Abstract
The study aimed to determine the associations of Peak Inspiratory Flow (PIF), inhalation technique and adherence with health status and exacerbations in participants with COPD using DPI maintenance therapy. This cross-sectional multi-country observational real-world study included COPD participants aged ≥40 years using a DPI for maintenance therapy. PIF was measured three times with the In-Check DIAL G16: (1) typical PIF at resistance of participant’s inhaler, (2) maximal PIF at resistance of participant’s inhaler, (3) maximal PIF at low resistance. Suboptimal PIF (sPIF) was defined as PIF lower than required for the device. Participants completed questionnaires on health status (Clinical COPD Questionnaire (CCQ)), adherence (Test of Adherence to Inhalers (TAI)) and exacerbations. Inhalation technique was assessed by standardised evaluation of video recordings. Complete data were available from 1434 participants (50.1% female, mean age 69.2 years). GOLD stage was available for 801 participants: GOLD stage I (23.6%), II (54.9%), III (17.4%) and IV (4.1%)). Of all participants, 29% had a sPIF, and 16% were shown able to generate an optimal PIF but failed to do so. sPIF was significantly associated with worse health status (0.226 (95% CI 0.107–0.346), worse units on CCQ; p = 0.001). The errors ‘teeth and lips sealed around mouthpiece’, ‘breathe in’, and ‘breathe out calmly after inhalation’ were related to health status. Adherence was not associated with health status. After correcting for multiple testing, no significant association was found with moderate or severe exacerbations in the last 12 months. To conclude, sPIF is associated with poorer health status. This study demonstrates the importance of PIF assessment in DPI inhalation therapy. Healthcare professionals should consider selecting appropriate inhalers in cases of sPIF.
Similar content being viewed by others
Introduction
COPD is a chronic and progressive lung disease, impacting the lives of ~384 million patients worldwide1,2. The main pharmacotherapeutic treatment for COPD is maintenance therapy with long-acting bronchodilators3. The most used devices for administration of long-acting bronchodilators are dry powder inhalers (DPIs)4.
Despite the variety of available therapies, studies reveal that COPD tends to be undertreated5. This may be partially explained by the incorrect use of inhalers, which results in critical errors, a limitation of the dose of medication that is delivered into the airways and eventually, undertreatment. This means that even if the patient is adherent to prescribed treatment regimens, a clinical response may not be sufficiently achieved6. A successful treatment of COPD with maintenance therapy depends on a complex constellation of factors, among which all aspects regarding breathing manoeuvres.
Taking into consideration that DPIs are breath-actuated devices, it is crucial that patients can generate a sufficient peak inspiratory flow rate (PIF) to enable optimal drug delivery to the airways7,8,9. The relation between PIF and drug delivery is based on the fact that a minimum inspiratory flow is required to de-agglomerate the medication-containing powder from its lactose carrier within the DPI, so that particles of <5 μm in diameter are released from the inhaler device. Several independent predictors of patients being unable to generate an optimal PIF have been identified, such as female gender, shorter height, and older age10,11.
Although patients might be able to generate sufficient flow for a given device with maximal effort and attention to technique, this flow is often not generated in daily life12. The typical PIF of a patient is defined as the PIF achieved with the inhaler device that the patient uses in everyday life. If the typical PIF is lower than the optimal PIF for a given device, it seems likely that the patient does not generate enough inspiratory flow to overcome the internal resistance of the device. In such cases, medication will not be optimally inhaled, potentially lowering the efficacy of the maintenance therapy. Therefore, it is recommended to select a device with lower internal resistance for patients with a limited ability to generate sufficient PIF. A reduced PIF with a mismatch in chosen inhaler device predicts all-cause and COPD readmissions in patients with COPD9.
Another factor that is likely to reduce the efficacy of therapy, is having a poor inhalation technique13,14,15,16. Commonly made inhalation technique errors by patients with COPD include drug priming without inhalation, failure to exhale before inhalation, exhalation into the inhaler, an inadequate generation of inspiratory flow, lack of chin lift and making multiple inhalations with one actuation12,17. In addition to successful inhalation, medication adherence is an important prerequisite for the effectiveness of maintenance therapy18. Adherence to prescribed treatments is generally low in primary care patients with COPD19. Non-adherence can be categorized as sporadic (e.g. patient forgets to take the medication), deliberate (e.g. due to patient’s perception of medication necessity20, but it can also be unconscious (e.g. due to inappropriate handling of the DPI)21.
Of particular concern to real-life practice is the fact that, although the majority of patients are treated in primary care in many countries, little is known about the prevalence of suboptimal PIF and/or inhalation technique errors in COPD patients in this healthcare setting. Also, the associations between a suboptimal PIF, inadequate inhalation technique and medication adherence with effectiveness of COPD maintenance therapy remain largely unexamined. PIF and inhalation technique errors are rarely assessed in primary care for the principle aim of evaluating and selecting an inhaler device. Moreover, in COPD, in contrast to asthma, it is unknown whether some inhalation technique errors are more critical than others for the effectiveness of treatment15,16,22. In this study, critical errors will be determined based on their association with health status.
Together, all these factors are likely to increase the risk of patients not optimally benefiting from maintenance therapy.
To understand how the interactions of these factors together may negatively affect health status of COPD patients and contribute to the risk of exacerbations, we evaluated this in the PIFotal COPD study. The aim of this study is to determine the association of PIF, inhalation technique errors and adherence with health status and exacerbations in COPD patients receiving maintenance therapy with a DPI.
Methods
Study design
The PIFotal COPD study was a cross-sectional observational real-world study in five European countries (Greece, the Netherlands, Poland, Portugal, Spain) and Australia23. Participants were recruited and included in the study between October 2020 and May 2021. PIFotal was registered in a public database prior to execution (clinicaltrials.gov identifier NCT04532853). Local medical ethics committees reviewed and approved the study protocol, and all subjects gave written informed consent. A flow chart of study procedures is illustrated in Fig. 1.
Participants were invited for clinical examination (Step 1) and eligibility was verified (Step 2). Prior to participation, participants provided written informed consent (Step 3). Subsequently, their PIF was assessed, namely typical PIF (Step 4), a patient’s maximal PIF against the resistance of their own device (Step 5) and a patient’s maximal PIF at low resistance (Step 6). Next, participants filled out questionnaires to assess health status, number of exacerbations, self-reported medication adherence, medication use, and demographic and clinical covariates (Step 7). Subsequently, participants inhaled their usual medication, which was video recorded for later assessment (Step 8). Lastly, participants received tailored inhalation instructions based on the inhalation errors they made (Step 9) after which the clinical assessment was finished (Step 10).
Study population
Inclusion/exclusion criteria were limited to ensure a real-world setting as much as possible. Participants with a clinical diagnosis of COPD, aged 40 years or older, who were treated with a DPI as maintenance therapy for their COPD in the previous 3 months or longer, were eligible for participation. Participants were excluded from participation if they were unable to give informed consent, were participating in other trials with COPD medication, if they had an exacerbation in the 6 weeks prior to participation or if they had a life-threatening disease with a life expectancy <6 months.
Peak inspiratory flow (PIF)
PIF (L/min) was assessed with the In-Check DIAL G16 (Clement Clarke, UK), a multi-patient device using disposable, single-patient mouthpieces with inspiratory one-way valves. The In-Check DIAL G16 can be set to resemble resistance of the participant’s inhaler. If a participant used multiple inhalers, the assessment priority determined the typical measurements with PIF and inhaler measurements. The priority assigned to the devices to determine the primary inhaler can be found in Supplementary Table 1.
With the In-Check DIAL G16, PIF was assessed in three ways: (1) typical PIF at the resistance of the own inhaler, (2) maximal PIF at the resistance of the own inhaler and (3) maximal PIF at low resistance24,25. For the typical PIF measurement participants were asked to inhale with the In-Check DIAL as they normally would with their DPI. For both maximal PIF measurements, participants were instructed to exhale fully and then inhale as hard and fast as possible. Maximal measurements were performed twice. The maximal of the two attempts was included in the data analysis. A suboptimal PIF was defined as typical PIF being lower than required for the device (Table S1), and optimal PIF as typical PIF equal to or higher than required for the device.
Participants with a typical PIF that was equal to or higher than the optimal PIF for their device were pragmatically named the ‘can and will do’ group. Participants with a typical PIF below the optimal PIF for their device, but able to perform a maximal PIF that is equal to or higher than the optimal PIF, were named the ‘can, but will not do’ group. Participants with both their typical and their maximal PIF below the optimal PIF for their device were named the ‘cannot do’ group.
Inhalation technique and adherence
Inhalation technique was observed and documented by video recording which was rated offline for errors by two independent observers. They used checklists on inhaler-specific and inhaler-independent commonly made errors, based on recommendations of the Netherlands Lung Alliance (www.inhalatorgebruik.nl) or, if unavailable for specific devices, the Aerosol Drug Management Improvement Team (www.inhalers4u.org). Inhalation technique was evaluated by grouping errors in steps together in 11 categories (Supplementary Table 2). To assess which errors could be regarded as critical in this analysis, all inhalation technique error groups (Supplementary Table 2) were tested for their association with moderate and severe exacerbations and health status (CCQ-score).
Adherence was calculated based on the answers on the 12-item Test of Adherence to Inhalers (TAI-12). Because of the more precise testing of inhalation technique in the study, item 12, concerning physician-observed critical inhalation technique errors, was replaced with the objective assessment of inhalation technique video. Items 1 to 10 could be scored 1–5 points each. Item 11 and inhalation technique could be scored with 1 or 2 points. Only if participants scored the maximum number of points on all items (total 50 points), he or she was considered adherent. We further used three different representations of non-adherence as exploratory predictors: sporadic non-adherence (TAI-12 items 1 to 5 < 25), deliberate non-adherence (TAI-12 items 6 to 10 < 25) and unconscious non-adherence (TAI-12 item 11 and video assessment <4)26.
Health status, exacerbations and healthcare resources
COPD-related health status was measured with the 10-item self-administered CCQ27, consisting of three domains: symptoms, functional status and mental health. The CCQ-score is the mean score of 10 item-scores, where each item is scored on a 7-point Likert scale indicating the severity of symptoms. Exacerbations in the previous 12 months were counted from the medical records (32%) or reported by the participants (68%), and were evaluated as either moderate, severe or combined. Moderate exacerbations were defined as exacerbations treated with oral corticosteroids or antibiotics without hospital admission and severe exacerbations were defined as exacerbations requiring hospital admission.
The CAT was self-administered by the participant and consists of 8 items with 5-point Likert scales to rate symptoms (e.g. frequency of coughing), disability, quality of sleep and energy.
Statistical analysis
The primary objective was to determine the associations of PIF, inhalation technique errors, and overall medication adherence with health status. There were two secondary objectives to determine the associations of PIF, inhalation technique errors, and overall medication adherence with the number of exacerbations (A) and to determine the proportion of participants with suboptimal PIF and different inhalation technique errors for clusters of inhaler devices (according to internal inhaler resistance) (B).
Inhalation technique videos were scored via a list of potential observed technique errors, specified per inhaler. For this article, the relation between observed errors and health status was assessed. We first constructed univariate models, and, to prevent type I statistical errors, inhalation errors with p-values < 0.1 were further considered as critical errors in the multi-level models. Three inhalation errors from this list occurred related to CCQ scores with a p-value < 0.1 and were further considered as critical errors in the analyses.
For each outcome-predictor combination, a multi-level regression model was fitted, allowing for a random effect at the level of the participants’ general practitioner (n = 621). Multiple imputation was used to handle missing data. Each candidate confounder was tested separately for bias potential, defined as the change in coefficient of the fixed effect under study. All candidate confounders were added to the model one by one, sorted by bias potential in a descending order. Whenever the bias potential was ≥5%, the candidate confounder was retained in the model. A list of all candidate confounders can be found in Supplementary Table 3 and an overview of confounders included in the models can be found in Supplementary Table 4.
Since for each outcome measure associations were assessed for 5 variables (suboptimal PIF, overall adherence and 3 critical errors), we adjusted the p-values for multiple testing using the false discovery rate according to Simes28. All statistical analyses were done using Stata version 15/MP.
Sample size calculation
A sample size calculation was performed prior to study execution. A sample size of 1200 participants was estimated to be sufficient to achieve sufficient (≥80%) statistical power. It was assumed that the difference between optimal and suboptimal PIF would lead to a Clinical COPD Questionnaire (CCQ) score difference of 0.2 points29. This minimal detectable difference yielded a sample size of 1176 participants, with an α of 5% and a power of 80%.
Ethics approval
The PIFotal COPD study protocol received approvals from the following institutional ethics committees/institutional review boards: Australia: Human Research Ethics Committee (HREC 3) University of Sydney; Greece: Research Ethics Committee University of Crete; Poland: Komisja Bioetyczna przy Beskidziej Izble Lekarskiej – Bielsko Biala; Komisji Bioetycznej przy Śląskiej Izbie Lekarskiej; Silesian Medical Society (Śląska Izba Lekarska); Bioethics Committee at Lower Silesian Medical Association; Bioethics Committee at the Medical University of Biaystok; Portugal: North Health Regional Administration (ARS Norte); Matosinhos Local Health Unit (ULS Matosinhos); Guimarães Hospital; Center Health Regional Administration (ARS Centro); Regional Health Administration of Lisbon and Tagus Valley (ARS LVT); Spain: Comité de Ética de la Investigación (CEI) Islas Baleares; CEI Hospital Universitario de Gran Canaria; The Netherlands: Medisch Ethische Toetsingscommissie (METC) Assen exempted this study.
Patient and public involvement
No patients were directly involved in the conceptualization and design of the study. A scientific advisory board has been set up to provide advice on the study protocol, the conduct of the study, data to be collected, statistical analysis and interpretation of the data. All members of the scientific advisory board are distinguished researchers and/or clinicians in the field of respiratory medicine and care for patients with COPD. For the contributing sites, the data collection raised awareness of the importance of a suboptimal PIF and/or inhalation technique errors in COPD patients and their medication adherence rates. Likewise, the participants could receive inhalation technique instructions during the visit. We plan on sharing our findings with clinicians, patients, and the public.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Results
Study population
A total of 1434 participants with COPD from Australia, Greece, the Netherlands, Poland, Portugal and Spain were included in the study (Supplementary Table 5) and provided signed informed consent. Supplementary Fig. 1 shows a flow chart of the selection of the study population. Of these participants, 50.1% were female and the mean (SD) age was 69.2 (9.3) years. COPD severity was available for 801 participants and classified as GOLD stage I in 23.6%, II in 54.9%, III in 17.4% and IV in 4.1%. Participant characteristics are shown in Table 1.
The primary inhaler used by the participants at the study visit are illustrated in Fig. 2. DPIs with a medium and medium-low internal resistance made up for over half (54%) of the DPIs used in the study. The majority of participants (66.2%) had received their last inhalation instruction more than a year prior to study entry, and 16% had never received an instruction on how to use their inhaler.
Mean (SD) CCQ-score and CAT-score were 1.7 (1.1) and 13.6 (7.8), respectively. In the previous 12 months, there were a total of 680 moderate and 77 severe exacerbations reported in 331 participants.
Critical inhalation errors and adherence
The frequency of observed inhalation technique errors in this study is shown in Fig. 3.
Three inhalation technique errors were associated with CCQ and were thus identified as critical errors: (1) ‘teeth and lips sealed around mouthpiece’ (p = 0.093), (2) ‘breathe in’ (p = 0.001) and (3) ‘breathe out calmly after inhalation’ (p = 0.093). There was no significant relation between inhalation technique errors and exacerbations. Inhalation technique errors were common with a total of 4792 errors observed: 17.6% of these were a critical error (n = 846). Over half of the participants (51.7%) made one or more critical error.
Medication adherence was limited in this study population, as measured with modified TAI: 73.6% was classified as non-adherent. A total of 39.4% was classified as sporadic non-adherent, 25.5% as deliberate non-adherent and 53.7% as unconscious non-adherent.
Associations of PIF, inhalation technique errors, adherence and health status
A suboptimal PIF (0.226 (95% CI 0.107–0.346, p = 0.001) and the critical error ‘breathe in’ (0.151 (95% CI 0.037–0.265, p = 0.023) were associated with worse CCQ-score after correcting for confounders. Adherence and other inhalation technique errors were not significantly associated with this measure of health status (Fig. 4).
Associations of PIF, inhalation technique errors, adherence and exacerbations
No significant association was found between moderate and severe exacerbations in the prior 12 months and suboptimal PIF after correcting for multiple testing (Fig. 5). Furthermore, adherence and inhalation errors were also not associated with the number of moderate or severe exacerbations (Fig. 5).
Peak inspiratory flow (PIF) and inhaler resistance
The proportion of participants with a suboptimal PIF did not increase with the internal resistance of the inhaler (Supplementary Fig. 2). There was, however, a difference per inhaler resistance cluster and the three identified critical errors (Supplementary Fig. 3). The error ‘teeth and lips sealed around mouthpiece’ was most frequently made by participants with a medium-low resistance device. On more detailed inspection, it was found that this error was almost solely made by participants using the Ellipta device. It was observed that users of the Ellipta inhaler often opened the protective cap to the incorrect side, before putting the inhaler between the lips. When opening the protective cap to the incorrect side, the air vents of the Ellipta point downwards instead of the correct upwards position, which may lead to medication loss via these air vents. Therefore, this step was often scored as being incorrect for Ellipta inhaler users.
The error ‘breathe in’ was most frequently made by participants with a low resistance device. The error ‘breathe out calmly after inhalation’ was most frequently made by participants with a low resistance device.
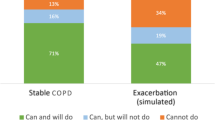
Figure 6 shows which inhaler resistances were used by the three patient groups: ‘can do and will do’, ‘can do, but will not do’ and ‘cannot do’. This illustrates that for each inhaler resistance there is a proportion of users who cannot generate sufficient inspiratory flow for their device.
Discussion
The PIFotal study demonstrated that a suboptimal PIF is associated with worse health status. Nearly a third of participants (29%) did not generate an optimal PIF for their own device during a typical inhalation manoeuvre, suggesting that PIF may be a critical inhaler factor not to be overlooked when selecting an inhaler device. Furthermore, the considerable proportion (16%) of participants in the ‘can, but will not do’ group, i.e. participants who were able to generate an optimal PIF but failed to do so, indicates that there is potential for interventions targeting PIF such as by improving inhalation technique. In addition, 13% of participants were unable to produce a sufficient PIF, indicating healthcare professionals should consider selecting different inhalers (with lower internal resistance or inhalers with zero internal resistance such as pMDIs or SMIs) in these cases. A more detailed breakdown of the alternative DPIs that are available for participants who are currently on a low resistance device is provided in Supplementary Table 6. A graphical overview of the findings in the PIFotal study can be found in Supplementary Fig. 4.
No significant relation was found between moderate and severe exacerbations and sPIF. However, participants with suboptimal PIF on average had a 73% higher severe exacerbation rate compared with participants with optimal PIF (p = 0.167). This finding is in line with the literature for patients discharged from hospital following a disease exacerbation and indicates the need to assess whether a patient’s PIF is satisfactory at every given opportunity, to prevent a sustained worsening of the patient’s health status.
The proportion of participants with a suboptimal PIF does not increase with increasing resistance of the inhaler.
This might appear to be counter intuitive. Our video analysis showed that a proportion of participants inhaled too forcefully especially with low resistance inhalers, which can potentially lead to an unwanted higher drug deposition in the oropharynx and potentially greater oral bioavailability affecting the therapeutic index30. Another explanation could be that participants feel more resistance when using the high resistance devices and therefore put more effort in their inhalation to overcome the resistance. They feel they need to “work” to inhale their medication and consequently do so.
Adherence to inhaled medication has been shown to be an important factor in disease outcomes31, and in our study, this indeed appeared to be related to CCQ scores and exacerbations. After correcting the associations for potential confounders, these associations did not continue to be significant. An explanation could be that we assessed adherence cross-sectionally and related this to disease outcomes. The nature of this study does not allow to describe causality. Another explanation could be that the assessment of adherence using the TAI is not specific enough, as currently electronic monitoring is considered the gold standard. However, one could discuss that the TAI has been validated and has been proven related to outcomes. Nevertheless, these studies did not correct for the breadth of variables we have been able to do in PIFotal. Therefore, the relations between (different types) of adherence need to be studied further. Response bias may also have played a role as those who were completely non-adherent despite a poor disease status are expected to show lower response rates. This study highlights that there is a complex relation between adherence and outcomes, and future adherence studies should measure potential confounders carefully to be able to disentangle the specific contribution of adherence to outcomes.
This study assessed inhaler technique errors in over 1400 participants with COPD using video recording and independent scoring. We assessed all inhaler errors and linked them to outcomes. Previous studies in COPD used predefined critical errors based on literature or expert opinion16. The errors reported in the large COPD study by Molimard et al. showed some overlapping errors to be related to outcomes, but there were also differences16. Our study did not highlight the importance of correct exhalation before inhalation, as was the case in Molimard’s study. If a patient does not exhale fully, forceful inhalation might not be possible due to lack of the inhaled volume30. In our study, this theoretical error was not related to outcomes. This warrants further discussion on the matter of which errors are actual critical errors. Further, future research needs to consider the role of patient perceptions and habits in using their device, on their actual technique, i.e. whether they feel, from experience that they need to use a deep and forceful breath or not when using their inhaler. It should not be underestimated that there is in fact a different sensation that participants experience in using devices of different internal resistance.
The findings in PIFotal complement previous findings on inhalation technique32, particularly from the CRITIKAL asthma study, emphasizing the importance of targeting inhaler training to reduce key critical errors22. This seems confirmed by a very recent publication, demonstrating that the incorporation of PIF-guided inhalation therapy into COPD treatment plan could reduce the risk of severe acute exacerbation33.
The strengths of PIFotal include the real-world design carried out in multiple countries and including a large group of participants with COPD.
We cannot exclude residual confounding, although our analyses were adjusted for all selected potential confounders based on literature and clinical expertise. One of the anticipated biases was the bias by indication for ICS-containing medication for those participants who experience exacerbations. We cannot exclude indication bias to have played a role either. Those with worse disease status may have received more ICS-containing regimes leading to device selection. However, sensitivity analyses showed that the association between a suboptimal PIF and CCQ was most accentuated and statistically significant in participants receiving triple therapy through an Ellipta inhaler (0.705 (0.175–1.235, p = 0.0092) (Supplementary Fig. 5), which supports our findings, although effect modification by full medication regimen for suboptimal PIFR on CCQ was not significant (p = 0.4186) (Supplementary Table 7).
Significant associations were found between suboptimal PIF and health status. It is, however, worth emphasizing that because of the current research design, the relations should be interpreted with caution, as they may not have a causal character.
Another limitation is that all measurements were performed in different countries. Although the multinational approach is very much a strength of PIFotal, it seems possible that inhalation instructions differ between countries. Although all investigators confirmed that the instruction for a maximal PIF was ‘for participants to do the very best they can’, and all investigators confirmed that this message had come across to all study participants, it is possible that small semantic differences in the instructions have occurred.
One could challenge our statistical method of multiple imputation to handle missing data. Fortunately, there was only missing data on PIF in 3.1% of the cases, implying that the risks accompanying multiple imputation were small.
Although it is not a limitation, it is worth emphasizing that this study was performed during the COVID-19 pandemic. This may have influenced some of the outcomes, for instance, the rate of exacerbations may not be entirely representative for a regular non-pandemic year34, and may also present a bias in terms of participants willing to partake in research at this time.
In this study, suboptimal PIF was significantly associated with poorer health status. It highlights the importance of selecting the optimal device for the patient, and PIF being a key parameter in this device selection. There was a trend but no significant association of sPIF and exacerbations. PIF is an important factor not to be overlooked when prescribing a DPI to COPD patients. The proportion of participants with a suboptimal PIF (29%) stresses the importance of taking PIF into account when selecting an DPI inhaler device. This is especially the case in the 13% of participants who cannot generate a sufficient inspiratory flow. As suboptimal PIF is shown to be associated with poorer health status, it is deemed valuable to assess whether PIF is sufficient at every given opportunity, as by doing so, healthcare professionals may be able to prevent sustained worsening of patients’ health status. Further research may elucidate which interventions targeting PIF are of use in selecting inhalation medication devices for COPD patients.
Data availability
The data that support the findings of this study are available on request from the corresponding author J.K.
Code availability
The custom code that is deemed central to the findings and conclusion in this manuscript are available on request from the corresponding author J.K.
References
Lopez, A. D. et al. Chronic obstructive pulmonary disease: current burden and future projections. Eur. Respir. J. 27, 397–412 (2006).
GOLD. 2020 REPORT Prevention of Chronic Obstructive Pulmonary Disease 1–141 (2020).
Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. www.goldcopd.org (2020).
Levy, M. L. et al. Understanding dry powder inhalers: key technical and patient preference attributes. Adv. Ther. 36, 2547–2557 (2019).
Barr, R. G. et al. Comorbidities, patient knowledge, and disease management in a national sample of patients with COPD. Am. J. Med. 122, 348–355 (2009).
Bonini, M. & Usmani, O. S. The importance of inhaler devices in the treatment of COPD. COPD Res. Pract. 1, 9 (2015).
Ghosh, S., Ohar, J. A. & Drummond, M. B. Peak inspiratory flow rate in chronic obstructive pulmonary disease: implications for dry powder inhalers. J. Aerosol. Med. Pulm. Drug Deliv. 30, 381–387 (2017).
Ghosh, S., Pleasants, R. A., Ohar, J. A., Donohue, J. F. & Drummond, M. B. Prevalence and factors associated with suboptimal peak inspiratory flow rates in COPD. Int. J. COPD 14, 585–595 (2019).
Loh, C. H., Peters, S. P., Lovings, T. M. & Ohar, J. A. Suboptimal inspiratory flow rates are associated with chronic obstructive pulmonary disease and all-cause readmissions. Ann. Am. Thorac. Soc. 14, 1305–1311 (2017).
Duarte, A. G. et al. Spirometry measurement of peak inspiratory flow identifies suboptimal use of dry powder inhalers in ambulatory patients with COPD. Chronic Obstr. Pulm. Dis.: J. COPD Found. 6, 246 (2019).
Malmberg, L. P., Rytilä, P., Happonen, P. & Haahtela, T. Inspiratory flows through dry powder inhaler in chronic obstructive pulmonary disease: age and gender rather than severity matters. Int. J. Chronic Obstr. Pulm. Dis. 5, 257–262 (2010).
Sulaiman, I. et al. Irregular and ineffective: a quantitative observational study of the time and technique of inhaler use. J. Allergy Clin. Immunol.: Pract. 4, 900–909.e2 (2016).
Capstick, T. G. & Clifton, I. J. Inhaler technique and training in people with chronic obstructive pulmonary disease and asthma. Expert Rev. Respir. Med. 6, 91–103 (2012).
Sanchis, J., Gich, I., Pedersen, S. & Sci, M. Systematic review of errors in inhaler use has patient technique improved over time? https://doi.org/10.1016/j.chest.2016.03.041 (2016).
Kocks, J. W. H. et al. Systematic review of association between critical errors in inhalation and health outcomes in asthma and COPD. Prim. Care Respir. Med. 28, 43 (2018).
Molimard, M. et al. Chronic obstructive pulmonary disease exacerbation and inhaler device handling: real-life assessment of 2935 patients. Eur. Respir. J. 49, 1601794 (2017).
Usmani, O. S. et al. Critical inhaler errors in asthma and COPD: a systematic review of impact on health outcomes. Respir. Res. 19, 1–20 (2018).
Mueller, S. et al. Non-persistence and non-adherence to long-acting COPD medication therapy: a retrospective cohort study based on a large German claims dataset. Respir. Med. 122, 1–11 (2017).
Price, D. et al. Factors associated with appropriate inhaler use in patients with COPD—lessons from the REAL survey. Int. J. Chronic Obstr. Pulm. Dis. 13, 695 (2018).
Duarte-De-Araújo, A., Teixeira, P., Hespanhol, V. & Correia-De-Sousa, J. COPD: understanding patients’ adherence to inhaled medications. Int. J. COPD 13, (2018).
Sulaiman, I. et al. Objective assessment of adherence to inhalers by patients with chronic obstructive pulmonary disease. https://doi.org/10.1164/rccm.201604-0733OC. (2017).
Price, D. B. et al. Inhaler errors in the CRITIKAL study: type, frequency, and association with asthma outcomes. J. Allergy Clin. Immunology: Pract. 5, 1071–1081.e9 (2017).
Leving, M. et al. Impact of PIF, inhalation technique and medication adherence on health status and exacerbations in COPD: protocol of a real-world observational study (PIFotal COPD study). Pulm. Ther. 7, 591–606 (2021).
Sanders, M. J. Guiding inspiratory flow: development of the in-check DIAL G16, a tool for improving inhaler technique. Pulmonary Medicine. 2017, (2017)
Harb, H. S., Laz, N. I., Rabea, H. & Abdelrahim, M. E. A. Prevalence and predictors of suboptimal peak inspiratory flow rate in COPD patients. Eur. J. Pharm. Sci. 147, 105298 (2020).
Plaza, V. et al. Validation of the “test of the adherence to inhalers” (TAI) for asthma and COPD patients. J. Aerosol Med. Pulm. Drug Deliv. 29, 142–152 (2016).
Ställberg, B., Nokela, M., Ehrs, P. O., Hjemdal, P. & Jonsson, E. W. Validation of the clinical COPD questionnaire (CCQ) in primary care. Health Qual. Life Outcomes 7, 26 (2009).
Simes, R. J. An improved Bonferroni procedure for multiple tests of significance. Biometrika 73, 751–754 (1986).
Kocks, J. W. H. et al. Health status measurement in COPD: the minimal clinically important difference of the clinical COPD questionnaire. Respir. Res. 7, 62 (2006).
Laube, B. L. et al. What the pulmonary specialist should know about the new inhalation therapies. Eur. Respir. J. 37, 1308–1331 (2011).
Mäkelä, M. J., Backer, V., Hedegaard, M. & Larsson, K. Adherence to inhaled therapies, health outcomes and costs in patients with asthma and COPD. Respir. Med. 107, 1481–1490 (2013).
Vanoverschelde, A., van der Wel, P., Putman, B. & Lahousse, L. Determinants of poor inhaler technique and poor therapy adherence in obstructive lung diseases: a cross-sectional study in community pharmacies. BMJ Open Respir. Res. 8, 1–9 (2021).
Chen, S. Y. et al. Peak-inspiratory-flow-rate guided inhalation therapy reduce severe exacerbation of COPD. Front. Pharmacol. 12, 1–9 (2021).
Alsallakh, M. A. et al. Impact of COVID-19 lockdown on the incidence and mortality of acute exacerbations of chronic obstructive pulmonary disease: national interrupted time series analyses for Scotland and Wales. BMC Med. 19, 1–10 (2021).
Acknowledgements
The study sponsor was the General Practitioners Research Institute; data collection and analysis were performed by General Practitioners Research Institute. Boehringer Ingelheim was the funding and scientific partner. The members of the PIFotal study group would like to acknowledge Dr. Judith Cohen for her assistance with review, drafting and editing of the paper, and Dr. Jaco Voorham from Data to Insights Research Solutions for his assistance with the statistical analyses. They would also like to thank all contributing researchers: Maria João Barbosa, Ana Margarida Cruz, Liliana Silva, Duarte Araújo, Eurico Silva, Daniel Castro, João Ramires, Ana Fernandes, Catarina Carvalho, Raquel Castro, Jerzy Zientek, Ewa Pasko, Witold Drzastwa, Tomasz Kachel, Kornelia Ciekalska, Krzysztof Wytrychowski, Bernard Panaszek, Krzysztof Kowal, Ebian Brill, Willemien Feenstra, Geert Struik, Hans Schuurman, Mariette van Oostrum, Hennie Holwerda Meekma, Boudewijn Dierick, George Amofa, Esther Kuipers, Lennard Ringnalda, Boris Tyndall, Mark Drenth, Peter Mast, Hilbert Talsma, Raoul Wolfs, Cobie Hoogeboom, Hanneke van Andel, Paul Stoutenberg, Nancy van de Laak, Tessa Hillaert, Laura Holtzer, Natascha Fehrmann, Anniek Makkinga – Maassen van den Brink, Annemarie Hilbink, Erik Feenstra, Murat Tek, Sabrina Burer, Jan van Ginkel, Rinze Boersma, Alyssa Bonger, Miguel Roman Rodriguez, Marina García Pardo, Alejandra Valero Suau, Laura López Velasco, Cecilia Amato, Francisco Palmer Simó, Alberto Abenza, Rosa Llull Vila, Bartolomé Llompart Van Belzen, Silvia Jimeno Martínez, Francesc Moranta Ribas, Margarita Perelló Oliver, Yolanda Gómez López, Patricia Ibañez Gómez, María Nieves Mendieta Lagos, Laura Bueno López, Virginia María Mirabal Sánchez, Ana Delia Rodríguez Delgado, Nils Fischer, Alicia González Sansó, Nayra Ramírez Mendoza, Valeria Gazzaneo, Paula Merced Guillama Rodríguez, Virginia Naranjo Guerrero, Jose Angel Suarez Caballero, Isidoro Souto Bethencourt, Juan R. Dominguez Beatell, Elena Vanesa Rojas Manrique, Maria Jose Sanz Orejas, Cary Perez Lorenzo, Jesús Antonio Pérez Jiménez, Silvia Lara Afonso Trujillo, Bartolomé Dominguez Del Río Boada, Stavroula Papageorgakopoulou, Eleytheria Vakouti, Claire Gkatzoudi, Thodoris Krasanakis, Dimitris Kounalakis, Izoldi Bouloukaki, Nikolaos Tsakountakis, Emmanouela Chronaki, Katherine Mary Borg and Kamila Abutalieva for their time and efforts to perform the study measurements and complete patient inclusion, even in the challenging times of the pandemic. Finally, they would like to thank the participants who generously gave their time to participate in the study.
Author information
Authors and Affiliations
Contributions
M.L., H.W., A.d.l.H., S.B.-A., R.D., A.G., F.L., J.M., D.P., M.R.R., I.T., O.U., B.W. and J.K. conceptualized the study. M.L., J.v.C., J.c.d.S., B.C., L.D., E.D., M.G.P., R.G., I.v.G.-P., I.v.d.H., M.H., Y.J., M.K., T.M., J.M., B.M., K.S., N.S., B.W., H.W. and J.K. and the contributing sites collected the data. M.L., J.v.C., L.D., E.D., I.v.G.-P., I.v.d.H., M.H., Y.J., M.K., J.M. B.M., K.S., N.S., B.W., H.W. and J.K. analysed the data. All authors ensured the accuracy of analysis. The first draft of the manuscript, which was revised, edited and agreed for submission by all authors. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. J.K. is the guarantor.
Corresponding author
Ethics declarations
Competing interests
J.K. reports grants, personal fees and non-financial support from AstraZeneca, GSK and Boehringer Ingelheim; grants and personal fees from Chiesi Pharmaceuticals and TEVA; grants from Mundipharma; personal fees from MSD and COVIS Pharma; and also holds 72.5% of shares in the General Practitioners Research Institute. H.W. has received grants from Boehringer Ingelheim, which is the financial and scientific partner of GPRI for the submitted study, and from AstraZeneca, Novartis and Chiesi for scientific projects in the area of COPD/asthma. S.B.-A. has received grants from TEVA, and personal fees from TEVA, Boehringer Ingelheim, AstraZeneca, GSK, Sanofi and Mylan. J.C.d.S. reports or personal fees from AstraZeneca, Bial, Boehringer Ingelheim, GSK, Medinfar, Mundipharma and Sanofi. B.C. received honorarium from GSK and Sanofi. J.v.C., L.D., I.v.G.-P., I.v.d.H., Y.J., M.K., B.M., K.S., N.S., M.H., B.M. and M.T.L. were employed by General Practitioners Research Institute (GPRI) at the time of the study. In the past three years (2019–2021), GPRI conducted investigator- and sponsor-initiated research funded by non-commercial organizations, academic institutes, and pharmaceutical companies (including AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Mundipharma, Novartis, and Teva). R.D. has received grants and personal fees from TEVA, Boehringer Ingelheim, AstraZeneca, GSK, Chiesi, Focus Care, and Glenmark. R.G. has received personal fees from AstraZeneca, GSK and Chiesi. E.D. holds 27.5% of shares in the General Practitioners Research Institute. M.G.P. receives grants from AstraZeneca, GSK and Boehringer Ingelheim. A.G. and A.d.l.H. are employees of Boehringer Ingelheim. F.L. received grants and personal fees from GSK, personal fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Menarini International, Novartis, Orion, and Trudell International, outside the submitted work. T.M. is an Assoicate Editor at npj Primary Care Respiratory Medicine. J.M. received grants from Boehringer Ingelheim, during the conduct of the study; and grants from AstraZeneca, Chiesi, Novartis, and GSK, outside the submitted work. D.P. reports grants and personal fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Mylan, Novartis, Regeneron Pharmaceuticals, Sanofi Genzyme, Theravance and Zentiva (Sanofi Generics); grants from the British Lung Foundation, Respiratory Effectiveness Group, UK National Health Service, and AKL Research and Development Ltd; personal fees from Cipla, GlaxoSmithKline, Kyorin, Merck, Mundipharma, Airway Vista Secretariat, EPG Communication Holdings Ltd, FIECON Ltd, Fieldwork International, OM Pharma SA, PeerVoice, Phadia AB, Spirosure Inc, Strategic North Limited, Synapse Research Management Partners S.L., Talos Health Solutions, and WebMD Global LLC; non-financial support from Efficacy and Mechanism Evaluation programme and Health Technology Assessment; stock/stock options from AKL Research and Development Ltd, which produces phytopharmaceuticals; owns 74% of the social enterprise Optimum Patient Care Ltd (Australia and UK) and 92.61% of Observational and Pragmatic Research Institute Pte Ltd (Singapore); and 5% shareholding in Timestamp, which develops adherence monitoring technology. M.R.-R. receives grants and personal fees from AstraZeneca and GSK; and personal fees from Boehringer Ingelheim, Chiesi, Menarini, Mundipharma, Novartis, Pfizer, TEVA and BIAL. I.T. reports grants and personal fees from GSK, AstraZeneca, Boehringer Ingelheim, Menarini, Novartis, Chiesi and Elpen. O.U. reports grants and personal fees from AstraZeneca, Boehringer Ingelheim, Edmond Pharma, Chiesi and GSK; grants from Edmond Pharma; and personal fees from Napp, Mundipharma, Sandoz, Takeda, Cipla, COVIS, Novartis, Mereobiopharma, Orion, and Menarini. S.B.-A. and T.M. are Associate Editors at npj Primary Care Respiratory Medicine, and I.T. is Editor in Chief. These authors were not involved in the journal’s review of, or decisions related to, this manuscript.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
W. H. Kocks, J., Wouters, H., Bosnic-Anticevich, S. et al. Factors associated with health status and exacerbations in COPD maintenance therapy with dry powder inhalers. npj Prim. Care Respir. Med. 32, 18 (2022). https://doi.org/10.1038/s41533-022-00282-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41533-022-00282-y
This article is cited by
-
Aerosol Plumes of Inhalers Used in COPD
Pulmonary Therapy (2024)
-
Demystifying Dry Powder Inhaler Resistance with Relevance to Optimal Patient Care
Clinical Drug Investigation (2024)
-
Identifying critical inhalation technique errors in Dry Powder Inhaler use in patients with COPD based on the association with health status and exacerbations: findings from the multi-country cross-sectional observational PIFotal study
BMC Pulmonary Medicine (2023)
-
Clinical recommendations for dry powder inhaler use in the management of COPD in primary care
npj Primary Care Respiratory Medicine (2022)