Abstract
Oxidative stress supposedly plays a role in the pathogenesis of Parkinson’s disease (PD). Uric acid (UA), a powerful antioxidant, is lowered in PD while allantoin, the oxidation product of UA and known biomarker of oxidative stress, was not systematically studied in PD. We aim to compare serum and cerebrospinal fluid (CSF) levels of UA, allantoin, and allantoin/UA ratio in de novo PD patients and controls, and evaluate their associations with clinical severity and the degree of substantia nigra degeneration in PD. We measured serum and CSF levels of UA, allantoin, and allantoin/UA ratio in 86 PD patients (33 females, mean age 57.9 (SD 12.6) years; CSF levels were assessed in 51 patients) and in 40 controls (19 females, 56.7 (14.1) years). PD patients were examined using Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS), Montreal Cognitive Assessment (MoCA), Scales for Outcomes in Parkinson Disease-Autonomic (SCOPA-AUT), the University of Pennsylvania Smell Identification Test (UPSIT), one-night video-polysomnography, and dopamine transporter single-photon emission computed tomography (DAT-SPECT). Serum allantoin and allantoin/UA ratio were significantly increased in the PD group compared to controls (p < 0.001 and p = 0.002, respectively). Allantoin/UA ratios in serum and CSF were positively associated with the SCOPA-AUT score (p = 0.005 and 0.031, respectively) and RBD presence (p = 0.044 and 0.028, respectively). In conclusion, serum allantoin and allantoin/UA ratio are elevated in patients with de novo PD. Allantoin/UA ratio in serum and CSF is associated with autonomic dysfunction and RBD presence, indicating that higher systemic oxidative stress occurs in PD patients with more diffuse neurodegenerative changes.
Similar content being viewed by others
Introduction
Parkinson’s disease (PD) is the most common form of neurodegenerative synucleinopathy. The main pathological features of PD are the loss of dopaminergic neurons in substantia nigra pars compacta leading to the depletion of dopamine and the formation of alpha-synuclein positive intracytoplasmatic inclusions referred to as Lewy bodies in surviving neurons1. Clinical manifestations of PD are heterogeneous, and several disease phenotypes were defined based on the spectrum of motor and non-motor symptoms. Unbiased clustering analyses showed that patients with dysautonomia and rapid eye movement (REM) sleep behavior disorder (RBD), referred to as the “diffuse malignant” subtype, have a higher risk of cognitive impairment and faster disease progression as compared to patients with only motor involvement, known as the “mild motor-predominant” subtype2,3.
There is increasing evidence that implicates oxidative stress as a key driver of the complex mechanism underlying neurodegeneration in PD4. Oxidative stress may be involved in the pathogenesis of PD through several mechanisms such as oxidation of dopamine, calcium influx through L-type calcium channels and its associated excitotoxicity, mitochondrial dysfunction, protein misfolding and aggregation, or ferroptosis5. Oxidative stress arises when the production of reactive oxygen species (ROS) exceeds clearance by endogenous antioxidants6. Uric acid (UA), the end product of purine metabolism in humans, is one of the powerful antioxidants in the extracellular compartment with putative neuroprotective effects. UA is a scavenger of singlet oxygen, peroxy radicals, and hydroxyl radicals7. PD is associated with lower serum UA levels, particularly in the later stages of the disease8. A series of epidemiological studies suggested that higher levels of UA are associated with a decreased risk of disease development and a slower rate of clinical progression in PD9,10. In line with these studies, a recent dose-response meta-analysis reported that higher serum UA levels reduced the risk for PD11. Consequently, the effects of UA were studied in preclinical disease models. UA within astrocytes prevented dopaminergic cell death and atrophy induced by oxidative and mitochondrial toxins in cellular PD models12. UA showed attenuated toxic effects on dopaminergic cells in a mouse intrastriatal 6-hydroxydopamine model of PD13. Because of the evolutionary loss of the functional uricase enzyme, humans are unable to further catabolize UA to the more soluble compound allantoin. However, UA can be nonenzymatically oxidized into allantoin by ROS under conditions of increased oxidative stress14. Consequently, allantoin has emerged as a reliable biomarker for assessing the oxidative status both in vitro and in vivo15. Whereas serum UA level is influenced by many factors such as diet, renal function, drugs, and variants in the genes coding urate transporters16, allantoin and/or allantoin/UA ratio may be more accurate biomarkers compared to UA alone. In contrast to UA, allantoin has been only marginally studied in PD. One study revealed no differences in 24-hour urine excretion of UA, allantoin, and allantoin/UA ratio between PD and healthy subjects17. In our previous study, we observed increased serum allantoin and allantoin/UA ratio in patients with isolated RBD (iRBD), which is in most cases the prodromal stage of neurodegenerative synucleinopathies, including PD18. This study aimed to compare serum and cerebrospinal fluid (CSF) levels of UA, allantoin, and allantoin/UA ratio in PD and healthy controls, and to evaluate associations of these biochemical parameters with the severity of motor and non-motor symptoms, including the presence of RBD and the degree of substantia nigra degeneration in PD.
Results
Of 111 patients consecutively included in the BIO-PD study between 11/2015 and 11/2021, 103 had serum samples available in the biobank; 17 samples were excluded due to the use of medication affecting UA levels. Thus, serum samples from 86 patients were analyzed while CSF was available in 51 of them. Serum and CSF samples from 50 controls included between 11/2015 and 06/2021 fulfilling the inclusion criteria were available in our biobank, while 10 samples were excluded due to a history of using medication affecting UA levels, yielding 40 control samples for comparison. Mean freezer storage-times were comparable for PD and control samples (3.3 (SD 1.7) vs 3.1 (SD 1.3) years; p = 0.50).
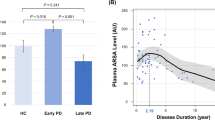
Demographic data of the PD and control groups, as well as results of biochemical analyses are listed in Table 1; The PD subgroup with available CSF had demographic and clinical parameters comparable to the total group (Supplementary Table 1). RBD was diagnosed in 21 (24%, 16 males, 5 females) PD patients. There was a significant effect of sex (p < 0.001; higher in men) and BMI (p < 0.001; positive association) on serum UA concentrations while no between-group difference was found. Serum allantoin concentrations and serum allantoin/UA ratio were significantly increased in the PD group compared to controls (p < 0.001 and p = 0.002, respectively); there was a significant effect of storage-time on serum allantoin concentration (p = 0.037; positive association) and a significant effect of sex on serum allantoin/UA ratio (p = 0.007; higher in women, Fig. 1).
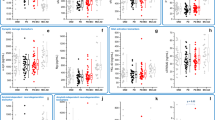
CSF UA and allantoin concentrations were significantly correlated with their serum concentrations (r = 0.65, p < 0.001 and r = 0.38, p < 0.001, respectively; Supplementary Table 2). There was a significant effect of sex and age on CSF concentrations of UA (p < 0.001 with higher concentrations in men and p = 0.04, respectively) and allantoin (p = 0.03 with higher concentrations in men and p < 0.001, respectively) and significant effect of age on CSF allantoin/UA ratio (p = 0.01); associations with age were positive for all parameters. No between-group differences in CSF parameters were found.
Adjusted for age and sex, allantoin/ UA ratios in serum and CSF were positively associated with SCOPA-AUT score (p = 0.005 and 0.031, respectively) and RBD presence (p = 0.044 and 0.028, respectively), whereby the associations with SCOPA-AUT were mostly driven by the gastrointestinal and urinary subscores (Supplementary Table 3). CSF allantoin levels and allantoin/ UA ratio were positively associated with disease duration (p < 0.001 and 0.010, respectively). CSF UA levels were positively associated with olfactory function (p = 0.013; Table 2). PD patients with RBD had higher allantoin/ UA ratios in the serum (0.006 ± 0.003 vs. 0.005 ± 0.001, p = 0.039) and CSF (0.015 ± 0.006 vs. 0.011 ± 0.004, p = 0.038) compared to patients without RBD.
Sensitivity analysis comparing PD and controls excluded due to medications affecting UA levels showed the same pattern of findings as the main analysis. The excluded PD group had higher serum allantoin concentrations (p < 0.001) and serum allantoin/UA ratio (p < 0.001) compared to the excluded control group; no between-group differences were observed for the CSF parameters (Supplementary Table 4).
Discussion
Our study investigated the serum and CSF levels of UA, allantoin, and allantoin/UA ratio in patients with PD and their associations with clinical parameters. We found significantly increased levels of serum allantoin and allantoin/UA ratio in PD patients compared to healthy controls, indicating increased oxidative stress. Allantoin/UA ratios in serum and CSF were positively associated with autonomic dysfunction assessed using the SCOPA-AUT questionnaire and with RBD presence. CSF UA levels were positively associated with olfactory function.
Degree of motor and autonomic impairment as well as the prevalence of RBD of our cohort are comparable to previous studies in newly diagnosed PD patients19,20 while cognitive performance is similar to the healthy Czech population in this age group21, indicating that the patient group is a representative early PD sample.
The determination of allantoin is difficult due to its low concentrations in body fluids, high polarity, and lack of chromophore for UV detection. In previous studies, allantoin was measured in diverse clinical samples, mostly in plasma, with highly variable mean concentrations in healthy controls ranging from 0.9 to 22.0 μmol/l22. We employed the UHPLC-MS/MS method using an isotopically labeled internal standard that proved to be precise23.
Elevated levels of allantoin, supposedly related to increased systemic oxidative stress, were previously reported in patients with various disorders, including bacterial meningitis, Behçet’s disease, recurrent aphthous stomatitis or chronic renal failure24,25. Plasmatic allantoin and allantoin/UA ratio were unrelated to levels of lipid peroxidation product malondialdehyde (MDA)26 and may be associated with the protective antioxidant activities under systemic oxidative stress. In line with our results, previous research on oxidative stress markers in PD showed significantly higher levels of blood 8-hydroxyguanosine, nitrite, and MDA and lower levels of catalase, glutathione, and total-cholesterol, indicating the exhaustion of the antioxidative defense system in PD27.
In contrast to results from two meta-analyses that revealed lower UA levels in PD patients8,28, we did not find a significant difference in serum and CSF UA levels between PD patients and controls. This may be due to the inclusion of newly diagnosed, untreated PD patients - a clinical population that did not show consistent alterations in UA levels in previous studies29. Indeed, a meta-analysis confirmed that lower UA levels may be detectable only in later disease stages8. Due to its neuroprotective effects, UA was proposed as a potential therapeutic target in PD12,13. However, a clinical trial with urate-elevating compound inosine in early PD has not shown any difference in the rate of clinical disease progression over placebo30. Moreover, a genome-wide association study of genetic variants underlying urate levels found no causal associations between UA and PD risk, age at onset, or disease progression severity31. Hence, UA may not be directly involved in the pathogenesis of PD but rather its decreased levels could represent an epiphenomenon32.
In PD patients only one study examining allantoin was conducted until now and no difference between PD and healthy subjects in 24-hour urine excretion of UA, allantoin, and allantoin/UA ratio was found17. In contrast to the latter finding, our previous study revealed increased serum allantoin and allantoin/UA ratio in patients with iRBD, implying increased systemic oxidative stress in prodromal synucleinopathy18. In the current study, we have also found increased serum allantoin and allantoin/UA ratio in PD, but the effect was smaller, yielding 45% increase in serum allantoin in PD compared to its 86% increase in iRBD males. Although allantoin levels were slightly higher in PD patients with RBD compared to those without RBD, we speculate that a larger increase of allantoin/UA ratio in iRBD compared to PD implies that high serum allantoin in synucleinopathies is most pronounced in the prodromal phase and gradually declines during later disease stages when low UA levels become apparent.
We have observed moderate associations of UA, allantoin or allantoin/UA ratios in serum and CSF with non-motor symptoms, i.e. autonomic and olfactory function and RBD presence. These results are only exploratory and need to be treated with caution since statistical correction for multiple comparisons was not applied. Autonomic dysfunction as well as RBD are associated with a diffuse spread of synuclein pathology in the nervous system, i.e. the diffuse malignant disease subtype33. Hence, the increased allantoin/UA ratio may be the systemic reaction to the spread of α-synuclein in the peripheral nervous system34. The association with autonomic symptoms were mostly found in the gastrointestinal and urinary domains, which may be related to their early occurrence in synucleinopathies. Interestingly, the association between high allantoin levels and autonomic dysfunction mirrors previous findings related to low UA and non-motor symptoms such as fatigue, attention deficit, anxiety, and cardiovascular disturbances in PD32,35, which we, however, could not replicate. We can speculate that higher oxidative stress levels herald a more severe course in the early stage of disease and it may be worth to further study allantoin/UA ratio as a prognostic biomarker in PD.
In our current work, higher UA and allantoin levels in serum and CSF were observed in PD patients taking medication affecting UA levels (mostly thiazide diuretics). In contrast to UA and allantoin levels, allantoin/UA ratio seems to be relatively independent on the use of medication affecting UA in PD and controls. This fact together with its stronger associations with the severity of non-motor symptoms indicates that allantoin/UA ratio may be a more universal and useful marker in synucleinopathies compared to UA or allantoin alone.
We did not find significant between-group differences in allantoin or allantoin/UA ratio in CSF. The fact that between group difference in allantoin and allantoin/UA levels in serum was not paralleled in CSF could reflect a higher extent of oxidative stress on a systemic level possibly reflecting alpha-synuclein spread in the peripheral nervous system in early-stage PD. The antioxidative defense in the CNS may also involve different substances than UA. Despite not being increased, the CSF allantoin/UA ratio was significantly associated with SCOPA-AUT score and RBD status in PD patients. It is, however, not clear whether CSF levels of UA and allantoin are truly related to dysautonomia and RBD, or rather these associations reflect just high cross-correlation between serum and CSF levels of UA and allantoin. This finding is in line with previous reports showing that CSF UA is predominantly determined by serum UA, and is modified by the blood-brain barrier integrity36. Yet, under pathological conditions, additional formation of UA from the catabolism of nucleotides, nucleosides, and purine precursors within CNS may occur, as was suggested in patients with bacterial meningitis37. Theoretically, allantoin in the CSF could increase at later PD stages with higher oxidative stress in the CNS compartment, which may be supported by the positive association between CSF allantoin and disease duration in our current study.
To our knowledge, this is the first study investigating allantoin and allantoin/UA ratio in serum and CSF in PD patients. The limitation of this study is the relatively small size of the control group. Additionally, only a clinical control group could be used for comparison due to difficulties in obtaining CSF from healthy volunteers. This clinical control group partially consisted of patients with altered CNS functioning, such as sleep disorders, anxiety, or chronic pain, conditions where little is known about oxidative stress involvement. However, the inclusion of heterogeneous control samples from patients with various disorders minimizes the likelihood of significant bias.
In conclusion, we have shown that serum allantoin and allantoin/UA ratio are elevated in patients with de novo untreated PD. Moreover, we found a positive association of allantoin/UA ratio in serum and CSF with autonomic dysfunction and RBD, indicating that allantoin and allantoin/UA ratio may be biomarkers of a more severe PD subtype with accentuated non-motor symptoms.
Methods
Participants
This study is part of a longitudinal project “biomarkers in PD (BIO-PD)” aimed at collecting a large representative sample of de novo PD patients38. All PD patients were diagnosed based on the Movement Disorder Society clinical diagnostic criteria for PD39 and comprehensively examined before the initiation of dopaminergic treatment.
Paired serum/CSF samples from age- and sex-comparable spinal anesthesia subjects and symptomatic controls with noninflammatory, non-neurodegenerative conditions (defined as CSF cell count ≤15 elements/unit of volume and CSF protein concentration ≤1 g/l) were selected from our CSF biobank operated according to Consensus Guidelines for CSF and Blood Biobanking for CNS Biomarker Studies40. To minimize potential bias associated with a homogeneous control group, the selected controls included patients with various neurological disorders including functional movement disorder (n = 14), narcolepsy (n = 3), idiopathic hypersomnia (n = 3), headache (n = 1), nerve root compression (n = 1), anxiety (n = 1), spinal stenosis (n = 1), and patients undergoing surgery in spinal anesthesia for nonmalignant urologic disorders including prostatic hyperplasia (n = 9), hydrocele (n = 6), bladder tumor (n = 6), urethral stricture (n = 3), and phimosis (n = 2).
To be eligible for this study, all participants had to be free from medical conditions (i.e. gout, chronic kidney disease, restless legs syndrome, and multiple sclerosis) and medications (i.e, thiazide diuretics or xanthine oxidase inhibitors) that potentially affect UA levels. In controls, a history of neurodegenerative disorders manifesting with a movement disorder or dementia was also an exclusion criterion. Another requirement was the availability of >200 μl serum and (in the case of controls) CSF aliquots stored in the biobank.
This study was approved by the ethics committee of the General University Hospital in Prague. Participants with PD were informed about the study and provided written informed consent; participants from the control group provided samples for biomarker research as per universal biobank informed consent.
Clinical Examinations
The protocol in PD patients included a structured interview, Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) part III41, Montreal Cognitive Assessment (MoCA)21, Scales for Outcomes in Parkinson Disease-Autonomic (SCOPA-AUT) questionnaire42, olfactory testing using the University of Pennsylvania Smell Identification Test (UPSIT)43, one-night video-polysomnography, and dopamine transporter single-photon emission computed tomography (DAT-SPECT). DAT-SPECT was performed using the [123I]-2-b-carbomethoxy-3b-(4-iodophenyl)-N-(3-fluoropropyl) nortropane ([123I]FP-CIT, DaTscan®, GE Healthcare, Little Chalfont, Buckinghamshire, UK) tracer according to European Association of Nuclear Medicine (EANM) procedure guidelines44. The acquisition parameters were as follows: rotational radius 13–15 cm, image matrix 128 × 128, angular sampling with 120 projections at 3° interval and 40 s per view, zoom 1.3, energy window 159 ± 10% keV. Reconstruction of the raw SPECT projection data was performed using the ordered subset expectation maximization (OSEM) algorithm with 8 iterations and 10 subsets including Chang attenuation correction (μ = 0.11 cm−1) and 3D Butterworth post-filtering with FWHM = 8 mm45. Automated semi-quantitative analysis was performed using the BasGan V2 software and specific binding ratios (SBR) in both putamina were calculated according to the formula [(putamen binding–background binding)/background binding]; the lower value from both hemispheres was used for further analyses46. RBD was diagnosed based on clinical history and video-polysomnography according to the International Classification of Sleep Disorders, third edition (ICSD-3)47.
Laboratory analyses
In PD patients, venous blood and optionally CSF samples were drawn at the baseline visit in the morning after an overnight fast. In controls, sampling was performed in the morning after an overnight fast either at the neurology or urology department. Lumbar punctures were performed using 22 G atraumatic needles in PD patients and neurologic controls and by 25 G needles in patients undergoing spinal anesthesia.
Sera were separated within 60 minutes after collection and then aliquoted. CSF was immediately centrifuged at 3000 rpm under 4 °C, and the supernatant was aliquoted. All samples were frozen at −80 °C until further biochemical analysis.
The concentration of UA in serum and CSF was determined using the Beckman Coulter AU system with Beckman Coulter Uric Acid kits (Beckman Coulter, Brea, CA, USA) in a standard biochemical laboratory at the Institute of Rheumatology in Prague.
Allantoin concentration in serum and CSF was determined by ultra-high performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS) with an isotopically labeled internal standard. Before the analysis, protein precipitation was used. Samples were processed as follows: 60 µL of 100% acetonitrile (containing 1.33 μM allantoin-13C2, 15N4 as an internal standard) was added to 20 µL of the sample. The mixture was vortexed and centrifuged at 16500×g for 6 min. 50 µL of supernatant was transferred into an LC vial. For the UHPLC-MS/MS analysis, a UHPLC system Agilent 1290 with a Triple Quad 6460 mass spectrometer (Agilent Technologies, Waldbronn, Germany) was used. The hydrophilic interaction liquid chromatography (HILIC) conditions consisted of the Acquity BEH Amide column; isocratic elution by a mobile phase composed of acetonitrile and 0.1% formic acid 90/10 (v/v). The flow rate of the mobile phase was maintained at 0.3 mL/min, and the injection volume was 2 µL. The temperature of the column was kept at 30 °C and samples were thermostated at 10 °C. The MS/MS spectrometer was operated in a positive mode. The applied conditions of the electrospray ion source were as follows: gas temperature: 300 °C, gas flow: 7 L/min, sheath gas temperature: 300 °C, sheath gas flow: 8 L/min, nebulizer pressure: 310 kPa and capillary voltage: 6000 V. The MS/MS measurement was performed in multiple reaction-monitoring mode (MRM). Two MRM transitions were monitored for allantoin: Quantifier transition was 159 > 116 (collision energy 2 V and fragmentor voltage 50 V) and a qualifier transition was 159 > 99 (collision energy 8 V and fragmentor voltage 50 V). The transition 165 > 120 (collision energy 2 V and fragmentor voltage 50 V) was monitored for allantoin-13C2, 15N4.This method, which has been validated in terms of linearity, lower limit of quantification (LLOQ), upper limit of quantification (ULOQ), accuracy, precision, selectivity, recovery, carry-over effect, matrix effects, robustness, and stability of both quality control (QC) samples and clinical patient samples, was demonstrated to be suitable for its intended purpose. The UHPLC-MS/MS method with one-step sample preparation provided high sensitivity and high sample throughput23.
Statistical analyses
Comparisons of UA, allantoin, and allantoin/UA ratio in the serum and CSF between PD and controls were performed using a univariate general linear model with group and sex as fixed factors, and with age, body mass index (BMI), and freezer storage-time as covariates. Difference in sample storage-times may influence the concentration of biomolecules and was thus suggested to be included as a covariate in longitudinal cohort studies48. Post-hoc multiple comparisons were performed using Tukey’s HSD. Chi-square statistics were used to analyze binary variables. Pearson partial correlation coefficients controlling for age and sex were calculated to examine relationships between biochemical and clinical variables. Correlation analyses were considered exploratory and corrections for multiple comparisons were thus not applied. IBM SPSS statistics version 25 (IBM, Armonk, NY, USA) was used for statistical analysis; graphs were plotted using Graphpad Prism version 9.0 (Graphpad software, San Diego, CA, USA).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Individual participant data that underlie the findings of this study are available upon request to the corresponding author by qualified researchers (i.e., affiliated to a respected university or research institution/hospital).
Code availability
Not relevant.
References
Spillantini, M. G. et al. Alpha-synuclein in Lewy bodies. Nature 388, 839–840 (1997).
Fereshtehnejad, S. M., Zeighami, Y., Dagher, A. & Postuma, R. B. Clinical criteria for subtyping Parkinson’s disease: biomarkers and longitudinal progression. Brain 140, 1959–1976 (2017).
Riboldi, G. M., Russo, M. J., Pan, L., Watkins, K. & Kang, U. J. Dysautonomia and REM sleep behavior disorder contributions to progression of Parkinson’s disease phenotypes. NPJ Parkinsons Dis. 8, 110 (2022).
Trist, B. G., Hare, D. J. & Double, K. L. Oxidative stress in the aging substantia nigra and the etiology of Parkinson’s disease. Aging Cell 18, e13031 (2019).
Yu, Z. et al. The significance of uric acid in the diagnosis and treatment of Parkinson disease: An updated systemic review. Med. (Baltim.) 96, e8502 (2017).
Sies, H. Oxidative stress: a concept in redox biology and medicine. Redox Biol. 4, 180–183 (2015).
Ames, B. N., Cathcart, R., Schwiers, E. & Hochstein, P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc. Natl Acad. Sci. 78, 6858–6862 (1981).
Wen, M. et al. Serum uric acid levels in patients with Parkinson’s disease: A meta-analysis. PLoS One 12, e0173731 (2017).
Ascherio, A. et al. Urate as a predictor of the rate of clinical decline in Parkinson disease. Arch. Neurol. 66, 1460–1468 (2009).
Schwarzschild, M. A. et al. Serum Urate as a Predictor of Clinical and Radiographic Progression in Parkinson Disease. Arch. Neurol. 65, 716–723 (2008).
Chang, H., Wang, B., Shi, Y. & Zhu, R. Dose-response meta-analysis on urate, gout, and the risk for Parkinson’s disease. npj Parkinson’s Dis. 8, 160 (2022).
Cipriani, S. et al. Urate and its transgenic depletion modulate neuronal vulnerability in a cellular model of Parkinson’s disease. PLoS One 7, e37331 (2012).
Chen, X. et al. Disrupted and transgenic urate oxidase alter urate and dopaminergic neurodegeneration. Proc. Natl Acad. Sci. 110, 300–305 (2013).
Kaur, H. & Halliwell, B. Action of biologically-relevant oxidizing species upon uric acid. Identification of uric acid oxidation products. Chem.-Biol. Interact. 73, 235–247 (1990).
Grootveld, M. & Halliwell, B. Measurement of allantoin and uric acid in human body fluids. A potential index of free-radical reactions in vivo? Biochem. J. 243, 803–808 (1987).
Dalbeth, N., Gosling, A. L., Gaffo, A. & Abhishek, A. Gout. Lancet 397, 1843–1855 (2021).
Sampat, R. et al. Potential mechanisms for low uric acid in Parkinson disease. J. Neural Transm. 123, 365–370 (2016).
Hasíková, L. et al. Patients with REM sleep behavior disorder have higher serum levels of allantoin. Parkinsonism Relat. Disord. 90, 38–43 (2021).
Simuni, T. et al. Baseline prevalence and longitudinal evolution of non-motor symptoms in early Parkinson’s disease: the PPMI cohort. J. Neurol. Neurosurg. Psychiatry 89, 78–88 (2018).
Mollenhauer, B. et al. Nonmotor and diagnostic findings in subjects with de novo Parkinson disease of the DeNoPa cohort. Neurology 81, 1226–1234 (2013).
Kopecek, M. et al. Montreal cognitive assessment (MoCA): Normative data for old and very old Czech adults. Appl Neuropsychol. Adult 24, 23–29 (2017).
Kanďár, R. The ratio of oxidized and reduced forms of selected antioxidants as a possible marker of oxidative stress in humans. Biomed. Chromatogr. 30, 13–28 (2016).
Kozlik, P., Hasikova, L., Stiburkova, B., Zavada, J. & Kalikova, K. Rapid and reliable HILIC-MS/MS method for monitoring allantoin as a biomarker of oxidative stress. Anal. Biochem 589, 113509 (2020).
Kastenbauer, S., Koedel, U., Becker, B. F. & Pfister, H. W. Oxidative stress in bacterial meningitis in humans. Neurology 58, 186–191 (2002).
Yardim‐Akaydin, S., Sepici, A., Özkan, Y., Şimşek, B. & Sepici, V. Evaluation of allantoin levels as a new marker of oxidative stress in Behçet’s disease. Scand. J. Rheumatol. 35, 61–64 (2006).
Kand'ár, R., Žáková, P. & Mužáková, V. Monitoring of antioxidant properties of uric acid in humans for a consideration measuring of levels of allantoin in plasma by liquid chromatography. Clin. Chim. Acta 365, 249–256 (2006).
Wei, Z., Li, X., Li, X., Liu, Q. & Cheng, Y. Oxidative Stress in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Front Mol. Neurosci. 11, 236 (2018).
Shen, L. & Ji, H. F. Low uric acid levels in patients with Parkinson’s disease: evidence from meta-analysis. BMJ Open 3, e003620 (2013).
Koros, C. et al. Serum uric acid level as a putative biomarker in Parkinson’s disease patients carrying GBA1 mutations: 2-Year data from the PPMI study. Parkinsonism Relat. Disord. 84, 1–4 (2021).
Schwarzschild, M. A. et al. Effect of Urate-Elevating Inosine on Early Parkinson Disease Progression: The SURE-PD3 Randomized Clinical Trial. Jama 326, 926–939 (2021).
Coneys, R., Storm, C. S., Kia, D. A., Almramhi, M. & Wood, N. W. Mendelian Randomisation Finds No Causal Association between Urate and Parkinson’s Disease Progression. Mov. Disord. 36, 2182–2187 (2021).
Grażyńska, A. et al. The Influence of Serum Uric Acid Level on Non-Motor Symptoms Occurrence and Severity in Patients with Idiopathic Parkinson’s Disease and Atypical Parkinsonisms-A Systematic Review. Medicina (Kaunas) 57, (2021).
De Pablo-Fernandez, E. et al. Association of Autonomic Dysfunction With Disease Progression and Survival in Parkinson Disease. JAMA Neurol. 74, 970–976 (2017).
Chen, Z., Li, G. & Liu, J. Autonomic dysfunction in Parkinson’s disease: Implications for pathophysiology, diagnosis, and treatment. Neurobiol. Dis. 134, 104700 (2020).
Moccia, M. et al. Is serum uric acid related to non-motor symptoms in de-novo Parkinson’s disease patients? Parkinsonism Relat. Disord. 20, 772–775 (2014).
Bowman, G. L., Shannon, J., Frei, B., Kaye, J. A. & Quinn, J. F. Uric acid as a CNS antioxidant. J. Alzheimers Dis. 19, 1331–1336 (2010).
Becker, B. F., Kastenbauer, S., Ködel, U., Kiesl, D. & Pfister, H. W. Urate oxidation in CSF and blood of patients with inflammatory disorders of the nervous system. Nucleosides Nucleotides Nucleic Acids 23, 1201–1204 (2004).
Dušek, P. et al. Clinical characteristics of newly diagnosed Parkinson’s disease patients included in the longitudinal BIO-PD study. Česká a slovenská neurologie a neurochirurgie 83, 633–639 (2020).
Postuma, R. B. et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 30, 1591–1601 (2015).
Teunissen, C. E. et al. A consensus protocol for the standardization of cerebrospinal fluid collection and biobanking. Neurology 73, 1914–1922 (2009).
Goetz, C. G. et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov. Disord. 23, 2129–2170 (2008).
Visser, M., Marinus, J., Stiggelbout, A. M. & Van Hilten, J. J. Assessment of autonomic dysfunction in Parkinson’s disease: the SCOPA-AUT. Mov. Disord. 19, 1306–1312 (2004).
Doty, R. L., Shaman, P. & Dann, M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol. Behav. 32, 489–502 (1984).
Darcourt, J. et al. EANM procedure guidelines for brain neurotransmission SPECT using (123)I-labelled dopamine transporter ligands, version 2. Eur. J. Nucl. Med Mol. Imaging 37, 443–450 (2010).
Dušek, P. et al. Relations of non-motor symptoms and dopamine transporter binding in REM sleep behavior disorder. Sci. Rep. 9, 15463 (2019).
Calvini, P. et al. The basal ganglia matching tools package for striatal uptake semi-quantification: description and validation. Eur. J. Nucl. Med Mol. Imaging 34, 1240–1253 (2007).
American Academy of Sleep, M. International classification of sleep disorders, (American Academy of Sleep Medicine, Darien, IL, 2014).
Enroth, S., Hallmans, G., Grankvist, K. & Gyllensten, U. Effects of Long-Term Storage Time and Original Sampling Month on Biobank Plasma Protein Concentrations. EBioMedicine 12, 309–314 (2016).
Acknowledgements
This study was supported by the Czech Ministry of Health (Grant NU21-04-0053, and MH CZ–DRO-VFN64165), and National Institute for Neurological Research (Programme EXCELES, ID Project No. LX22NPO5107 (MEYS): Financed by European Union – Next Generation EU) and by the project (Ministry of Health, Czech Republic) for consensual development of research organization 023728.
Author information
Authors and Affiliations
Contributions
All authors were involved in drafting the manuscript or revising it critically for content. L.H. wrote the manuscript, contributed to the design of the study, organized the allantoin measurement and other biochemical measurements. J.Z. designed the project. T.S. contributed to the design of the study. L.K. contributed to the biological sample collection, P.K. and K.K. carried out allantoin measurements. J.T. and D.Z. carried out DAT-SPECT analysis, K.Š. and E.R. contributed to the biological sample and clinical data collection, P.D. supervised the project, contributed to the design of the project, conducted statistical data analysis, contributed to the biological sample and clinical data collection and wrote the manuscript. The final manuscript has been seen and approved by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hasíková, L., Závada, J., Serranová, T. et al. Serum but not cerebrospinal fluid levels of allantoin are increased in de novo Parkinson’s disease. npj Parkinsons Dis. 9, 60 (2023). https://doi.org/10.1038/s41531-023-00505-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41531-023-00505-0