Abstract
Over 1400 patients using dry powder inhalers (DPIs) to deliver COPD maintenance therapies were recruited across Europe and Australia. Their peak inspiratory flow (PIF) was measured, inhaler technique was observed, and adherence to treatment assessed. From relating the findings with patient health status, and thereby identifying critical errors, key clinical recommendations for primary care clinicians were determined, namely – measure PIF before prescribing a DPI to ensure inhalation manoeuvre ability is well-matched with the device. Some patients could benefit from inhalation training whereas others should have their DPI changed for one better suited to their inspiratory ability or alternatively be prescribed an active device (such as a soft mist inhaler or pressurized metered dose inhaler). Observing the inhalation technique was valuable however this misses suboptimal PIF (approaching one fourth of patients with a satisfactory observed manoeuvre had a suboptimal PIF for their DPI). Assess adherence as deliberate non-adherence can point to a mismatch between a patient and their inhaler (deliberate non-adherence was significantly associated with PIFs below the minimum for the DPI). In-person observation of inhalation technique was found to be inferior to video rating based on device-specific checklists. Where video assessments are not possible, observation training for healthcare professionals would therefore be valuable particularly to improve the ability to identify the critical errors associated with health status namely ‘teeth and lips sealed around mouthpiece’, ‘breathe in’ and ‘breathing out calmly after inhalation’. However, it is recommended that observation alone should not replace PIF measurement in the DPI selection process.
Trial registration: https://clinicaltrials.gov/ct2/show/NCT04532853.
Similar content being viewed by others
Introduction
The effectiveness of dry powder inhaler (DPI) maintenance therapy in chronic obstructive pulmonary disease (COPD) patients is associated with a complex constellation of factors, as assessed in the cross-sectional observational multinational PIFotal study1,2. This study found that suboptimal peak inspiratory flow (sPIF) and inhalation technique errors were associated with poor health status in COPD patients, whereas DPI adherence was not associated to poor health status2. According to the most recent International Primary Care Respiratory Group research prioritisation exercise3, significant evidence gaps remain within the study of respiratory diseases, coinciding with a lack of evidence-based guidelines, quality standards and training to support primary care for patients with respiratory diseases, such as COPD. Drawing on the views of primary healthcare professionals (HCPs) worldwide, there is a need for more effective clinical education in order to deliver best-practice primary care. With this in mind, we provide clinical recommendations from the PIFotal study – summarizing for primary care clinicians, a holistic approach to the clinical care of COPD patients using DPI maintenance therapies – taking into consideration PIF, inhalation technique and adherence to treatment.
The PIFotal study indicated that 29% of the COPD patients had insufficient inspiratory flow (a PIF lower than required for their DPI) during a typical day-to-day inhalation manoeuvre2. DPIs are breath-actuated devices and the amount of medication reaching the lungs depends upon the aerosol characteristics created by the patient’s inspiratory manoeuvre overcoming the internal resistance of the device and dispersing the dry powder medication, separating drug from carrier particles4. When the inspiratory effort is insufficient drug deposition in the lungs is reduced, compromising the effectiveness of the prescribed medication4. Therefore, for patients who exhibit insufficient inspiratory flow, there is a need to either deliver tailored instructions targeting at producing sufficient inspiratory flow5 or these patients should be switched to an alternative inhaler better suited to the patient’s inspiratory ability; also considering devices which do not depend upon a patient’s inspiratory ability, such as a soft mist inhaler (SMI), pressurized metered dose inhaler (pMDI), or a portable nebulizer. From a prescriber’s perspective, selecting a DPI fitting both patients’ needs and preferences is a complex decision, which should be reached together with the patient. However, among other factors such as inhalation technique, inhaler design and the environmental impact (including the plastic burden), PIF is one of the key factors to consider as sPIF can result in higher all-cause and COPD hospital readmissions6. It is known that inspiratory ability is compromised in COPD patients who experience exacerbations, and that exacerbations are associated with the inability to generate sufficient PIF for a DPI7. There is, however, little information about the best clinical practice to tailor individualised device selection vis-à-vis the necessity of taking PIF into consideration. Second, it is unclear whether it is important to measure PIF e.g., with portable assessment tools such as the In-Check DIAL G16 (Clement Clarke, UK) or whether observation of the inspiratory flow manoeuvre is sufficient to evaluate whether a DPI is well-matched to the patient.
Another important consideration in device selection is the patient’s ability, likelihood, desire to be adherent to their treatment regimen. A prior observational study found that deliberate non-adherence was linked to the inability to generate sufficient inspiratory flow for a DPI8. More importantly, patients with low PIF (<35 L/min) stopped using their inhaler more often than patients with sufficient PIF8. The association between PIF and adherence was therefore explored in PIFotal, where the medication adherence was poor in the overall study population2.
The PIFotal study revealed that inhalation technique errors were common and associated with worse health status2. In addition, a higher cumulative number of inhalation technique errors was associated with higher COPD-related healthcare costs9. These findings highlight the importance of correct DPI handling in managing COPD. Nevertheless, inhalation technique is often not assessed when selecting a DPI10, and there is currently no consensus about how such assessments should take place in daily clinical practice. Therefore, in this study we provide recommendations for clinical practice to assess inhalation technique in primary care.
Methods
Study design
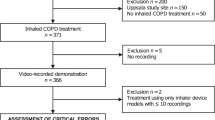
The PIFotal study (clinicaltrials.gov identifier NCT04532853) was a cross-sectional observational real-world study in six countries (Australia, Greece, the Netherlands, Poland, Portugal, Spain)1. Patients were included in the study between October 2020 and May 2021. Local medical ethics committees reviewed and approved the study protocol, and all patients provided written informed consent. A description of the study procedures is available in Supplementary Fig. 1.
Study population
A minimalist approach to the inclusion/exclusion criteria was used in order to ensure a real-world setting as much as possible. Patients were eligible for participation when clinically diagnosed with COPD, aged 40 years or older and treated with a DPI as maintenance therapy for their COPD in the previous 3 months or longer. Patients were excluded when they were unable to provide informed consent, were participating in other trials with COPD medication, experienced an exacerbation in the 6 weeks prior to participation, or had a life-threatening disease with a life expectancy <6 months.
Peak inspiratory flow (PIF)
PIF (L/min) was objectively assessed with the In-Check DIAL G16 (Clement Clarke, UK), a multi-patient device set to resemble the internal resistance of the patient’s inhaler during an inhalation manoeuvre (with 6 settings of either a pMDI, low, medium low, medium, medium-high, or high resistance DPI device). If a patient used multiple DPIs for their maintenance therapy, a priority list based on the prevalence of DPIs in the participating countries (prioritising the more common inhalers to obtain the most representative and generalisable data) determined which DPI and corresponding internal resistance was used for the PIF assessment (Table S2)
PIF was assessed in three ways: 1) day-to-day typical PIF at the resistance of the patient’s DPI, 2) maximum PIF at the resistance of the patient’s DPI 3) maximum PIF at low internal resistance2. For the typical PIF measurement, participants were asked to inhale once with the In-Check DIAL G16 as they would normally do when using their DPI. For both maximum PIF measurements, participants were instructed to breathe out completely to empty the lungs, and then inhale as forcefully and fast as possible. Maximum PIF measurements were performed twice, and the highest PIF measurement was included in the data analysis. The following definitions of PIF were used in the analysis:
-
‘sPIF’: as typical PIF being lower than optimal for their device (for the cut-off values, Supplementary Table 1)
-
‘Low PIF’: as typical PIF being lower than minimally required for their device (for the cut-off values, Supplementary Table 1)
Furthermore, three clinically relevant groups were defined based on the PIF measurements:
-
‘Can and will do’: patients with a typical PIF ≥ than the optimal PIF for their device
-
‘Can, but will not do’: patients with a typical PIF below the optimal PIF for their device, but able to generate maximum PIF ≥ the optimal PIF
-
‘Cannot do’: patients with both their typical and maximum PIF < optimal PIF for their device
Inhalation technique errors
Inhalation technique was observed and documented by video recording which was rated offline for errors by two independent observers. Checklists on inhaler-specific and inhaler-independent commonly made errors were used that were based on recommendations of the Netherlands Lung Alliance (www.inhalatorgebruik.nl) or, if unavailable for specific devices, the Aerosol Drug Management Improvement Team (www.inhalers4u.org). Differences between the two independent observers were resolved by discussion. In case non-consensus was reached, a third independent expert was consulted to reconcile the disagreement. The inhalation technique errors were dichotomous variables (‘yes’ / ‘no’ error observed). Inhalation steps marked as not applicable for the device were ‘no’ error. Inhalation technique was evaluated by grouping errors in steps together in 12 categories (Supplementary Table 2). For this study, we specifically focused on the errors ‘Breathe in incorrect’, ‘Teeth and lips sealed mouthpiece incorrect,’ and ‘Breathing out after inhalation incorrect’, as these errors were deemed ‘critical’ based on their frequency and significant association with worse health status in the PIFotal study2.
Medication adherence
Adherence to the inhalers was assessed with the 12-item Test of Adherence to Inhalers11. Items 1 to 10 were answered by the patients and scored on a 5-point Likert scale (1–5). Item 11 was answered by the HCP performing the study visit. Due to precise offline assessments of inhalation technique, item TAI-12, concerning in-person clinician reported ‘critical’ inhalation technique errors, was replaced with the offline assessment of inhalation technique based on the video recordings. Of specific focus in this study were deliberate non-adherence, defined as TAI items 6 to 10 score <25, and item TAI-12 (i.e., the HCP assessed whether the patient revealed a critical inhalation technique error or if inhalation technique was correct during the visit).
Health status
COPD-related health status was measured with the 10-item self-administered Clinical COPD Questionnaire (CCQ), consisting of three domains: symptoms, functional status, and mental health12. The CCQ score is the mean score of 10 item-scores, where each item is scored on a 7-point Likert scale (0–6), with higher scores indicating worse health status.
Statistical analyses
Patient characteristics (including demographic variables, medication regimen, comorbidities) and PIF, adherence, inhalation technique error frequency and health status were described using descriptive statistics.
In order to define clinical practices aimed to optimise the use of DPIs, the following analyses were conducted, and recommendations proposed:
-
1.
The proportion of patients within the three clinically relevant groups (can and will do, can but will not do, cannot do) was identified, and to simulate the impact of an exacerbation, the proportions were derived following a 20% reduction in a typical and maximal PIF13.
-
2.
The association between the objectively measured sPIF with the In-Check DIAL G16 (Clement Clarke, UK), the observed error ‘Breathe in’ and health status (CCQ) was assessed with a linear regression model adjusted for potential confounders (Supplementary Table 3). Patients were categorized into four groups based on their PIF (optimal or suboptimal) and whether the error ‘Breathe in’ was observed (yes/no), which was regressed on the clinical outcome CCQ.
-
3.
The association between deliberate non-adherence and low PIF (below the minimum flow for the device) was assessed with a logistic regression model adjusted for potential confounders (Supplementary Table 3). The odds-ratios (OR) and 95% confidence interval (95% CI) of having a low PIF was compared for deliberate non-adherent (TAI items 6 to 10) and adherent patients.
-
4.
The agreement between clinician reported ‘critical’ inhalation technique (TAI item 12) and ‘critical’ error rating based on the video recordings using checklists was determined.
A sample size calculation was performed before study execution for the main study objectives1,2, and not specifically for these post-hoc analyses defined to deduce clinical recommendations from the PIFotal study. All statistical analyses were performed using Stata version 17.
The PIFotal COPD study protocol received approvals from the following institutional ethics committees/institutional review boards: Australia: Human Research Ethics Committee (HREC 3) University of Sydney; Greece: Research Ethics Committee University of Crete; Poland: Komisja Bioetyczna przy Beskidziej Izble Lekarskiej – Bielsko Biala; Komisji Bioetycznej przy Śląskiej Izbie Lekarskiej; Silesian Medical Society (Śląska Izba Lekarska); Bioethics Committee at Lower Silesian Medical Association; Bioethics Committee at the Medical University of Biaystok; Portugal: North Health Regional Administration (ARS Norte); Matosinhos Local Health Unit (ULS Matosinhos); Guimarães Hospital; Center Health Regional Administration (ARS Centro); Regional Health Administration of Lisbon and Tagus Valley (ARS LVT); Spain: Comité de Ética de la Investigación (CEI) Islas Baleares; CEI Hospital Universitario de Gran Canaria; The Netherlands: Medisch Ethische Toetsingscommissie (METC) Assen exempted this study.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Results
Study population
A total of 1434 patients were included in the study and provided signed informed consent. An overview of the selection of the study population can be found in Supplementary Fig. 2.
Patient characteristics are shown in Table 1. Of these patients, 50.1% were female and the mean (SD) age was 69.2 (9.3) years.
The proportion of patients with suboptimal PIF by device resistance, and potential interventions based on PIF
PIF measurements were available for 1389 patients. 71% (n = 987) of the patients were able to generate sufficient inspiratory effort for their device (‘can and will do’), whereas 16% (n = 219) were able to generate sufficient inspiratory effort but failed to do so (‘can, but will not do’), and 13% (n = 183) of the patients were revealed insufficient inspiratory effort (‘cannot do’) (Fig. 1, left).
‘Can and will do’: patients with a typical PIF ≥ than the optimal PIF for their device; ‘Can, but will not do’: patients with a typical PIF below the optimal PIF for their device, but able to generate maximum PIF ≥ the optimal PIF; ‘Cannot do’: patients with both their typical and maximum PIF < optimal PIF for their device.
In a scenario resembling the compromised PIF during an exacerbation, with a 20% reduction of the typical and the maximum PIF13, 24% of the total population who were initially in the ‘can and will do’ PIF category, would potentially not be able to perform sufficient inspiratory effort to perform a proper inhalation manoeuvre. Specifically, 62% of the patients in the ‘can, but will not do’ group would be categorized as ‘cannot do’ because of an exacerbation. Thus, 34% of all patients in this study would not be able to perform a proper inhalation with their current device during an exacerbation, compared to 13% of patients who were not able to do this under ‘usual’ conditions (Fig. 1), Supplementary Table 4).
Patients in the ‘can and will do’ PIF category generated sufficient inspiratory effort for their device, however, regular assessments of PIF are recommended to ensure consistent inspiratory effort over time. In addition, the impact of exacerbations (Fig. 1) should be considered for these patients (a quarter of patients could be compromised during an exacerbation).
Patients in the ‘can, but will not do’ PIF category, who were able to generate sufficient inspiratory effort for their device but failed to do so, could benefit from inhalation technique training to improve their device use. Switching to an alternative DPI (first checking if the patient can achieve the optimal PIF required) or an active device (SMI or pMDI) could be considered particularly if the patient has a higher exacerbation risk.
Patients in the ‘cannot do’ group could benefit from a different inhaler; either an alternative DPI better suited to the patient’s inspiratory ability, or an active device (SMI or pMDI) (Fig. 2).
Association between suboptimal PIF measured with an In-Check Dial G16 and the observed ‘Breathe in’ error with health status
In 44.8% of the patients, both the measured PIF was optimal, and the observed inspiratory manoeuvre was correct. 14.9% of the patients exhibited both an sPIF and were observed to have an ‘Breathe in’ technique error (Fig. 3). In 40.3% of the patients, there was a discrepancy between the objective PIF assessment and the observed ‘breathe in’ error.
Even when PIF was observed to be sufficient, as assessed with video (n = 817), 24% of patients (n = 195) had sPIF, as objectively measured with In-Check G-16. Notably, patients with the ‘breathe in’ step scored as correct with video, but with sPIF objectively measured with an In-Check Dial G-16, had significantly worse health status compared to patients with optimal PIF and sufficient inspiratory effort based on the video (CCQ score 0.19; 95 % CI [0.03, 0.35]; p = 0.02) (Fig. 3).
As observation alone does not accurately identify patients with sPIF (a factor associated with significantly worse health status2). The recommendation therefore would be to measure PIF objectively (where possible) and follow the decision tree in Fig. 2.
Association between deliberate non-adherence and low PIF
Circa 8% of the patients had a low PIF (below the minimal PIF value required for their device, Table 2). Deliberately non-adherent patients were almost twice as likely to have a low PIF compared to those who were adherent (OR 1.94, 95% CI [1.26, 3.00] p = <0.01; Table 2).
It is therefore recommended to question patients on adherence as deliberate non-adherence could be an indicator of low PIF, this being especially useful when there is a lack of resources to measure PIF.
The agreement between ‘critical’ inhalation technique errors from TAI-12 and from the video recordings using checklists
The agreement between in-person inhalation technique assessment by the HCP (TAI-12) and the standardised assessment of video recordings (focussing on errors ‘Teeth and lips sealed around mouthpiece incorrect’; ‘Breathe in incorrect’; ‘Breathing out calmly after inhalation incorrect’)2 by two trained researchers was low: 54% agreement (Table 3).
In order to detect ‘critical’ inhalation technique errors, in-person observation was found to be inferior to video rating based on standardised device-specific checklists. Where video assessments are not possible, it is recommended that clinicians should be trained to improve their ability to identify ‘critical’ errors namely ‘teeth and lips sealed around mouthpiece’, ‘breathe in’ and ‘breathing out calmly after inhalation’.
Discussion
This study provided insight into the substantial proportion of patients with COPD with insufficient inspiratory flow for their DPI, 29% in a stable condition potentially rising to a possible 53% in case of exacerbations. The first clinical recommendation regarding device selection to improve health status of patients with COPD on DPI maintenance therapy in primary care, would be to measure PIF, in addition to observing patient inhaler technique. As sPIF is associated with poorer health status, patients with insufficient inspiratory ability should be switched to an alternative inhaler better suited to the patient’s inspiratory ability, especially if the patient had a higher exacerbation risk. The alternative device, if a DPI, should be checked to see if the patient can generate the optimal flow required. Questioning regarding adherence is informative since deliberate non-adherence is associated with a PIF below minimum for device operation. In case of deliberate non-adherence, we recommend observing the inhalation manoeuvre with the view to correcting critical errors and switching the device. Finally, clinicians should be trained to accurately detect the specific ‘critical’ inhalation technique errors in patients with COPD using a DPI. If possible, recording the inhalation technique and scoring afterwards is optimal to improve the accuracy of the assessment.
We observed that especially sPIF, and to a lesser extent the inhalation technique error ‘Breathe in’, were associated with worse health status. These results are in agreement with a previous observational study that found sPIF to be predictive of all-cause and COPD-related hospital readmission in COPD patients6. sPIF could be partially accurately observed, but our findings indicate that objective PIF measurements can provide a better evaluation of a patient’s ability to use and benefit from a DPI. In the PIFotal study, insufficient inspiratory effort has been marked as a critical error for patients with COPD using a DPI. This finding is consistent with evidence from the CRITIKAL study, where this error was found to be associated with uncontrolled symptom control and increased exacerbation rate in patients with asthma14. The PIFotal study underlines the need for objective PIF measurements in clinical settings, especially in primary care where these measurements are currently scarce due to equipment and time constraints. However, the In-Check DIAL G16 could be considered a simple method of inhalation technique training, with relatively low one-off costs of around €50, excluding disposable mouthpieces. When objective measurements are not feasible, HCPs could consider patients factors and disease characteristics as determinants of PIF (such as older age, female gender, frailty)15 and their adherence to identify the most suitable device for their patients15.
Deliberately non-adherent patients were almost twice as likely to have a PIF below the minimum level required for their DPI compared to those who were adherent (OR 1.94, 95% CI [1.26, 3.00] p = <0.01, Table 2). This finding is in line with a previous study8 and might be explained by limited treatment efficacy in the case of low PIF. We hypothesize a potential vicious circle; where low PIF might lead to insufficient treatment dose into the lungs and thus limited treatment efficacy, which could result in deliberate non-adherence. Subsequently, the non-adherence might hamper a clinical response to the prescribed treatment regimen, worsen the health status, and even lead to a further reduction of PIF. However, the direction of this association could not be established with our cross-sectional study design. Although we need to be cautious when interpreting these findings, the association between deliberate non-adherence and low PIF further emphasizes the importance of PIF measurements in clinical practice.
Finally, we found that the interpretation of ‘critical’ inhalation technique errors differed widely between the in-person assessment by the HCP during the study visit, and the standardised assessment of the video recordings. The inhalation technique in the PIFotal study was recorded and subsequently assessed offline, evaluated by two independent researchers, and a consensus meeting (with a third expert) was held if needed. The approach of recording the inhalation manoeuvre allowed to pause and replay the video, which increased the focus on the inhalation technique details which are usually hard to assess during a consultation. In this way, any potential nuance in the inhalation technique can be found and be scored in more detail, especially considering that a typical inhalation manoeuvre takes place in less than five seconds. Aside from that, the video recordings could be used as a tool to improve the accuracy of error detection. The device-specific checklists used in this study would be feasible for clinical practice, with no costs and with minimal time required to ensure that all inhaler technique steps – required for delivery of the medication – are evaluated and corrected if needed. The feasibility and effectiveness of in-person observation of (dry powder) inhaler technique has been confirmed in patients with asthma. An intervention conducted in community pharmacies, targeting inhaler technique errors with a brief training based on standardized inhaler technique checklists, significantly improved inhaler technique and asthma outcomes16.
The strengths of PIFotal include the real-world design, the multinational character of the study, and our large sample of participants with COPD. This allowed us to study a wide range of DPI devices that contributes to the external validity of our findings. The robustness of the PIFotal analysis, and practical recommendations, will help HCPs improve the management of COPD.
Since this was a cross-sectional study, the direction of associations cannot be established. Furthermore, caution is needed when interpreting associations between sPIF and outcomes as PIF is a marker of muscle strength and disease severity. Although we adjusted for a comprehensive set of potential confounders, including disease severity, residual confounding might be present.
Although the international approach is considered a strength of PIFotal, and study procedures were carried out following standardized protocols, the multi-country setting may have introduced heterogeneity in our data. Given our decision tree (Fig. 2), it should be acknowledged that matching the DPI to the patients’ needs should not be solely based on PIF, but that (among others) shared decision-making regarding inhalers17, quality of disease control and inhalation technique are factors to consider5.
We provide clinical recommendations for primary care clinicians to improve their care of COPD patients by limiting the potential negative consequences of mismatching patients with inhalers. A substantial proportion of COPD patients with insufficient inspiratory flow could benefit from training targeting their peak inspiratory flow, or switching to an alternative DPI better suited to the patient’s inspiratory ability, or active devices such as pMDIs or an SMI. Objective PIF measurements (against the resistance of the patient’s DPI), rather than inspiratory manoeuvre observation ideally should guide the DPI selection process in primary care. HCPs should regularly evaluate the patient’s adherence, as deliberate non-adherence was associated with low PIF. Lastly, we concluded that HCPs should be trained to improve the ability to identify ‘critical’ inhalation technique errors in patients with COPD using a DPI.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author (JK) on request.
References
Leving, M. et al. Impact of PIF, inhalation technique and medication adherence on health status and exacerbations in COPD: protocol of a real-world observational study (PIFotal COPD Study). Pulm. Ther. 7, 591–606 (2021).
Kocks, J. W. H. et al. Factors associated with health status and exacerbations in COPD maintenance therapy with dry powder inhalers. NPJ Prim. Care Respir. Med. 32, 18 (2022).
Abdel-Aal, A. et al. Prioritising primary care respiratory research needs: results from the 2020 International Primary Care Respiratory Group (IPCRG) global e-Delphi exercise. npj Prim. Care Respiratory Med. 32, 1–12 (2022).
Telko, M. J. & Hickey, A. J. Dry Powder inhaler formulation. Respir. Care 50, 1209–1227 (2005).
Peché, R., Attar‐zadeh, D., Scullion, J. & Kocks, J. Matching the Inhaler to the Patient in COPD. J. Clin. Med. 10, 5683 (2021).
Loh, C. H., Peters, S. P., Lovings, T. M. & Ohar, J. A. Suboptimal inspiratory flow rates are associated with chronic obstructive pulmonary disease and all-cause readmissions. Ann. Am. Thorac. Soc. 14, 1305–1311 (2017).
van der Palen, J. Peak inspiratory £ow through Diskus and Turbuhaler, measured by means of a peak inspiratory £ow meter (In-Check DIAL s). Respir Med. 97, 285–289 (2003).
Sulaiman, I. et al. Objective Assessment of adherence to inhalers by patients with chronic obstructive pulmonary disease. Am. J. Respir. Critical Care Med. 195. https://doi.org/10.1164/rccm.201604-0733OC (2017).
Leving, M. et al. Suboptimal peak inspiratory flow and critical inhalation errors are associated with higher COPD-related healthcare costs. Int. J. Chron. Obstruct. Pulmon. Dis. 17, 2401–2415 (2022).
Chapman, K. R., Voshaar, T. H. & Virchow, J. C. Inhaler choice in primary practice. Eur. Respiratory Rev. 14, 117–122 (2005).
Plaza, V. et al. Validation of the ‘Test of the Adherence to Inhalers’ (TAI) for asthma and COPD patients. J. Aerosol Med. Pulm. Drug Deliv. 29, 142–152 (2016).
Ställberg, B., Nokela, M., Ehrs, P. O., Hjemdal, P. & Jonsson, E. W. Validation of the clinical COPD questionnaire (CCQ) in primary care. Health Qual. Life Outcomes 7, 1–9 (2009).
Ghosh, S., Ohar, J. A. & Drummond, M. B. Peak inspiratory flow rate in chronic obstructive pulmonary disease: implications for dry powder inhalers. J. Aerosol Med. Pulm. Drug Deliv. 30, 381–387 (2017).
Price, D. B. et al. Inhaler errors in the CRITIKAL study: type, frequency, and association with asthma outcomes. J. Allergy Clin. Immunol. Pr. 5, 1071–1081.e9 (2017).
Leving, M. T., Kocks, J., Bosnic-Anticevich, S., Dekhuijzen, R. & Usmani, O. S. Relationship between peak inspiratory flow and patient and disease characteristics in individuals with COPD—a systematic scoping review. Biomedicines 10, 458 (2022).
Basheti, I. A., Armour, C. L., Bosnic-Anticevich, S. Z. & Reddel, H. K. Evaluation of a novel educational strategy, including inhaler-based reminder labels, to improve asthma inhaler technique. Patient Educ. Couns. 72, 26–33 (2008).
Metting, E. I., van Dijk, L., El Messlaki, H., Luers, J. & Kock, J. Development of a shared decision-making tool to support patients and their healthcare provider in choosing the best inhaler device. Eur. Respiratory J. 52, OA1643 (2018).
Acknowledgements
The study sponsor was the General Practitioners Research Institute; data collection and analysis were performed by General Practitioners Research Institute. Boehringer Ingelheim was the funding and scientific partner. The members of the PIFotal study group would like to acknowledge Dr. Jaco Voorham from Data to Insights Research Solutions for his assistance with the statistical analyses, Dr Wilma Zijlema for her assistance with the review, drafting and editing of the paper, and Dr. Hans Wouters for his contribution to the project administration in the initial phase of the project. They would also like to thank all contributing researchers: Maria João Barbosa, Ana Margarida Cruz, Liliana Silva, Duarte Araújo, Eurico Silva, Daniel Castro, João Ramires, Ana Fernandes, Catarina Carvalho, Raquel Castro, Jerzy Zientek, Ewa Pasko, Witold Drzastwa, Tomasz Kachel, Kornelia Ciekalska, Krzysztof Wytrychowski, Bernard Panaszek, Krzysztof Kowal, Ebian Brill, Willemien Feenstra, Geert Struik, Hans Schuurman, Mariette van Oostrum, Hennie Holwerda Meekma, Boudewijn Dierick, George Amofa, Esther Kuipers, Lennard Ringnalda, Boris Tyndall, Mark Drenth, Peter Mast, Hilbert Talsma, Raoul Wolfs, Cobie Hoogeboom, Hanneke van Andel, Paul Stoutenberg, Nancy van de Laak, Tessa Hillaert, Laura Holtzer, Natascha Fehrmann, Anniek Makkinga – Maassen van den Brink, Annemarie Hilbink, Erik Feenstra, Murat Tek, Sabrina Burer, Jan van Ginkel, Rinze Boersma, Alyssa Bonger, Miguel Roman Rodriguez, Marina García Pardo, Alejandra Valero Suau, Laura López Velasco, Cecilia Amato, Francisco Palmer Simó, Alberto Abenza, Rosa Llull Vila, Bartolomé Llompart Van Belzen, Silvia Jimeno Martínez, Francesc Moranta Ribas, Margarita Perelló Oliver, Yolanda Gómez López, Patricia Ibañez Gómez, María Nieves Mendieta Lagos, Laura Bueno López, Virginia María Mirabal Sánchez, Ana Delia Rodríguez Delgado, Nils Fischer, Alicia González Sansó, Nayra Ramírez Mendoza, Valeria Gazzaneo, Paula Merced Guillama Rodríguez, Virginia Naranjo Guerrero, Jose Angel Suarez Caballero, Isidoro Souto Bethencourt, Juan R. Dominguez Beatell, Elena Vanesa Rojas Manrique, Maria Jose Sanz Orejas, Cary Perez Lorenzo, Jesús Antonio Pérez Jiménez, Silvia Lara Afonso Trujillo, Bartolomé Dominguez Del Río Boada, Stavroula Papageorgakopoulou, Eleytheria Vakouti, Claire Gkatzoudi, Thodoris Krasanakis, Dimitris Kounalakis, Izoldi Bouloukaki, Nikolaos Tsakountakis, Emmanouela Chronaki, Katherine Mary Borg and Kamila Abutalieva for their time and efforts to perform the study measurements and complete patient inclusion, even in the challenging times of the pandemic. Finally, they would like to thank the participants who generously gave their time to participate in the study.
Author information
Authors and Affiliations
Contributions
M.T.L., S.B.-A., R.D., A.G., F.L., J.M., D.P., M.R.-R., I.T., O.U., and J.W.H.K. conceptualized the study. M.T.L., J.v.C., J.c.d.S., B.C., L.D., M.G.P., R.G., I.v.d.H., M.H., Y.J., T.M., B.M., K.S., N.S., I.T., and J.W.H.K. and the contributing sites collected the data. M.T.L., J.v.C., L.D., I.v.d.H., Y.J., J.M., B.M., K.S., N.S., and J.W.H.K. analysed the data. All authors ensured the accuracy of the analysis. The first draft of the manuscript, which was revised, edited and agreed for submission by all authors. The corresponding author attest that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. J.W.H.K. is the guarantor.
Corresponding author
Ethics declarations
Competing interests
M.T.L., J.v.C., L.D., I.v.d.H., Y.J., B.M., K.S., N.S. were employed by General Practitioners Research Institute (GPRI) at the time of the study. In the past three years (2019–2021), GPRI conducted investigator- and sponsor-initiated research funded by non-commercial organizations, academic institutes, and pharmaceutical companies (including AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Mundipharma, Novartis, and Teva). S.B.-A. has received grants from TEVA, and personal fees from TEVA, Boehringer Ingelheim, AstraZeneca, GSK, Sanofi and Mylan. J.C.d.S. reports or personal fees from AstraZeneca, Bial, Boehringer Ingelheim, GSK, Medinfar, Mundipharma and Sanofi. B.C. received honorarium from GSK and Sanofi. R.D. has received grants and personal fees from TEVA, Boehringer Ingelheim, AstraZeneca, GSK, Chiesi, Focus Care, and Glenmark. M.G.P. receives grants from AstraZeneca, GSK and Boehringer Ingelheim. A.G. and R.A.-E. are employees of Boehringer Ingelheim. R.G. has received personal fees from AstraZeneca, GSK and Chiesi. F.L. received grants and personal fees from GSK, personal fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Menarini International, Novartis, Orion, and Trudell International, outside the submitted work. T.M. has no competing interests to declare. J.M. received grants from Boehringer Ingelheim, during the conduct of the study; and grants from AstraZeneca, Chiesi, Novartis, and GSK, outside the submitted work. D.P. reports grants and personal fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Mylan, Novartis, Regeneron Pharmaceuticals, Sanofi Genzyme, Theravance and Zentiva (Sanofi Generics); grants from the British Lung Foundation, Respiratory Effectiveness Group, UK National Health Service, and AKL Research and Development Ltd; personal fees from Cipla, GlaxoSmithKline, Kyorin, Merck, Mundipharma, Airway Vista Secretariat, EPG Communication Holdings Ltd, FIECON Ltd, Fieldwork International, OM Pharma SA, PeerVoice, Phadia AB, Spirosure Inc, Strategic North Limited, Synapse Research Management Partners S.L., Talos Health Solutions, and WebMD Global LLC; non-financial support from Efficacy and Mechanism Evaluation programme and Health Technology Assessment; stock/stock options from AKL Research and Development Ltd, which produces phytopharmaceuticals; owns 74% of the social enterprise Optimum Patient Care Ltd (Australia and UK) and 92.61% of Observational and Pragmatic Research Institute Pte Ltd (Singapore); and 5% shareholding in Timestamp, which develops adherence monitoring technology. M.R.-R. receives grants and personal fees from AstraZeneca and GSK; and personal fees from Boehringer Ingelheim, Chiesi, Menarini, Mundipharma, Novartis, Pfizer, TEVA and BIAL. I.T. reports grants and personal fees from GSK, AstraZeneca, Boehringer Ingelheim, Menarini, Novartis, Chiesi and Elpen. OU reports grants and personal fees from AstraZeneca, Boehringer Ingelheim, Edmond Pharma, Chiesi and GSK; grants from Edmond Pharma; and personal fees from Napp, Mundipharma, Sandoz, Takeda, Cipla, COVIS, Novartis, Mereobiopharma, Orion, and Menarini. J.W.H.K. reports grants, personal fees and non-financial support from AstraZeneca, GSK and Boehringer Ingelheim; grants and personal fees from Chiesi Pharmaceuticals and TEVA; grants from Mundipharma; personal fees from MSD and COVIS Pharma; and also holds 72.5% of shares in the General Practitioners Research Institute. IT is Editor-in-Chief of npj Primary Care Respiratory Medicine, and S.B.-A. and T.M. are Associate Editors. I.T., S.B.A., and TM were not involved in the journal’s review of, or decisions related to, this manuscript.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Leving, M.T., Bosnic-Anticevich, S., van Cooten, J. et al. Clinical recommendations for dry powder inhaler use in the management of COPD in primary care. npj Prim. Care Respir. Med. 32, 59 (2022). https://doi.org/10.1038/s41533-022-00318-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41533-022-00318-3
This article is cited by
-
Aerosol Plumes of Inhalers Used in COPD
Pulmonary Therapy (2024)
-
Identifying critical inhalation technique errors in Dry Powder Inhaler use in patients with COPD based on the association with health status and exacerbations: findings from the multi-country cross-sectional observational PIFotal study
BMC Pulmonary Medicine (2023)