval, E. et al. Molecular mechanism of hypoxia-induced chondrogenesis and its application in in vivo cartilage tissue engineering. Biomaterials 33, 6042–6051 (2012).
Markway, B. D., Cho, H. & Johnstone, B. Hypoxia promotes redifferentiation and suppresses markers of hypertrophy and degeneration in both healthy and osteoarthritic chondrocytes. Arthritis Res. Ther. 15, R92 (2013).
Stiers, P.-J., van Gastel, N. & Carmeliet, G. Targeting the hypoxic response in bone tissue engineering: a balance between supply and consumption to improve bone regeneration. Mol. Cell Endocrinol. 432, 96–105 (2016).
Zhou, Y. et al. Hypoxia induces osteogenic/angiogenic responses of bone marrow-derived mesenchymal stromal cells seeded on bone-derived scaffolds via ERK1/2 and p38 pathways. Biotechnol. Bioeng. 110, 1794–1804 (2013).
Lee, W. Y., Lui, P. P. & Rui, Y. F. Hypoxia-mediated efficient expansion of human tendon-derived stem cells in vitro. Tissue Eng. Part A 18, 484–498 (2012).
Zhang, J. & Wang, J. H. Human tendon stem cells better maintain their stemness in hypoxic culture conditions. PLoS ONE 8, e61424 (2013).
Fehrer, C. et al. Reduced oxygen tension attenuates differentiation capacity of human mesenchymal stem cells and prolongs their lifespan. Aging Cell 6, 745–757 (2007).
Basciano, L. et al. Long term culture of mesenchymal stem cells in hypoxia promotes a genetic program maintaining their undifferentiated and multipotent status. BMC Cell Biol. 12, 12 (2011).
Gaspar, D. et al. Local pharmacological induction of angiogenesis: drugs for cells and cells as drugs. Adv. Drug Deliv. Rev. 146, 126–154 (2019).
Kokubo, M. et al. Characterization of layered chondrocyte sheets created in a co-culture system with synoviocytes in a hypoxic environment. J. Tissue Eng. Regen. Med. 11, 2885–2894 (2017).
Tanaka, Y. et al. Autologous preconditioned mesenchymal stem cell sheets improve left ventricular function in a rabbit old myocardial infarction model. Am. J. Transl. Res. 8, 2222–2233 (2016).
Hosoyama, T. et al. Cardiosphere-derived cell sheet primed with hypoxia improves left ventricular function of chronically infarcted heart. Am. J. Transl. Res. 7, 2738–2751 (2015).
Brahimi-Horn, M. C. & Pouysségur, J. Oxygen, a source of life and stress. FEBS Lett. 581, 3582–3591 (2007).
Parrinello, S. et al. Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nat. Cell Biol. 5, 741–747 (2003).
Pomatto, L. C. D. et al. Limitations to adaptive homeostasis in an hyperoxia-induced model of accelerated ageing. Redox Biol. 24, 101194 (2019).
Lee, P. J. & Choi, A. M. Pathways of cell signaling in hyperoxia. Free Radic. Biol. Med. 35, 341–350 (2003).
Huang, Y. C., Parolini, O., Deng, L. & Yu, B. S. Should hypoxia preconditioning become the standardized procedure for bone marrow MSCs preparation for clinical use? Stem Cells 34, 1992–1993 (2016).
Tsiapalis, D. & Zeugolis, D. I. Hypoxia preconditioning of bone marrow mesenchymal stem cells before implantation in orthopaedics. J. Am. Acad. Orthop. Surg. 27, e1040–e1042 (2019).
Peroglio, M., Gaspar, D., Zeugolis, D. I. & Alini, M. Relevance of bioreactors and whole tissue cultures for the translation of new therapies to humans. J. Orthop. Res. 36, 10–21 (2018).
Humphrey, J. D., Dufresne, E. R. & Schwartz, M. A. Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell Biol. 15, 802–812 (2014).
Jeon, J. E. et al. Effect of preculture and loading on expression of matrix molecules, matrix metalloproteinases, and cytokines by expanded osteoarthritic chondrocytes. Arthritis Rheum. 65, 2356–2367 (2013).
Meinert, C., Schrobback, K., Hutmacher, D. W. & Klein, T. J. A novel bioreactor system for biaxial mechanical loading enhances the properties of tissue-engineered human cartilage. Sci. Rep. 7, 16997 (2017).
Mouw, J. K., Connelly, J. T., Wilson, C. G., Michael, K. E. & Levenston, M. E. Dynamic compression regulates the expression and synthesis of chondrocyte-specific matrix molecules in bone marrow stromal cells. Stem Cells 25, 655–663 (2007).
Screen, H. R. C., Shelton, J. C., Bader, D. L. & Lee, D. A. Cyclic tensile strain upregulates collagen synthesis in isolated tendon fascicles. Biochem. Biophys. Res. Commun. 336, 424–429 (2005).
Huisman, E., Lu, A., McCormack, R. G. & Scott, A. Enhanced collagen type I synthesis by human tenocytes subjected to periodic in vitro mechanical stimulation. BMC Musculoskelet. Disord. 15, 386 (2014).
Youngstrom, D. W., LaDow, J. E. & Barrett, J. G. Tenogenesis of bone marrow-, adipose-, and tendon-derived stem cells in a dynamic bioreactor. Connect Tissue Res. 57, 454–465 (2016).
Kuo, C. K. & Tuan, R. S. Mechanoactive tenogenic differentiation of human mesenchymal stem cells. Tissue Eng. Part A 14, 1615–1627 (2008).
Thomopoulos, S. et al. Fibrocartilage tissue engineering: the role of the stress environment on cell morphology and matrix expression. Tissue Eng. Part A 17, 1039–1053 (2011).
Fernandez-Yague, M. A. et al. Biomimetic approaches in bone tissue engineering: integrating biological and physicomechanical strategies. Adv. Drug Deliv. Rev. 84, 1–29 (2015).
Ng, J., Spiller, K., Bernhard, J. & Vunjak-Novakovic, G. Biomimetic approaches for bone tissue engineering. Tissue Eng. Part B Rev. 23, 480–493 (2017).
Tran, S. C., Cooley, A. J. & Elder, S. H. Effect of a mechanical stimulation bioreactor on tissue engineered, scaffold-free cartilage. Biotechnol. Bioeng. 108, 1421–1429 (2011).
Hoenig, E. et al. High amplitude direct compressive strain enhances mechanical properties of scaffold-free tissue-engineered cartilage. Tissue Eng. Part A 17, 1401–1411 (2011).
Ku, C. H. et al. Collagen synthesis by mesenchymal stem cells and aortic valve interstitial cells in response to mechanical stretch. Cardiovasc Res. 71, 548–556 (2006).
Waldman, S. D., Couto, D. C., Grynpas, M. D., Pilliar, R. M. & Kandel, R. A. A single application of cyclic loading can accelerate matrix deposition and enhance the properties of tissue-engineered cartilage. Osteoarthr. Cartil. 14, 323–330 (2006).
Lee, A. et al. Hypoxia modulates the development of a corneal stromal matrix model. Exp. Eye Res. 170, 127–137 (2018).
Falanga, V., Zhou, L. & Yufit, T. Low oxygen tension stimulates collagen synthesis and COL1A1 transcription through the action of TGF-β1. J. Cell Physiol. 191, 42–50 (2002).
Gaspar, D., Fuller, K. P. & Zeugolis, D. I. Polydispersity and negative charge are key modulators of extracellular matrix deposition under macromolecular crowding conditions. Acta Biomater. 88, 197–210 (2019).
Kumar, P. et al. Macromolecularly crowded in vitro microenvironments accelerate the production of extracellular matrix-rich supramolecular assemblies. Sci. Rep. 5, 8729 (2015).
Satyam, A. et al. Macromolecular crowding meets tissue engineering by self-assembly: a paradigm shift in regenerative medicine. Adv. Mater. 26, 3024–3034 (2014).
Lareu, R. R. et al. Collagen matrix deposition is dramatically enhanced in vitro when crowded with charged macromolecules: the biological relevance of the excluded volume effect. FEBS Lett. 581, 2709–2714 (2007).
Shendi, D. et al. Hyaluronic acid as a macromolecular crowding agent for production of cell-derived matrices. Acta Biomater. 100, 292–305 (2019).
Graceffa, V. & Zeugolis, D. I. Carrageenan enhances chondrogenesis and osteogenesis in human bone marrow stem cell culture. Eur. Cell Mater. 37, 310–332 (2019).
Patrikoski, M. et al. Effects of macromolecular crowding on human adipose stem cell culture in fetal bovine serum, human serum, and defined xeno-free/serum-free conditions. Stem Cells Int. 2017, 6909163 (2017).
Cigognini, D. et al. Macromolecular crowding meets oxygen tension in human mesenchymal stem cell culture - a step closer to physiologically relevant in vitro organogenesis. Sci. Rep. 6, 30746 (2016).
Lee, M. H. et al. ECM microenvironment unlocks brown adipogenic potential of adult human bone marrow-derived MSCs. Sci. Rep. 6, 21173 (2016).
Ma, L. et al. Comparative proteomic analysis of extracellular matrix proteins secreted by hypertrophic scar with normal skin fibroblasts. Burns Trauma 2, 76–83 (2014).
Zeiger, A. S., Loe, F. C., Li, R., Raghunath, M. & Van Vliet, K. J. Macromolecular crowding directs extracellular matrix organization and mesenchymal stem cell behavior. PLoS ONE 7, e37904 (2012).
De Pieri, A., Rana, S., Korntner, S. & Zeugolis, D. I. Seaweed polysaccharides as macromolecular crowding agents. Int. J. Biol. Macromol. 164, 434–446 (2020).
Prewitz, M. C. et al. Extracellular matrix deposition of bone marrow stroma enhanced by macromolecular crowding. Biomaterials 73, 60–69 (2015).
Minton, A. P. The influence of macromolecular crowding and macromolecular confinement on biochemical reactions in physiological media. J. Biol. Chem. 276, 10577–10580 (2001).
Zhou, H.-X., Rivas, G. & Minton, A. P. Macromolecular crowding and confinement: biochemical, biophysical, and potential physiological consequences. Annu. Rev. Biophys. 37, 375–397 (2008).
Minton, A. P. Excluded volume as a determinant of macromolecular structure and reactivity. Biopolymers 20, 2093–2120 (1981).
Hatters, D. M., Minton, A. P. & Howlett, G. J. Macromolecular crowding accelerates amyloid formation by human apolipoprotein C-II. J. Biol. Chem. 277, 7824–7830 (2002).
van den Berg, B., Ellis, R. J. & Dobson, C. M. Effects of macromolecular crowding on protein folding and aggregation. EMBO J. 18, 6927–6933 (1999).
Minton, A. P. & Wilf, J. Effect of macromolecular crowding upon the structure and function of an enzyme: Glyceraldehyde-3-phosphate dehydrogenase. Biochemistry 20, 4821–4826 (1981).
Zhang, C., Shao, P. G., van Kan, J. A. & van der Maarel, J. R. C. Macromolecular crowding induced elongation and compaction of single DNA molecules confined in a nanochannel. Proc. Natl Acad. Sci. USA 106, 16651–16656 (2009).
Zimmerman, S. B. & Murphy, L. D. Macromolecular crowding and the mandatory condensation of DNA in bacteria. FEBS Lett. 390, 245–248 (1996).
Akabayov, B., Akabayov, S. R., Lee, S.-J., Wagner, G. & Richardson, C. C. Impact of macromolecular crowding on DNA replication. Nat. Commun. 4, 1615–1615 (2013).
Miyoshi, D. & Sugimoto, N. Molecular crowding effects on structure and stability of DNA. Biochimie 90, 1040–1051 (2008).
Minton, A. P. in Methods Enzymol, Vol. 295, 127–149 (Academic, 1998).
Chebotareva, N. A., Kurganov, B. I. & Livanova, N. B. Biochemical effects of molecular crowding. Biochemistry (Mosc). 69, 1239–1251, https://doi.org/10.1007/s10541-005-0070-y (2004).
Chen, C., Loe, F., Blocki, A., Peng, Y. & Raghunath, M. Applying macromolecular crowding to enhance extracellular matrix deposition and its remodeling in vitro for tissue engineering and cell-based therapies. Adv. Drug Deliv. Rev. 63, 277–290 (2011).
Dewavrin, J.-Y., Hamzavi, N., Shim, V. P. W. & Raghunath, M. Tuning the architecture of three-dimensional collagen hydrogels by physiological macromolecular crowding. Acta Biomater. 10, 4351–4359 (2014).
Lareu, R. R., Arsianti, I., Subramhanya, K. H., Yanxian, P. & Raghunath, M. In vitro enhancement of collagen matrix formation and crosslinking for applications in tissue engineering: a preliminary study. Tissue Eng. 13, 385–391 (2007).
Harve, K. S., Raghunath, M., Lareu, R. R. & Rajagopalan, R. Macromolecular crowding in biological systems: Dynamic light scattering (DLS) to quantify the excluded volume effect (EVE). Biophys. Rev. Lett. 01, 317–325 (2006).
Mes, E., Kok, W., Poppe, H. & Tijssen, R. Comparison of methods for the determination of diffusion coefficients of polymers in dilute solutions: the influence of polydispersity. J. Polym. Sci. B Polym. Phys. 37, 593–603 (1999).
Satyam, A., Kumar, P., Cigognini, D., Pandit, A. & Zeugolis, D. I. Low, but not too low, oxygen tension and macromolecular crowding accelerate extracellular matrix deposition in human dermal fibroblast culture. Acta Biomater. 44, 221–231 (2016).
Kumar, P., Satyam, A., Cigognini, D., Pandit, A. & Zeugolis, D. I. Low oxygen tension and macromolecular crowding accelerate extracellular matrix deposition in human corneal fibroblast culture. J. Tissue Eng. Regen. Med. 12, 6–18 (2018).
Bakarich, S. E., Gorkin, R. III, Panhuis, M. I. H. & Spinks, G. M. 4D printing with mechanically robust, thermally actuating hydrogels. Macromol. Rapid Commun. 36, 1211–1217 (2015).
Ang, X. M. et al. Macromolecular crowding amplifies adipogenesis of human bone marrow-derived mesenchymal stem cells by enhancing the pro-adipogenic microenvironment. Tissue Eng. Part A 20, 966–981 (2014).
Satyam, A. et al. Macromolecular crowding meets tissue engineering by self-assembly: a paradigm shift in regenerative medicine. Adv. Mater. 26, 3024–3034 (2014).
Kumar, P. et al. Accelerated development of supramolecular corneal stromal-like assemblies from corneal fibroblasts in the presence of macromolecular crowders. Tissue Eng. Part C. Methods 21, 660–670 (2015).
Magno, V. et al. Macromolecular crowding for tailoring tissue-derived fibrillated matrices. Acta Biomater. 55, 109–119 (2017).
Satyam, A., Tsokos, M. G., Tresback, J. S., Zeugolis, D. I. & Tsokos, G. C. Cell-derived extracellular matrix-rich biomimetic substrate supports podocyte proliferation, differentiation, and maintenance of native phenotype. Adv. Funct. Mater. 30, 1908752 (2020).
Peng, Y. et al. Human fibroblast matrices bio-assembled under macromolecular crowding support stable propagation of human embryonic stem cells. J. Tissue Eng. Regen. Med 6, e74–86 (2012).
Wong, C. W. et al. In vitro expansion of keratinocytes on human dermal fibroblast-derived matrix retains their stem-like characteristics. Sci. Rep. 9, 18561 (2019).
Benny, P., Badowski, C., Lane, E. B. & Raghunath, M. Improving 2D and 3D skin In vitro models using macromolecular crowding. J .Vis. Exp. 53642 (2016).
Benny, P., Badowski, C., Lane, E. B. & Raghunath, M. Making more matrix: enhancing the deposition of dermal–epidermal junction components in vitro and accelerating organotypic skin culture development, using macromolecular crowding. Tissue Eng. Part A 21, 183–192 (2015).
Chen, C. et al. The Scar-in-a-Jar: studying potential antifibrotic compounds from the epigenetic to extracellular level in a single well. Br. J. Pharm. 158, 1196–1209 (2009).
Rønnow, S. R. et al. Prolonged Scar-in-a-Jar: an in vitro screening tool for anti-fibrotic therapies using biomarkers of extracellular matrix synthesis. Respir. Res 21, 108 (2020).
Graupp, M. et al. Establishing principles of macromolecular crowding for in vitro fibrosis research of the vocal fold lamina propria. Laryngoscope 125, E203–E209 (2015).
Woodcock, H. V. et al. The mTORC1/4E-BP1 axis represents a critical signaling node during fibrogenesis. Nat. Commun. 10, 6, https://doi.org/10.1038/s41467-018-07858-8 (2019).
Tsiapalis, D. et al. The synergistic effect of low oxygen tension and macromolecular crowding in the development of extracellular matrix-rich tendon equivalents. Biofabrication 12, 025018 (2019).
Gaspar, D., Ryan, C. N. M. & Zeugolis, D. I. Multifactorial bottom-up bioengineering approaches for the development of living tissue substitutes. FASEB J. 33, 5741–5754 (2019).
ten Ham, R. M. T. et al. What does cell therapy manufacturing cost? A framework and methodology to facilitate academic and other small-scale cell therapy manufacturing costings. Cytotherapy 22, 388–397 (2020).
Culme-Seymour, E. J. et al. Cost of stem cell-based tissue-engineered airway transplants in the United Kingdom: case series. Tissue Eng. Part A 22, 208–213 (2016).
Aijaz, A. et al. Biomanufacturing for clinically advanced cell therapies. Nat. Biomed. Eng. 2, 362–376 (2018).
Lipsitz, Y. Y. et al. A roadmap for cost-of-goods planning to guide economic production of cell therapy products. Cytotherapy 19, 1383–1391 (2017).
Doulgkeroglou, M. N. et al. Automation, monitoring, and standardization of cell product manufacturing. Front. Bioeng. Biotechnol. 8, 811 (2020).
Hunsberger, J. G., Shupe, T. & Atala, A. An industry-driven roadmap for manufacturing in regenerative medicine. Stem Cells Transl. Med. 7, 564–568 (2018).
Chase, L. G. et al. Development and characterization of a clinically compliant xeno-free culture medium in good manufacturing practice for human multipotent mesenchymal stem cells. Stem Cells Transl. Med. 1, 750–758 (2012).
Hunsberger, J. G., Goel, S., Allickson, J. & Atala, A. Five critical areas that combat high costs and prolonged development times for regenerative medicine manufacturing. Curr. Stem Cell Rep. 3, 77–82 (2017).
Jørgensen, J., Mungapen, L. & Kefalas, P. Data collection infrastructure for patient outcomes in the UK - opportunities and challenges for cell and gene therapies launching. J. Mark. Access Health Policy 7, 1573164 (2019).
Bubela, T. et al. Bringing regenerative medicines to the clinic: the future for regulation and reimbursement. Regen. Med. 10, 897–911 (2015).
Rémuzat, C., Toumi, M., Jørgensen, J. & Kefalas, P. Market access pathways for cell therapies in France. J. Mark Access Health Policy 3 (2015).
Brennan, T. A. & Wilson, J. M. The special case of gene therapy pricing. Nat. Biotechnol. 32, 874–876 (2014).
Malik, N. N. Pay-for-performance pricing for a breakthrough heart drug: learnings for cell and gene therapies. Regen. Med 11, 225–227 (2016).
Pierna, M., Santos, M., Arias, F. J., Alonso, M. & Rodríguez-Cabello, J. C. Efficient cell and cell-sheet harvesting based on smart surfaces coated with a multifunctional and self-organizing elastin-like recombinamer. Biomacromolecules 14, 1893–1903 (2013).
Chen, B. et al. Dynamics of smooth muscle cell deadhesion from thermosensitive hydroxybutyl chitosan. Biomaterials 28, 1503–1514 (2007).
Dang, J. M. et al. Temperature-responsive hydroxybutyl chitosan for the culture of mesenchymal stem cells and intervertebral disk cells. Biomaterials 27, 406–418, https://doi.org/10.1016/j.biomaterials.2005.07.033 (2006).
Thirumala, S., Gimble, J. M. & Devireddy, R. V. Methylcellulose based thermally reversible hydrogel system for tissue engineering applications. Cells 2, 460–475 (2013).
Altomare, L., Cochis, A., Carletta, A., Rimondini, L. & Farè, S. Thermo-responsive methylcellulose hydrogels as temporary substrate for cell sheet biofabrication. J. Mater. Sci. Mater. Med. 27, 95 (2016).
Dworak, A. et al. Poly(2-substituted-2-oxazoline) surfaces for dermal fibroblasts adhesion and detachment. J. Mater. Sci. Mater. Med. 25, 1149–1163 (2014).
Oleszko, N. et al. Controlling the crystallinity of thermoresponsive poly(2-oxazoline)-based nanolayers to cell adhesion and detachment. Biomacromolecules 16, 2805–2813 (2015).
Higashi, N., Hirata, A., Nishimura, S. N. & Koga, T. Thermo-responsive polymer brushes on glass plate prepared from a new class of amino acid-derived vinyl monomers and their applications in cell-sheet engineering. Colloids Surf. B Biointerfaces 159, 39–46 (2017).
Lee, B. et al. Initiated chemical vapor deposition of thermoresponsive poly(N-vinylcaprolactam) thin films for cell sheet engineering. Acta Biomater. 9, 7691–7698 (2013).
Teichmann, J. et al. Thermo-responsive cell culture carriers based on poly(vinyl methyl ether)-the effect of biomolecular ligands to balance cell adhesion and stimulated detachment. Sci. Technol. Adv. Mater. 16, 045003 (2015).
Teichmann, J. et al. Human corneal endothelial cell sheets for transplantation: thermo-responsive cell culture carriers to meet cell-specific requirements. Acta Biomater. 9, 5031–5039 (2013).
Higuchi, A. et al. Temperature-dependent cell detachment on Pluronic gels. Biomacromolecules 6, 691–696 (2005).
Higuchi, A. et al. Temperature-induced cell detachment on immobilized pluronic surface. J. Biomed. Mater. Res A 79, 380–392 (2006).
Cerqueira, M. T. et al. Cell sheet technology-driven re-epithelialization and neovascularization of skin wounds. Acta Biomater. 10, 3145–3155 (2014).
Sato, M. et al. Combined surgery and chondrocyte cell-sheet transplantation improves clinical and structural outcomes in knee osteoarthritis. NPJ Regen. Med. 4, 4 (2019).
Fujii, Y. et al. Bone regeneration by human dental pulp stem cells using a helioxanthin derivative and cell-sheet technology. Stem Cell Res. Ther. 9, 24 (2018).
Nishida, K. et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N. Engl. J. Med. 351, 1187–1196 (2004).
Miyagawa, S. et al. Phase I clinical trial of autologous stem cell-sheet transplantation therapy for treating cardiomyopathy. J. Am. Heart Assoc. 6, e003918 (2017).
Kawamura, M. et al. Xenotransplantation of bone marrow-derived human mesenchymal stem cell sheets attenuates left ventricular remodeling in a porcine ischemic cardiomyopathy model. Tissue Eng. Part A 21, 2272–2280 (2015).
Masumoto, H. et al. Human iPS cell-engineered cardiac tissue sheets with cardiomyocytes and vascular cells for cardiac regeneration. Sci. Rep. 4, 6716 (2014).
Ohki, T. et al. Prevention of esophageal stricture after endoscopic submucosal dissection using tissue-engineered cell sheets. Gastroenterology 143, 582–588.e582 (2012).
Iwata, T. et al. Periodontal regeneration with autologous periodontal ligament-derived cell sheets - a safety and efficacy study in ten patients. Regen. Ther. 9, 38–44 (2018).
Itaba, N. et al. Human mesenchymal stem cell-engineered hepatic cell sheets accelerate liver regeneration in mice. Sci. Rep. 5, 16169 (2015).
Ahmed, M. et al. Dental pulp cell sheets enhance facial nerve regeneration via local neurotrophic factor delivery. Tissue Eng. Part A (2020, in press).
Yamamoto, K. et al. Middle ear mucosal regeneration by tissue-engineered cell sheet transplantation. NPJ Regen. Med. 2, 6 (2017).
Takagi, R. et al. Fabrication of human oral mucosal epithelial cell sheets for treatment of esophageal ulceration by endoscopic submucosal dissection. Gastrointest. Endosc. 72, 1253–1259 (2010).
Okura, H. et al. Cardiomyoblast-like cells differentiated from human adipose tissue-derived mesenchymal stem cells improve left ventricular dysfunction and survival in a rat myocardial infarction model. Tissue Eng. Part C. Methods 16, 417–425 (2010).
Fotia, C., Massa, A., Boriani, F., Baldini, N. & Granchi, D. Prolonged exposure to hypoxic milieu improves the osteogenic potential of adipose derived stem cells. J. Cell Biochem. 116, 1442–1453 (2015).
Parsons, M., Kessler, E., Laurent, G. J., Brown, R. A. & Bishop, J. E. Mechanical load enhances procollagen processing in dermal fibroblasts by regulating levels of procollagen C-proteinase. Exp. Cell Res. 252, 319–331, https://doi.org/10.1006/excr.1999.4618 (1999).
Tokuyama, E., Nagai, Y., Takahashi, K., Kimata, Y. & Naruse, K. Mechanical stretch on human skin equivalents increases the epidermal thickness and develops the basement membrane. PLoS ONE 10, e0141989 (2015).
Huang, C. H., Chen, M. H., Young, T. H., Jeng, J. H. & Chen, Y. J. Interactive effects of mechanical stretching and extracellular matrix proteins on initiating osteogenic differentiation of human mesenchymal stem cells. J. Cell Biochem. 108, 1263–1273 (2009).
Wang, J. et al. Mechanical stimulation orchestrates the osteogenic differentiation of human bone marrow stromal cells by regulating HDAC1. Cell Death Dis. 7, e2221 (2016).
Akhyari, P. et al. Mechanical stretch regimen enhances the formation of bioengineered autologous cardiac muscle grafts. Circulation 106, I137–142 (2002).
Graceffa, V. & Zeugolis, D. I. Macromolecular crowding as a means to assess the effectiveness of chondrogenic media. J. Tissue Eng. Regen. Med. 13, 217–231 (2019).
Bateman, J. F. & Golub, S. B. Assessment of procollagen processing defects by fibroblasts cultured in the presence of dextran sulphate. Biochem. J. 267, 573–577 (1990).
Goh, T. K. P. et al. Microcarrier culture for efficient expansion and osteogenic differentiation of human fetal mesenchymal stem cells. Biores Open Access 2, 84 (2013).
Bateman, J. F., Cole, W. G., Pillow, J. J. & Ramshaw, J. A. Induction of procollagen processing in fibroblast cultures by neutral polymers. J. Biol. Chem. 261, 4198–4203 (1986).
Rashid, R., Lim, N. S., Chee, S. M., Png, S. N. & Raghunath, M. Novel use for polyvinylpyrrolidone as a macromolecular crowder for enhanced extracellular matrix deposition and cell proliferation. Tissue Eng. Part C. Methods 20, 994–1002 (2014).
Acknowledgements
This work has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie, grant agreement No. 676338 and the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme, grant agreement No. 866126. This work has also received funding from Science Foundation Ireland, Career Development Award, grant agreement No. 15/CDA/3629, Science Foundation Ireland, Frontiers for the Future, grant agreement No. 19/FFP/6982 and Science Foundation Ireland/European Regional Development Fund, grant agreement No. 13/RC/2073.
Author information
Authors and Affiliations
Contributions
A.D.P. wrote the first draft of manuscript. D.I.Z. wrote and edited and manuscript. D.I.Z. obtained funding and supervised the work. All authors discussed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
De Pieri, A., Rochev, Y. & Zeugolis, D.I. Scaffold-free cell-based tissue engineering therapies: advances, shortfalls and forecast. npj Regen Med 6, 18 (2021). https://doi.org/10.1038/s41536-021-00133-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41536-021-00133-3
This article is cited by
-
Exploring the interaction between extracellular matrix components in a 3D organoid disease model to replicate the pathophysiology of breast cancer
Journal of Experimental & Clinical Cancer Research (2023)
-
Enhancement of mesenchymal stem cells’ chondrogenic potential by type II collagen-based bioscaffolds
Molecular Biology Reports (2023)
-
Modulation of Mesenchymal Stem Cells-Mediated Adaptive Immune Effectors’ Repertoire in the Recovery of Systemic Lupus Erythematosus
Stem Cell Reviews and Reports (2023)
-
Assembling the Puzzle Pieces. Insights for in Vitro Bone Remodeling
Stem Cell Reviews and Reports (2023)
-
Noncovalent functionalization of carbon nanotubes as a scaffold for tissue engineering
Scientific Reports (2022)