Abstract
Iron diantimonide is a material with the highest known thermoelectric power. By combining scanning transmission electron microscopic study with electronic transport neutron, X-ray scattering, and first principle calculation, we identify atomic defects that control colossal thermopower magnitude and nanoprecipitate clusters with Sb vacancy ordering, which induce additional phonon scattering and substantially reduce thermal conductivity. Defects are found to cause rather weak but important monoclinic distortion of the unit cell Pnnm → Pm. The absence of Sb along [010] for high defect concentration forms conducting path due to Fe d orbital overlap. The connection between atomic defect anisotropy and colossal thermopower in FeSb2 paves the way for the understanding and tailoring of giant thermopower in related materials.
Similar content being viewed by others
Introduction
Thermoelectric materials exploit thermoelectric effect where temperature difference is converted into electric power and vice versa1,2. High operating temperatures are favorable for significant figure of merit ZT = S2σT/κ, where T is temperature, S is thermopower, and σ (κ) are electrical (thermal) conductivities. When operating temperatures are not high, material must maximize its thermoelectric power factor (S2σ) where S provides considerable contribution, whereas in cryogenic environment electronic correlations could also be significant3,4,5,6,7,8.
The iron diantimonide is a correlated narrow-gap semiconductor9,10. It also features colossal thermopower S and highest known thermoelectric power factor at cryogenic temperatures; whereas both electronic diffusion and phonon-drag mechanism have been proposed, reported maximum S values vary between 0.1 and 50 mV K−1 and in some crystals quasi-one-dimensional (quasi-1D) conductivity has been observed9,11,12,13,14,15,16,17,18. Interstitial Fe atoms in the unit cell that create impurity states connected with phonon drag have been postulated but never observed16. The connection with fine details of crystal structure is not understood since, for example, FeAs2 features an order of magnitude higher thermal conductivity and should have higher thermopower within the phonon drag mechanism when compared to FeSb2, but experiments show S maxima about six times smaller in iron diarsenide14.
Atomic defects have been used to enhance high-temperature thermoelectric performance by lowering phonon thermal conductivity in the figure of merit19,20. Defect optimization in semiconductors is important in a wide range of technology-enabling materials21. In this work, for the first time, we present the direct visualization of structural defects in iron diantimonide and we show that colossal thermopower arises from Sb vacancies in the crystal structure. Moreover, thermopower, quasi-1D conductivity, and thermal conductivity are tunable by Sb defects and phase separation clusters with Sb vacancies of several nanometer size. Our results uncover hitherto unknown relation between crystal structure and colossal thermopower, thus providing basis for the comprehensive understanding of the nanostructural aspects related to the colossal thermopower control in iron diantimonide.
Results and discussion
Sample characterization
We first fabricated several crystals from Sb melt using different decanting methods, purity of raw elements, and crucibles (see Supplementary Note 4 and Table 7). Then we picked two crystals: one with expected high purity (S8) and the other with expected low purity (S3). To shed light on the atomic-scale structure, we performed high-resolution scanning transmission electron microscopy (STEM) with a high-angle annular dark field (HAADF) detector as its contrast is proportional to Z1.7 along the atom column, where Z is the atomic number. Figure 1a, b show the STEM-HAADF images taken from S3 and S8 crystals, respectively. The strong and weak dots in the images correspond to Sb and Fe atoms, respectively. The atomic arrangement in the images is consistent with the FeSb2 structure with Pnnm symmetry, as shown in the insets where the atomic projections are embedded in the magnified image. Due to the Z-contrast nature of the STEM-HAADF image, the peak intensity of each dot can be used to count the atoms along the column22. Higher peak intensity indicates more atoms along the column, while weaker peak intensity indicates less atoms, thus more vacancies along the column. It is seen that the peak intensity of Fe is quite uniform in crystal S8 (Fig. 1b), indicating the relative uniform distribution of Fe. However, the peak intensity of Fe varies in crystal S3, e.g., there are less Fe in area I than that in area II, as shown in the magnified image in the insets of Fig. 1a. We also observe Sb intensity variation, indicating the variation of Sb occupancies. The Sb occupancies or vacancies can be better resolved by refining each Sb column peak with the second-order polynomial function. Figure 1c, d shows the peak intensity maps of Sb for crystals S3 and S8, respectively. There are four Sb atoms in a FeSb2 unit cell. In crystal S3, the Sb peak intensity is the same in some area (inset III in Fig. 1c), consistent with the Pnnm symmetry. In the other area, however, the Sb peak intensity changes periodically and orders along Pnnm [010] direction, as shown in the inset II in Fig. 1c, where two Sb peaks (orange squares) are stronger than the other two Sb (green squares) within the unit cell. This indicates the ordering of Sb vacancies and reduction of the Pnnm symmetry in this area. In [100] projection, orange and red spheres represent high and low Sb occupancy, respectively, embedded in the inset II. The size of the phase separation clusters with Sb vacancy ordering is about a few nanometers. These clusters are similar to the nanoprecipitates observed in PbTe-AgSbTe2 system23,24. They induce additional phonon scattering, thus reduce the thermal conductivity and phonon mean free path (MFP) of crystal S3. For crystal S8, the Sb vacancy ordering is observed in nearly all locations and also along [010] direction. In area IV (Fig. 1d), two Sb peaks (orange) are stronger than the other two Sb (red). While in the area V, one Sb peak (yellow square) is stronger than the other three Sb (orange squares).
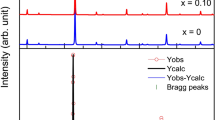
a, b STEM-HAADF image of crystal S3 (a) viewed along Pnnm [100] direction and crystal S8 viewed along Pnnm [001] direction (b). The insets are the magnified images from areas I and II in a (areas IV and V in b) with the Pnnm [100] in a (Pnnm [001] projection in b) projection of the structure embedded. Scale bar 2 nm. The contrast is approximately proportional to Z1.7 along the atomic column, thus the dots with strong and weak contrast correspond to Sb and Fe column, respectively. Red and green spheres represent Sb and Fe, respectively. c, d Sb peak intensity maps refined from a and b, respectively. Each square represents an Sb column with intensity increasing in black–blue–green–orange–yellow–white order. The insets are magnified maps from areas II to V. Note that the smooth Sb peak intensity oscillation from top to bottom in b and d could be attributed to thickness variation. e–g Synchrotron X-ray diffraction scans of crystal S8. H is the Miller index of the diffraction from (H00). Pnnm-forbidden peak is observed at [100] wavevector (f), about 3 orders of magnitude weaker than nearby Bragg peaks. Pnnm (h, i) and Pm (j, k) unit cell refinements obtained in single-crystal neutron diffraction experiment on low thermopower crystal, confirming structural distortion. l Pm unit cell of FeSb2 induced by atomic defects. Central octahedral atom Fe (dark) is surrounded by Sb (light). The defects are preferably on Sb11 atomic sites (white arrow-marked light balls).
Sb vacancy ordering results in a Pnnm-forbidden peak observed in single-crystal synchrotron X-ray diffraction (Fig. 1e–g) of crystal S8, consistent with weak structural distortion observed in neutron diffraction measurements (Fig. 1h–k). Neutron diffraction shows the presence of weak (h,0,l), h + l = odd and (0,k,l), k + l = odd type Bragg reflections, which are forbidden in Pnnm crystal structure previously refined for the stoichiometric FeSb29,10,25. The presence of these forbidden reflections hints that the symmetry of the crystal lattice is lower. Comprehensive structural refinement (Fig. 1h–k) and also Supplementary Note 1 and Tables 1–4 indicate that one of the two Sb sites, which are equivalent in Pnnm space group, shows displacive monoclinic distortions and site deficiency. This induces change in the structural symmetry (Pnnm → Pm), making two Sb sites inequivalent, i.e., Sb1 site is fully occupied while Sb11 site contains substantial number of vacancies that do not change with temperature (Fig. 1l). We find that the Sb11 site is 0.82(2) occupied while both Fe sites are 0.94(2) occupied; the chemical vacancies do not change with temperature, whereas intensity of Pnnm-forbidden peaks at 300 K is 2/3 of that at 5 K (see Supplementary Note 1 and Tables 1–4).
Electrical and thermal transport properties
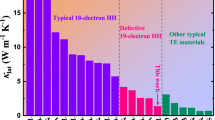
Having established the presence of atomic vacancies, next we focus on electrical and thermal transport properties of crystals S3 and S8 as well as of six additional crystals engineered to have different defect content. Figure 2a–c presents electronic and thermal transport difference among all eight iron diantimonide crystals. Crystals S7 and S8 have about 1–2 order of magnitude higher electrical resistivity when compared to crystals S1, S2, and S3 in the temperature region 10–20 K (Fig. 2a). Moreover, crystals S1, S2, and S3 have clear weak (semi)metallic resistivity in 60–300 K temperature region. Low-temperature thermopower S shows large variation (Fig. 2b); ∣S∣ maxima change by several orders of magnitude from S1 (14 μV K−1) to S8 (20 mV K−1). Figure 2c shows thermal conductivity κ vs temperature for all crystals. The small κ(T) maxima are coincident with small thermopower, consistent with phonon-drag mechanism15,16,17.
Temperature dependence of the a electrical resistivity, b thermopower, and c thermal conductivity for the investigated iron diantimonide crystals with different defect content (see text). d Thermal conductivity <50 K. The solid lines show the fitting by Callaway model. e [100] projection of crystal S3 with green and red spheres representing Fe and Sb atoms. The occupancy of Fe is 0.93. The occupancy of Sb at the row indicated by blue arrow lines is 0.85 (small spheres), while that indicated by brown arrow lines are 0.98 (large spheres). f Magnified image from area II in Fig. 1a. g Sb peak intensity map refined from f. h Simulated image calculated based on multislice method with frozen phonon approximation using structure model in e. i Sb peak intensity map from h. From the simulation, the contrast at Sb site changes with the occupancy of Sb, e.g., the contrast of Sb at the row indicated by blue arrow lines is weaker than that at the row indicated by brown arrow lines. j [001] projection of crystal S8. The occupancy of Fe is 0.95. The occupancy of Sb at the row indicated by blue arrow lines is 0.88, while that indicated by brown arrow lines is 0.97. k Magnified image from area IV in Fig. 1b. l Sb peak intensity map refined from k. m Simulated image based on structure model in j. n Sb peak intensity map from m. From the simulation, the contrast at Sb site changes with the occupancy of Sb, e.g., the contrast of Sb at the row indicated by blue arrow lines is weaker than that at the row indicated by brown arrow lines. o, p present composition of all the investigated crystals in a–c. A is the fitting parameter in Callaway model as discussed in the main text.
In what follows, we focus on the deviation from the ideal 1:2 stoichiometry. The contribution of different relaxation processes to τ and their difference among investigated crystals in the simplest form can be assessed from:
where τB, τD, and τU are the relaxation times for boundary scattering, impurities or defect scattering, and Umklapp processes, respectively26,27,28. The L, A, and B are fitting parameters in the fit of the experimental lattice thermal conductivity using Callaway model (Fig. 2d):
In this model, \(x=\frac{\hslash \omega }{{k}_{{\rm{B}}}T}\) is dimensionless, ω is the phonon frequency, kB is the Boltzmann constant, \(\hbar\) is the Plank constant, θD is the Debye temperature, and νs is the velocity of sound. Fit parameters are listed in Table 1. Since Umklapp scattering processes are of importance at high temperature, we mainly focus on parameters A and L. In crystals S5, S6, S7, and S8 with large phonon MFP, surface scattering is more important when compared to S1, S2, S3, and S4 where the impurity scattering processes are much stronger as inferred from A (Table 1), which features inverse scaling with τ. The decrease of MFP in the investigated crystals is clearly related to the defect scattering of phonons.
We note that, in the Callaway model, the parameter τB = L/ν represents the relaxation time determined by boundary scattering. The L is the phonon MFP at low temperature in the boundary scattering regime29, which is different from grain size. For the rod-like sample with the square cross-section and infinite length, it depends on the side dimension only. For the finite length and rectangular cross-section, there are correction factors, i.e., it not only depends on two side dimensions but also on their ratio and sample length29,30.
From the data collected on crystal S3 (Fig. 1h–k) and Supplementary Table 3, the refined crystal stoichiometry is Fe0.93Sb1.83. STEM-HAADF image simulations (Fig. 2e–n) show Fe0.93Sb1.83 for crystal S3 (in good agreement with neutron refinement) and Fe0.95Sb1.85 for crystal S822.
Next, from the thermal conductivity fits (Table 1), we note differences among crystals due to defect scattering, given by parameter A in the Callaway model. From the changes in parameter A, we estimate the defects content, i.e., the stoichiometry of all investigated crystals by assuming linear relation between defect scattering and defect concentration. The results are shown in Fig. 2o, p and in Table 1. The relative stoichiometry ratio of Sb:Fe differs from the ideal stoichiometric 2:1 in all crystals in linear manner, albeit with differences that are <2.3 atomic %.
Thermally activated resistivity >50 K (Fig. 2a) stems from the intrinsic energy gap; however, the resistivity shoulder in the region of \({S}_{\max }\) around 10 K is a fingerprint of the in-gap impurity states that couple to phonon drag16,18,31,32. Magnetoresistance (MR) is strong in the temperature range where such states are dominant in electronic transport; a single in-gap band gives only one peak in MR16. High-thermopower crystals S6 and S8 show one MR peak but in low-thermopower crystals S1 and S3 a broad MR is observed (Fig. 3a), suggesting a rather broad distribution of multiple in-gap states16. A low-temperature Curie tail present in crystal S3 (Fig. 3b) implies higher low-temperature magnetic moment associated with low Sb purity33, consistent with importance of Sb defects. Figure 3c shows heat capacity of all crystals. We observe a trend of the slope change associated with Debye temperature. In general, vacancy formation energy is related to Debye temperature: \({T}_{{\rm{D}}}=C\frac{{E}_{{\rm{v}}}^{1/2}}{{M}^{1/2}{{{\Omega }}}^{1/3}}\), where C is a constant, Ev is the vacancy formation energy, and M and Ω are atomic mass and volume, respectively34,35. Low vacancy formation energy is consistent with higher number of vacancies for low thermopower crystals (e.g., S3) when compared to high-thermopower crystals (e.g., S8). Results are summarized in Supplementary Note 2 and Table 5.
The Pnnm FeSb6 octahedra are edge-sharing along the shortest lattice parameter 3.194 Å. Closer inspection of Pm unit cell shows that the Sb11 atomic sites (Fig. 4a) are also separated by the lattice parameter length 3.194 Å along the edge-sharing octahedral direction. However, weak metallic conductivity at high temperature for low-thermopower crystals (Fig. 2a) is along the orthogonal, 6.536 Å lattice parameter direction where distance of Sb11 to Fe is shorter (b-direction in Fig. 4a)9,36. This is consistent with TEM-observed vacancy order direction (Fig. 1a–d) and quasi-1D conductivity in optics37,38. Occupancy of Fe d orbital (dn) translates into different unit cell parameter along the d orbital overlap. Higher (lower) occupancy corresponds to larger (shorter) unit cell parameter39,40. Detailed comparison of crystals S3 and S8 shows longer b lattice parameter in S3 (see Supplementary Note 3 and Table 6), implying differences in Fe d orbital occupation. A picture emerges where Sb11 vacancy defects create Fe-derived conducting in-gap states due to short Fe–Sb11 hopping distance (Fig. 4a). Low vacancy formation energy, i.e., higher vacancy content in low-thermopower crystals such as S3 (Fig. 3c), promotes stronger quasi-1D Fe d orbital overlap due to the absence of Sb along [010], weak metallicity, and metal–insulator transition on cooling12.
a Crystal structure of FeSb2 in the Pm space group. Note that Sb-deficient Sb11 atomic site are apical for Fe1, whereas they are within octahedral plane for Fe2. Bond distances to Fe1, Fe2, and Sb1 are shown in nm. Sb11 atomic sites are separated by the unit cell distance along Pmc axis (3.194 Å), whereas they are closer to Sb1 sites along the b-axis (2.878 Å). b–h Different arrangements of two Sb vacancies in the supercell and their energetics. The arrangement consistent with the Pm space group has the lowest energy, in agreement with the experiment. Projected density of states for i one and j two Sb vacancies. Notice that the antimony vacancy gives rise to an iron impurity band.
Band structure calculations
GW+DMFT calculations (see Supplementary Note 5) show that bands associated with quasi-1D dispersion along 6.536 Å are Fe derived: bottom of the conduction band is dominated by Fe xy orbital, whereas top of the valence bands is dominated by Fe xz/yz bands38. First-principle calculation results (Fig. 4b–h) confirm that structure distortion to the Pm space group is energetically favorable in Sb-deficient Pnnm FeSb2 unit cell. However, the Sb vacancies also induce Fe dangling bonds that might influence electronic structure through conducting impurity band at the Fermi level for high Sb defect concentration.
To explore how Sb atom vacancy and its ratio affect the electronic structure of FeSb2, we studied two cases of Sb vacancy ratios: one and two Sb vacancies in a 2 × 2 × 3 super-cell of FeSb2. To simulate the experimental situation, we only consider Sb vacancies at the Sb11 site. Hence, Sb vacancies for both cases occupy the Sb11 site only, and they correspond to 91.7 and 83.3% of Sb11 occupancy, respectively, where the case of two Sb vacancies is close to the experimental Sb11 occupancy, 82% at T = 300 K. For the case of one Sb vacancy, there is only one symmetrically non-equivalent configuration for introducing one Sb vacancy at the Sb11 site in the 2 × 2 × 3 super-cell. On the other hand, for the case of two Sb vacancies, there are seven symmetrically non-equivalent configurations. We have considered all of the seven configurations and found that the configuration with two Sb vacancies is the most energetically stable (Fig. 4b). From now on, we only focus on the most stable configuration for each Sb vacancy ratio.
Figure 4i, j shows the density of states of the most stable configurations for both Sb vacancy ratios. The Sb vacancies give rise to Fe dangling bonds that lead to metallic impurity bands at the Fermi level. The impurity bands have a dominant d orbital character of Fe possessing the dangling bonds. We would like to note that the density-functional theory calculation with the modified Becke–Johnson exchange potential method gives a clear bulk gap of ~0.25 eV in FeSb2 without any vacancies. As depicted in Fig. 4j, the case of two Sb vacancies shows broader bandwidth of the metallic impurity band than that of one Sb vacancy, indicating that, as the Sb vacancy ratio increases, the metallic impurity band is more dispersive. The case of one Sb vacancy does not show the quasi-1D conductivity. Therefore, we conclude that larger Sb atom vacancy content induces more anisotropy in the electronic structure and triggers the quasi-1D conducting path.
Now, we turn our attention to the thermoelectric power of FeSb2. In the experiment, the maximum value of thermoelectric power decreases as the energy of vacancy formation is lowered, i.e., with higher number of vacancies (Figs. 2b and 3c). This is consistent with a two-band model study with an ionized impurity donor state41. Hence, the increased Sb atom vacancies lower thermoelectric power, in agreement with the STEM-HAADF study. It would be of interest to investigate point defects and atomic structure of other marcasite materials with high-thermopower values, such as CrSb242,43.
In summary, we have achieved point defect control of thermoelectricity and have directly observed atomic structure of colossal thermopower material iron diantimonide. Sb defects induce monoclinic distortion and generate the in-gap states with Fe 3d orbital character, which, for sufficiently high number of defects, result in nanoprecipitates with Sb vacancy ordering and quasi-1D conducting path. Our study demonstrates the potential of low-level atomic defects to control thermopower magnitude and quasi-1D electronic conduction. This paves the way toward computational predictions of colossal thermopower induced by atomic point defect engineering.
Methods
Crystal synthesis
Single crystals of FeSb2 were grown as described before9,10,12, albeit with different starting purity of Fe and Sb and with variable methods of crystal decanting from Sb liquid. Whereas within one single batch thermopower values ∣\({S}_{\max }\)∣ exhibit variations, especially for low-purity starting materials such as Fe 99.5% and Sb 99.99% and lower, the use of high-purity starting materials, such as Fe 99.997% and Sb 99.9999%, elimination of quartz wool in excess Sb decanting and use of filter screw-top crucibles developed for flux crystal growth above the quartz melting point44 reduces the variation. This produces FeSb2 crystals with about 5–20 mV K−1 thermopower peak within single batch. On the other hand, we note that, among different batches with variable starting materials purity and decanting method, ρ(T) and S(T) measured on same crystal exhibit close correspondence to values presented in Fig. 2 in the main text.
Transport, thermal, and magnetic measurements
Crystals were oriented using a Laue camera and cut along the b-axis for magnetization, resistivity, and thermopower measurement in a Quantum Design MPMS-5 and PPMS-9.
Scanning tunneling microscopy
STEM-HAADF imaging and electron diffraction were performed using the double aberration-corrected JEOL-ARAM200CF microscope with a cold-field emission gun and operated at 200 keV. The images are filtered in frequency space by applying periodic mask to remove noise.
X-ray and neutron diffraction
Synchrotron single-crystal X-ray diffraction was performed at NSLS-II beamline 4-ID, using a photon energy of 11.44 keV with an incident beam intensity of 3 × 1012 photons s−1. Single-crystal neutron diffraction was performed at the HB3A four-circle diffractometer (FCD) equipped with a two-dimensional Anger camera detector at the High Flux Isotope Reactor at the Oak Ridge National Laboratoray. Neutron wavelength of 1.005 Å was used from the bent perfect Si-331 monochromator45. Room temperature laboratory single-crystal X-ray diffraction was performed at NHMFL Tallahassee using an Oxford Diffraction Xcalibur 2 charge-coupled device FCD with graphite-monochromated Mo Kα radiation.
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
References
Bell, L. E. Cooling, heating, generating power, and recovering waste heat with thermoelectric systems. Science 321, 1457–1461 (2008).
Snyder, G. J. & Toberer, E. S. Complex thermoelectric materials. Nat. Mater. 7, 105–114 (2008).
Heremans, J. P. Introduction to cryogenic solid state cooling. Proc. SPIE 9821, 98210G (2016).
He, J., Kanatzidis, M. G. & Dravid, V. P. High performance bulk thermoelectric via a panoscopic approach. Mater. Today 16, 166–176 (2013).
Hébert, S. et al. Searching for new thermoelectric materials: some examples among oxides, sulfides and selenides. J. Phys. Condens. Matter 28, 013001 (2016).
Tomczak, J. M., Haule, K. & Kotliar, G. Signatures of electronic correlations in iron silicide. Proc. Natl Acad. Sci. USA 109, 3243–3246 (2012).
Palsson, G. & Kotliar, G. Thermoelectric response near the density driven Mott transition. Phys. Rev. Lett. 80, 4775–4778 (1998).
Koshibae, W. & Maekawa, S. Effects of spin and orbital degeneracy on the thermopower of strongly correlated systems. Phys. Rev. Lett. 87, 236603 (2001).
Petrovic, C. et al. Anisotropy and large magnetoresistance in the narrow-gap semiconductor FeSb2. Phys. Rev. B 67, 155205 (2003).
Petrovic, C. et al. Kondo insulator description of spin state transition in FeSb2. Phys. Rev. B 72, 045103 (2005).
Bentien, A. et al. Colossal Seebeck coefficient in strongly correlated semiconductor FeSb2. Eur. Phys. Lett. 80, 17008 (2007).
Jie, Q. et al. Electronic thermoelectric power factor and metal-insulator transition in FeSb2. Phys. Rev. B 86, 115121 (2012).
Sun, P. et al. Huge thermoelectric power factor: FeSb2 versus FeAs2 and RuSb2. Appl. Phys. Express 2, 091102 (2009).
Sun, P. et al. Narrow band gap and enhanced thermoelectricity in FeSb2. Dalton Trans. 39, 1012–1019 (2010).
Tomczak, J. M. et al. Thermopower of correlated semiconductors: application to FeAs2 and FeSb2. Phys. Rev. B 82, 085104 (2010).
Battiato, M., Tomczak, J. M., Zhong, Z. & Held, K. Unified picture for the colossal thermopower in FeSb2. Phys. Rev. Lett. 114, 236603 (2015).
Takahashi, H. et al. Colossal Seebeck effect enhanced by quasi-ballistic phonons dragging massive electrons in FeSb2. Nat. Commun. 7, 12732 (2016).
Matsuura, H., Maebashi, H., Ogata, M. & Fukuyama, H. Effect of phonon drag on Seebeck coefficient based on linear response theory: application to FeSb2. J. Phys. Soc. Jpn. 88, 074601 (2019).
Biswas, K. et al. Strained endotaxial nanostructures with high thermoelectric figure of merit. Nat. Chem. 3, 160–166 (2011).
Kim, T. K. et al. Strain-mediated point defects in thermoelectric p-type bismuth telluride polycrystalline. Nano Energy 55, 486–493 (2019).
Walsh, A. & Zunger, A. Instilling defect tolerance in new compounds. Nat. Mater. 16, 964–967 (2017).
LeBeau, J. M., Findlay, S. D., Allen, L. J. & Stemmer, S. Standardless atom counting in scanning transmission electron microscopy. Nano Lett. 10, 4405–4408 (2010).
Hsu, K. F. et al. Cubic AgPbmSbTe2+m: bulk thermoelectric materials with high figure of merit. Science 303, 818–821 (2004).
Ke, X. et al. Microstructure and a nucleation mechanism for nanoprecipitates in PbTe-AgSbTe2. Phys. Rev. Lett. 103, 145502 (2009).
Hägg, G. X-ray Studies on the Binary Systems of Iron with Nitrogen, Phosphorus, Arsenic, Antimony and Bismuth (Almqvist & Wiksell, Uppsala, 1929).
Wang, K., Hu, R., Warren, J. & Petrovic, C. Enhancement of the thermoelectric properties in doped FeSb2 bulk crystals. J. Appl. Phys. 112, 013703 (2012).
Callaway, J. Model for lattice thermal conductivity at low temperatures. Phys. Rev. 113, 1046–1051 (1959).
Onn, D. G. et al. Some aspects of the thermal conductivity of isotopically enriched diamond single crystals. Phys. Rev. Lett. 68, 2806–2809 (1992).
Inyushkin, A. V. et al. Thermal conductivity of high purity synthetic single crystal diamonds. Phys. Rev. B 97, 144305 (2018).
McCurdy, A. K., Maris, H. J. & Elbaum, C. Anisotropic heat conduction in cubic crystals in the boundary scattering regime. Phys. Rev. B 2, 4077–4083 (1970).
Sun, P. et al. Highly dispersive electron relaxation and colossal thermoelectricity in the correlated semiconductor FeSb2. Phys. Rev. B 88, 245203 (2013).
Takahashi, H., Okazaki, R., Yasui, Y. & Terasaki, I. Low-temperature magnetotransport of the narrow-gap semiconductor FeSb2. Phys. Rev. B 84, 205215 (2011).
Takahashi, H., Yasui, Y., Terasaki, I. & Sato, M. Effects of ppm-level imperfection on the transport properties of FeSb2 single crystals. J. Phys. Soc. Jpn. 80, 054708 (2011).
Mukherjee, K. Monovacancy formation energy and Debye temperature of close-packed metals. Philos. Mag. 12, 915–918 (1965).
March, N. H. Vacancy formation energy and Debye temperature in close packed metals. Phys. Lett. 20, 231–232 (1966).
Hu, R. et al. Colossal positive magnetoresistance in a doped nearly magnetic semiconductor. Phys. Rev. B 77, 085212 (2008).
Perucchi, A. et al. Optical investigation of the metal-insulator transition in FeSb2. Eur. Phys. J. B 54, 175–183 (2008).
Homes, C. C. et al. Unusual electronic and vibrational properties in the colossal thermopower material FeSb2. Sci. Rep. 8, 11692 (2018).
Hull, G. W. & Hulliger, F. CuSe2, a marcasite type superconductor. Nature 220, 257–258 (1968).
Hulliger, F. in Structure and Bonding, Vol. 4 83–229 (Springer, Berlin, 1968).
Kang, C. J. & Kotliar, G. Study for material analogs of FeSb2: material design for thermoelectric materials. Phys. Rev. Mater. 2, 034604 (2018).
Sales, B. C. et al. Transport, thermal, and magnetic properties of the narrow-gap semiconductor CrSb2. Phys. Rev. B 86, 235136 (2012).
Du, Q., Guzman, D., Choi, S. & Petrovic, C. Crystal size effects on giant thermopower in CrSb2. Phys. Rev. B 101, 035125 (2020).
Petrovic, C., Canfield, P. C. & Mellen, J. Y. Growing intermetallic single crystals using in situ decanting. Philos. Mag. 92, 2448–2457 (2012).
Chakoumakos, B. et al. Four-circle single-crystal neutron diffractometer at the high flux isotope reactor. J. Appl. Crystallogr. 44, 655–658 (2011).
Acknowledgements
Work at Brookhaven National Laboratory is supported by the Office of Basic Energy Sciences, Materials Sciences and Engineering Division, U.S. Department of Energy under Contract No. DE-SC0012704 (Q.D., L.W., I.Z., Y.Z., and C.P.) and as a part of the Computational Materials Science Program (C.-J.K., G.L.P., K.H., and G.K.). This research used resources at the High Flux Isotope Reactor, a DOE Office of Science User Facility operated by the Oak Ridge National Laboratory. This research used beamline 4-ID of the National Synchrotron Light Source II, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under Contract No. DE-SC0012704. A portion of this work was performed at the National High Magnetic Field Laboratory, which is supported by National Science Foundation Cooperative Agreement No. DMR-1644779 and the State of Florida.
Author information
Authors and Affiliations
Contributions
C.P. designed research. Q.D. and C.P. made crystals. Q.D. carried out transport, magnetization, and thermal measurements and analysis with C.P. TEM measurement and analysis was done by L.W. and Y.Z. C.N. performed synchrotron X-ray diffraction measurement. H.C. and I.Z. carried out neutron diffraction measurements and solved new structure with input from L.W. and Y.Z. T.B. and T.S. carried out laboratory single-crystal measurements. C.-J.K., G.L.P., K.H., and G.K. carried out first-principle calculations. C.P. supervised the project and wrote the paper with Q.D. and with input from L.W., I.Z., C.-J.K., and G.K. The manuscript reflects contribution and ideas of all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Du, Q., Wu, L., Cao, H. et al. Vacancy defect control of colossal thermopower in FeSb2. npj Quantum Mater. 6, 13 (2021). https://doi.org/10.1038/s41535-020-00308-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41535-020-00308-z
This article is cited by
-
Accurate atomic scanning transmission electron microscopy analysis enabled by deep learning
Nano Research (2024)
-
Nanoscale inhomogeneity and the evolution of correlation strength in FeSe\(_{1-x}\)S\(_x\)
Nano Convergence (2023)
-
High Thermoelectric Figure of Merit of FeSb2−x Thin Films via Defect Engineering for Low-Temperature Cooling Applications
Journal of Electronic Materials (2021)