Abstract
Pathophysiological substrate(s) and progression of Parkinson’s disease (PD) with mild cognitive impairment (PD-MCI) are still matter of debate. Baseline cerebrospinal fluid (CSF) neurochemical profile and cognitive changes after 2 years were investigated in a retrospective series of PD-MCI (n = 48), cognitively normal PD (PD-CN, n = 40), prodromal Alzheimer’s disease (MCI-AD, n = 25) and cognitively healthy individuals with other neurological diseases (OND, n = 44). CSF biomarkers reflecting amyloidosis (Aβ42/40 ratio, sAPPα, sAPPβ), tauopathy (p-tau), neurodegeneration (t-tau, NfL, p-NfH), synaptic damage (α-syn, neurogranin) and glial activation (sTREM2, YKL-40) were measured. The great majority (88%) of PD-MCI patients was A-/T-/N-. Among all biomarkers considered, only NfL/p-NfH ratio was significantly higher in PD-MCI vs. PD-CN (p = 0.02). After 2 years, one-third of PD-MCI patients worsened; such worsening was associated with higher baseline levels of NfL, p-tau, and sTREM2. PD-MCI is a heterogeneous entity requiring further investigations on larger, longitudinal cohorts with neuropathological verification.
Similar content being viewed by others
Introduction
Cognitive dysfunctions are frequently reported in Parkinson’s disease (PD), even in newly diagnosed patients, potentially evolving into dementia1. Following the paradigm of Alzheimer’s disease (AD) clinical continuum2, the concept of mild cognitive impairment (MCI) as a prodromal stage of dementia has been also applied to PD3,4. In a recent meta-analysis, Parkinson’s disease with mild cognitive impairment (PD-MCI) has shown variable clinical presentations and progression to dementia5.
Different pathophysiological mechanisms are potentially involved in PD-MCI6. Although neuropathological data devoted to this specific entity are scanty and carried out in small series, Lewy body pathology, Alzheimer pathology, and cerebral amyloid angiopathy seem to be related to PD-MCI clinical phenotypes7,8.
The difficulties in identifying the neurobiological substrate(s) represent a matter of debate about the validity of the MCI construct in the clinical management of PD patients9. To give a contribution in this field, we retrospectively analyzed a cohort of patients with PD categorized as PD-MCI3 and as cognitively normal (PD-CN), for whom cerebrospinal fluid (CSF) samples collected at the diagnostic work-up were available. As a control group, we considered cognitively healthy individuals who underwent CSF analysis for other minor neurological disorders (OND). We also included, as a contrast group, patients with MCI due to AD (MCI-AD), who have, by definition, a typical CSF neurochemical profile and the highest risk of progression to dementia.
In these groups we measured a large panel of CSF biomarkers reflecting different pathophysiological pathways including amyloidosis (soluble amyloid precursor protein α and β, sAPPα,β; β-amyloid 1–42/1–40 ratio, Aβ42/Aβ40), tauopathy (181-phosphorylated tau, p-tau), amyloid-dependent neurodegeneration (total-tau, t-tau), amyloid-independent neurodegeneration (neurofilament light chain, NfL; phosphorylated neurofilament heavy chain, p-NfH), synaptic damage (total α-synuclein, α-syn; neurogranin, Ng), and glial activation (soluble triggering receptor expressed on myeloid cells 2, sTREM2; chitinase-3-like protein 1, YKL-40). In PD-CN and PD-MCI patients, we also verified the longitudinal changes in cognitive functioning by considering screening tests after 2 years.
The aims of our study were: (i) to investigate whether a well-characterized cohort of PD-MCI patients, compared to PD-CN individuals, show a differential neurochemical profile by measuring a large panel of CSF putative biomarkers reflecting different pathophysiological pathways; (ii) to verify changes in cognitive performances after 2 years, also investigating the potential predictive role of CSF biomarkers on cognitive outcome.
Results
Demographic and clinical features
We included 40 PD-CN, 48 PD-MCI, 25 MCI-AD, and 44 OND patients. The details of the main demographic and clinical features of each diagnostic group are summarized in Table 1. At baseline, the great majority of patients were drug-naïve (80% of PD-CN patients and 75% of PD-MCI patients). As expected, PD-MCI patients showed lower Montreal Cognitive Assessment (MoCA) and Mini-Mental State Examination (MMSE) scores, and higher Unified Parkinson’s Disease Rating Scale-part III (UPDRS-III) and Hoehn and Yahr (H&Y) scores, as compared to PD-CN individuals. Details referring to the baseline neuropsychological evaluation in the two diagnostic groups are reported in Table 2.
At the comprehensive clinical evaluation carried out after two years, the diagnosis of PD was confirmed in all patients. Out of 88 PD patients, neuropsychological assessment at follow-up was available for 65 (28 PD-CN and 37 PD-MCI). Cognitive worsening (loss of at least 2 points at both MMSE and MoCA) was observed in 30% of PD-MCI patients, as opposed to 43% of PD-MCI patients with unchanged scores. None of PD-CN individuals worsened both at MMSE and MoCA (Table 3). In Supplementary Table 1 follow-up scores obtained in all neuropsychological tests are reported.
CSF biomarkers
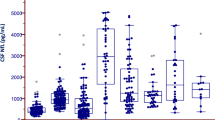
Biomarker’s concentrations in PD-CN, PD-MCI, MCI-AD, and OND are plotted in Fig. 1. Mean values of all CSF biomarkers in the four groups are reported in Supplementary Table 2.
a–c amyloid biomarkers: Aβ42/40, sAPPα, sAPPβ. d tauopathy biomarker: p-tau. e, f synaptic damage biomarkers: α-syn, Ng. g, h glial activation biomarkers: YKL-40, sTREM2. i amyloid-dependent neurodegeneration biomarker: t-tau. j–l amyloid-independent neurodegeneration biomarkers: NfL, p-NfH, NfL/p-NfH. Boxplots summarize the distribution of the data, where the box represents the interquartile range, the horizontal line inside the box represents the median, and the filled square within the box represents the mean. The whiskers extend to the minimum and maximum values within 90% of the data range. P-values have been corrected for multiple group comparisons. MCI-AD mild cognitive impairment due to Alzheimer’s disease, OND healthy cognitive patients with other neurological disorders, PD-CN cognitively normal Parkinson’s disease, PD-MCI Parkinson’s disease with mild cognitive impairment. Aβ42/Aβ40 β-amyloid 1-42/1-40 ratio, NfL neurofilament light chain, Ng neurogranin, p-NfH phosphorylated neurofilament heavy chain, p-tau phosphorylated-tau, sAPPα soluble amyloid precursor protein α, sAPPβ soluble amyloid precursor protein β, sTREM2 soluble triggering receptor expressed on myeloid cells 2, α-syn total α-synuclein, t-tau total tau, YKL-40 chitinase-3-like protein 1.
AD biomarkers
CSF Aβ42/40 ratio, p-tau, t-tau, sAPPα, and sAPPβ levels did not differ among PD-MCI, PD-CN, and OND. With respect to A/T/(N) profile, 3 (6%) out of PD-MCI patients and 2 (5%) out of PD-CN patients showed a CSF AD-like profile.
Amyloid-independent neurodegeneration biomarkers
CSF NfL showed a slight trend toward an increase in PD-MCI compared to PD-CN (p = 0.09), while CSF p-NfH levels were similar in PD-MCI, PD-CN, and OND. NfL/p-NfH ratio was significantly higher in PD-MCI vs. PD-CN (p = 0.02). This difference was still statistically significant after adjusting for age, sex, and disease duration (p adjusted = 0.036).
CSF NfL was significantly higher in MCI-AD compared to all other groups (p = 0.0001). CSF p-NfH and NfL/p-NfH ratios were significantly increased in MCI-AD with respect to PD-MCI and PD-CN.
Biomarkers of synaptic damage
CSF α-syn and Ng levels were similar in PD-MCI, PD-CN, and OND patients. As expected, they were higher in MCI-AD vs. the other groups.
Biomarkers of glial activation
CSF YKL-40 and sTREM2 did not differ among PD-MCI, PD-CN, and OND groups. Both were increased in MCI-AD compared to all other groups.
Correlations among CSF biomarkers
Spearman’s correlation coefficients among CSF biomarkers are reported in two heatmaps (Supplementary Figure). Ng and α-syn concentrations strongly correlated with each other; they correlated with t-tau in PD-MCI and PD-CN, with p-tau in PD-MCI, and with YKL-40 in PD-CN. In all groups, NfL and p-NfH levels, as well as sAPPα and sAPPβ, were strongly correlated.
CSF biomarkers association with clinical measures
CSF biomarkers did not show any correlation with baseline UPDRS-III, Levodopa Equivalent Daily Dose (LEDD), MMSE, and MoCA scores in PD groups.
In Fig. 2 the associations between CSF biomarkers and Z-score changes at neuropsychological tests are reported. In PD-MCI group, Z-score change was significantly associated with p-tau (ρ = −0.48, FDR-adjusted p = 0.01), t-tau (ρ = −0.38, FDR-adjusted p = 0.02), NfL (ρ = −0.46, FDR-adjusted p = 0.02), and NfL/p-NfH (ρ = −0.43, FDR-adjusted p = 0.03).
a p-tau, b t-tau, c NfL, d NfL/p-NfH. The reported correlations produced, in the different groups, Spearman correlation coefficients significantly different from 0 after FDR correction and p-values < 0.05 after a nonparametric regression that considered age, sex, and disease duration as covariates. In order to allow a better visual interpretation of the Spearman correlation, biomarker values are shown on a log2-scale, a log fit was also added for visual purposes. NfL neurofilament light chain, p-NfH phosphorylated neurofilament heavy chain, p-tau phosphorylated tau protein, t-tau total tau protein.
PD-MCI patients showing cognitive worsening had significantly higher levels of CSF p-Tau, NfL, and sTREM2, when compared to cognitively stable PD-MCI patients (p = 0.0017, p = 0.042, and p = 0.047, respectively, Fig. 3).
a difference in CSF p-tau between worsening PD-MCI and stable PD-MCI. b difference in CSF NfL between worsening PD-MCI and stable PD-MCI. c difference in CSF sTREM2 between worsening PD-MCI and stable PD-MCI. In boxplots, box height represents the interquartile range, the square represents the mean, the horizontal line represents the median and whiskers represent the 5–95% range. Biomarker values are shown in the log-scale for viewing purposes. NfL neurofilament light chain, p-tau phosphorylated tau protein, sTREM2 soluble triggering receptor expressed on myeloid cells 2.
Discussion
In this retrospective study, we wanted to verify if the CSF neurochemical profile, explored by means of a large panel of CSF biomarkers, and the clinical outcome of patients diagnosed as PD-MCI show differential features when compared to PD-CN individuals. To the best of our knowledge, such a large panel of CSF biomarkers has not been investigated before in the specific category of PD-MCI.
In our series, out of all biomarkers assessed, only the NfL/p-NfH ratio showed to be significantly higher in PD-MCI vs. PD-CN. After 2 years, one-third of PD-MCI patients showed a cognitive worsening, associated with baseline higher levels of NfL, p-tau, and sTREM2.
CSF biomarkers reflecting AD pathology are the most investigated biomarkers in PD. According to the A/T/(N) classification2, AD pathology is characterized by the presence of amyloidosis (A+) and tauopathy (T+). In CSF, brain amyloidosis is reflected by decreased Aβ42/Aβ40 ratio (A+) and tauopathy by increased p-tau levels (T+). Several post-mortem studies report the possible coexistence of AD pathology in PD brains7,8, also suggesting a relationship with the development of dementia in PD10. We thus assessed in our cohort CSF A/T/(N) profile, in order to evaluate the possible coexistence of AD pathology. CSF AD-like profile has been reported in 9–28% of Parkinson’s disease with dementia (PDD) series11,12. PD-MCI patients may show either normal13,14,15 or altered16,17,18,19 CSF AD biomarkers. In a previous work12, we found that 87% of PD-MCI subjects show a CSF A-/T-/(N-) profile, consistent with the findings of the present study. Previous data indicated that low baseline CSF Aβ42 levels may predict cognitive decline in PD20,21,22. In the present study, p-tau and t-tau seem to better predict cognitive worsening in PD-MCI. The association between CSF tau species and cognitive decline in PD has been previously reported in DATATOP23 and BioFINDER24 cohorts.
As a measure of axonal degeneration, we investigated CSF NfL, pNfH, and their ratio. Available data consistently show increased levels of NfL in neurodegenerative disorders with worse outcomes, including atypical parkinsonisms as compared to idiopathic PD25. Several reports consistently indicate that increased CSF NfL levels at baseline predict cognitive decline in PD patients14,15,26,27,28. Available findings concerning CSF NfL in PD-MCI are inconsistent, showing either increased15,26,27 or unchanged14 levels vs. cognitively healthy PD. In our cohort, the CSF NfL/p-NfH ratio could show a statistically significant difference between PD-MCI and PD-CN, and CSF NfL was significantly higher in PD-MCI patients showing cognitive worsening with respect to cognitively stable PD-MCI individuals. Additionally, both CSF NfL and NfL/p-NfH were positively associated with Z-score change of cognitive tests in PD-MCI, which further strengthens the role of CSF neurofilaments as markers of worse cognitive outcome in this group of patients.
CSF biomarkers assessing synaptic dysfunction, i.e., α-syn and Ng, did not differ between PD-MCI and PD-CN. In previous studies, CSF α-syn has been shown to be decreased in PD patients when compared to controls29,30, as a possible consequence of its accumulation within the Lewy bodies. However, data from different meta-analyses consistently reported poor diagnostic accuracy of CSF α-syn in discriminating PD31,32. Afterward, CSF α-syn has been found to increase in PD along with disease duration24,33, being also related to the clinical outcome (higher the levels, worse the motor outcome)22. With respect to the relationship between CSF α-syn levels and cognitive decline in PD, available data are inconsistent22,34,35,36,37. In a previous report, no difference in CSF α-syn levels was found between PD-MCI and cognitively unimpaired PD15. Since there is evidence about the increase of CSF α-syn levels with disease duration24,33, the relatively low levels of α-syn found both in PD-MCI and in PD-CN in our series might be also due to the short disease duration of the patients included. CSF α-syn increase has been documented in other neurodegenerative disorders unrelated to α-syn pathology, namely AD38, Creutzfeldt–Jakob disease39 and progressive supranuclear palsy40. Taken together, these findings indicate that CSF α-syn levels unspecifically reflect the ongoing synaptic damage, as already previously proposed22,41,42. However, studies specifically assessing CSF α-syn as a biomarker of synaptic damage in the group of PD-MCI, are actually scanty.
In our series, CSF Ng was increased in MCI-AD, in agreement with previous reports43,44, being unchanged both in PD-CN and PD-MCI. Previous studies in PD cohorts show either unchanged44,45 or decreased levels46,47,48. Ng has been defined as the biomarker of cognition, being specifically related to cognitive functioning not only in AD44, but also in normal aging49 and in PD48,50—the higher the levels, the worse the performance, as a reflection of cortical synaptic damage. Accordingly, in the majority of PD cases, we could justify unchanged CSF Ng levels as a consequence of little/no cortical involvement. With respect to the specific category of PD-MCI, available data are scanty44, making necessary further investigations on this entity.
CSF YKL-40 and sTREM2, reflecting astrocytic and microglial activation respectively, were unchanged both in PD-CN and PD-MCI. Increased levels of these inflammatory markers have been reported in PD patients with MoCA scores <2626. On the contrary, normal levels of CSF sTREM2 have been recently found in both PD-MCI and PD-CN51. In our series, increased baseline levels of sTREM2 were observed in PD-MCI patients showing cognitive worsening after two years. Accordingly, different investigations from the PPMI cohort reported that CSF sTREM2 has a role in predicting cognitive decline in PD26,52, being also increased in those patients with positive CSF tau signature51,52,53. All these findings, taken together, seem to suggest that CSF sTREM2 may be a promising predictor of worse cognitive outcome rather than a diagnostic biomarker in PD-MCI.
As a limitation of our work, we should acknowledge the short follow-up available for our cohort when assessing the progression rate of cognitive impairment in PD. This limitation is partially counterbalanced by the clinical homogeneity of the PD cohort and the thorough neuropsychological and CSF characterization.
Overall, in our retrospective cohort, when compared to cognitively unimpaired PD subjects, patients categorized as PD-MCI did not show a differential CSF neurochemical profile. Not all PD-MCI patients showed cognitive worsening after 2 years (although we should acknowledge the relatively short time window of observation), indicating some heterogeneity in this category, which is in line with previous literature demonstrating that up to 30% of PD-MCI patients can even revert to a normal cognition along a follow-up ranging from 3 to 5 years54,55. Of interest, baseline CSF p-tau, NfL, and sTREM2 were significantly higher in PD-MCI patients showing cognitive worsening, which suggests the possibility to consider these CSF biomarkers as prognostic factors. Our data highlight the need for further investigations on larger, longitudinal cohorts of PD-MCI patients including neuropathological verification.
Methods
Among patients referred to our Center in the period 2016–2019, we retrospectively selected a consecutive series of 88 PD patients, 25 MCI-AD patients, and 44 cognitively healthy patients with OND, i.e., mononeuropathy, dizziness, and headache, for whom CSF and clinical follow-up at 2 years could be available. Similar to what was done for Alzheimer’s disease, in our Center CSF analysis is a routine procedure also in PD patients, as approved by our local Ethics Committee (protocol No.19369/08/AV, registry No. 1287/08, date: 9 October 2008). The study was approved by our local Ethics Committee CER Umbria. All patients gave their written informed consent for the study participation.
PD patients
We included only PD patients with satisfactory motor performance, thus functionally independent, with relatively short disease duration, in order to have a quite homogeneous cohort and to avoid the impact of motor impairment on cognitive functioning. Therefore, the following criteria were considered. Inclusion criteria were: (i) diagnosis of clinically established PD, according to the Movement Disorder Society (MDS) criteria56, (ii) optimal response to dopaminergic challenge test, (iii) age at lumbar puncture (LP) between 60 and 80 years old, (iv) disease duration since symptoms onset <5 years, (v) early—moderate disease stage (H&Y57 < 3), (vi) de novo drug-naïve patients or patients treated with low-middle dosage of dopaminergic drugs, i.e., LEDD58 ≤ 600 mg per day. Exclusion criteria included: (i) L-dopa-induced dyskinesia and motor fluctuations, as well as behavioral disturbances, (ii) diagnosis of PDD, (iii) diagnosis of atypical parkinsonian syndromes, (iv) relevant vascular encephalopathy on brain imaging and recent major stroke (<6 months), (v) recent traumatic brain injury (<30 days); (vi) major systemic disorders. At baseline, PD patients underwent a thorough clinical evaluation including motor assessment and neuropsychological testing, as well as brain imaging to rule out other causes. Motor impairment and disease-related disability were assessed by means of UPDRS-III59 and H&Y57, respectively. Neuropsychological evaluation included MMSE and MoCA as cognitive screening tests, and a comprehensive neuropsychological battery exploring attention and working memory, executive functions, memory, language, and visuospatial functions. Based on neuropsychological evaluation, patients with no cognitive impairment were defined as PD-CN, while PD-MCI patients were defined according to MDS Task Force criteria, level II3. After 2 years, cognitive worsening was defined as a loss of at least 2 points in both MMSE and MoCA, based on available evidence from previous literature60,61,62.
MCI-AD patients
Inclusion criteria were: (i) diagnosis of AD according to the CSF biomarkers profile A+/T+, (ii) score at Clinical Dementia Rating (CDR) scale = 0.5, (iii) age at LP between 60 and 80 years.
OND patients
Inclusion criteria were: (i) minor neurological diseases for which CSF analysis was performed as part of the diagnostic work-up, (ii) CSF AD core biomarkers in the normal range, (iii) normal neuropsychological evaluation at baseline and at 2-year follow-up, (iv) no vascular encephalopathy, recent major stroke, brain injury and major systemic disorders, (v) no ongoing treatments with anti-inflammatory drugs.
CSF sampling and analysis
LP was performed according to international guidelines63. Briefly, 10–12 mL of CSF were collected in sterile polypropylene tubes and centrifuged at room temperature for 10 min (2000×g). Aliquots (0.5 mL) were frozen at −80 °C. All the analyses were carried out by means of commercially available kits. Aβ40, Aβ42, t-tau and p-tau were analyzed using Lumipulse G600-II fully automated chemiluminescent enzyme immunoassay system (Fujirebio Europe, Gent, Belgium). NfL concentrations were determined with NF-light (UmanDiagnostics, Umeå, Sweden), p-NfH with p-NfH (Euroimmun, Lubeck, Germany), Ng with Neurogranin (Trunc P75) (Euroimmun, Lubeck, Germany), t-α-syn with Alpha-Synuclein (Euroimmun, Lubeck, Germany), sAPPα with sAPP-alpha (Tecan, Männedorf, Switzerland), sAPPβ with sAPP-beta wild type (Tecan, Männedorf, Switzerland), sTREM2 with Human TREM2 (Abcam, Cambridge, United Kingdom), and YKL-40 with Human Chitinase 3-like 1 Quantikine (R&D Systems, Minneapolis, MN). All analyses were performed by board-certified laboratory technicians blinded to clinical data. The analyses were performed using one batch of reagents with intra-assay coefficients of variation below 10%.
Statistical analysis
The data analysis was performed by using OriginPro 9 and R v3.6. Normality of both clinical and biochemical features was assessed by means of the Shapiro–Wilk test. Considering the non-normal distribution of some clinical and biochemical variables, the Wilcoxon–Mann–Whitney sum rank test was used to compare biomarkers distributions among groups. All p-values were corrected for multiple group comparisons64. To combine the score changes of both MMSE and MoCA, a Z-score change was calculated65. Correlations among CSF biomarkers, and among CSF biomarkers and clinical measures (MoCA, MMSE, UPDRS-III and H&Y, LEDD, and Z-score change) were calculated by means of Spearman’s (ρ) correlation coefficient. When assessing the significance of the correlations between biomarkers and Z-score changes at two-year follow-up, p-values were adjusted for false-discovery-rate (FDR)64. Linear regression was also applied to further assess the significance of the differences observed among PD groups and the significance of the associations between biomarker levels and clinical parameters by assuming age, sex, and disease duration as covariates. Prior to linear regression, data were rank-transformed to account for non-normality. Logistic regression was applied to determine differences in CSF biomarkers levels between PD-MCI with and without cognitive worsening assuming age, sex, and disease duration as covariates.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
All relevant data generated during this study are available from the corresponding author on reasonable request.
References
Aarsland, D. et al. Parkinson disease-associated cognitive impairment. Nat. Rev. Dis. Prim. 7, 47 (2021).
Jack, C. R. et al. NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 14, 535–562 (2018).
Litvan, I. et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Mov. Disord. 27, 349–356 (2012).
Goldman, J. G., Aggarwal, N. T. & Schroeder, C. D. Mild cognitive impairment: an update in Parkinson’s disease and lessons learned from Alzheimer’s disease. Neurodegener. Dis. Manag. 5, 425–443 (2015).
Wallace, E. R., Segerstrom, S. C., van Horne, C. G., Schmitt, F. A. & Koehl, L. M. Meta-analysis of cognition in Parkinson’s disease mild cognitive impairment and dementia progression. Neuropsychol. Rev. 32, 149–160 (2022).
Halliday, G. M., Leverenz, J. B., Schneider, J. S. & Adler, C. H. The neurobiological basis of cognitive impairment in Parkinson’s disease. Mov. Disord. 29, 634–650 (2014).
Adler, C. H. et al. Heterogeneous neuropathological findings in Parkinson’s disease with mild cognitive impairment. Acta Neuropathol. 120, 827–828 (2010).
Knox, M. G. et al. Neuropathological findings in Parkinson’s disease with mild cognitive impairment. Mov. Disord. 35, 845–850 (2020).
Wen, M. C., Chan, L. L., Tan, L. C. S. & Tan, E. K. Mild cognitive impairment in Parkinson’s disease: a distinct clinical entity? Transl. Neurodegener. 6, 24 (2017).
Irwin, D. J. et al. Neuropathologic substrates of Parkinson’s disease dementia. Ann. Neurol. 72, 587–598 (2012).
van Steenoven, I. et al. Cerebrospinal fluid Alzheimer’s disease biomarkers across the spectrum of Lewy body diseases: results from a Large Multicenter Cohort. J. Alzheimers Dis. 54, 287–295 (2016).
Bellomo, G. et al. A/T/(N) profile in cerebrospinal fluid of Parkinson’s disease with/without cognitive impairment and dementia with Lewy bodies. Diagnostics 10, 1015 (2020).
Beyer, M. K. et al. Cerebrospinal fluid Aβ levels correlate with structural brain changes in Parkinson’s disease. Mov. Disord. 28, 302–310 (2013).
Bäckström, D. C. et al. Cerebrospinal fluid patterns and the risk of future dementia in early, incident Parkinson disease. JAMA Neurol. 72, 1175–1182 (2015).
Olsson, B. et al. Association of cerebrospinal fluid neurofilament light protein levels with cognition in patients with dementia, motor neuron disease, and movement disorders. JAMA Neurol. 76, 318–325 (2018).
Montine, T. J. et al. CSF Aβ42 and tau in Parkinson’s disease with cognitive impairment. Mov. Disord. 25, 2682–2685 (2010).
Yarnall, A. et al. ICLE-PD Study Group. Characterizing mild cognitive impairment in incident Parkinson disease: the ICICLE-PD study. Neurology 82, 308–316 (2014).
Yu, S.-Y. et al. Potential biomarkers relating pathological proteins, neuroinflammatory factors and free radicals in PD patients with cognitive impairment: a cross-sectional study. BMC Neurol. 14, 113 (2014).
Skogseth, R. E. et al. Associations between cerebrospinal fluid biomarkers and cognition in early untreated Parkinson’s disease. Parkinsons Dis. 5, 783–792 (2015).
Siderowf, A. et al. CSF amyloid β 1-42 predicts cognitive decline in Parkinson disease. Neurology 75, 1055–1061 (2010).
Parnetti, L. et al. Differential role of CSF alpha-synuclein species, tau, and Aβ42 in Parkinson’s disease. Front. Aging Neurosci. 75, 1055–1061 (2014).
Hall, S. et al. CSF biomarkers and clinical progression of Parkinson disease. Neurology 84, 57–63 (2015). Jan 6.
Liu, C. et al. CSF tau and tau/Aβ42 predict cognitive decline in Parkinson’s disease. J. Parkinsonism Relat. Disord. 21, 271–276 (2015).
Hall, S. et al. Longitudinal measurements of cerebrospinal fluid biomarkers in Parkinson’s disease. Mov. Disord. 31, 898–905 (2016).
Gaetani, L. et al. Neurofilament light chain as a biomarker in neurological disorders. J. Neurol. Neurosurg. Psychiatry 90, 870–881 (2019).
Bartl, M. et al. Parkinson’s Progression Markers Initiative. Biomarkers of neurodegeneration and glial activation validated in Alzheimer’s disease assessed in longitudinal cerebrospinal fluid samples of Parkinson’s disease. PLoS ONE 16, e0257372 (2021).
Lerche, S. et al. CSF NFL in a Longitudinally Assessed PD Cohort: age effects and cognitive trajectories. Mov. Disord. 35, 1138–1144 (2020).
Aamodt, W. W. et al. Neurofilament light chain as a biomarker for cognitive decline in Parkinson disease. Mov. Disord. 36, 2945–2950 (2021).
Parnetti, L. et al. Cerebrospinal fluid Tau/α-synuclein ratio in Parkinson’s disease and degenerative dementias. Mov. Disord. 26, 1428–1435 (2011).
Parnetti, L. et al. Cerebrospinal fluid lysosomal enzymes and alpha-synuclein in Parkinson’s disease. Mov. Disord. 29, 1019–1027 (2014).
Gao, L. et al. Cerebrospinal fluid alpha-synuclein as a biomarker for Parkinson’s disease diagnosis: a systematic review and meta-analysis. Int. J. Neurosci. 125, 645–654 (2015).
Eusebi, P. et al. Diagnostic utility of cerebrospinal fluid α-synuclein in Parkinson’s disease: a systematic review and meta-analysis. Mov. Disord. 32, 1389–1400 (2017).
Majbour, N. et al. Longitudinal changes in CSF alpha-synuclein species reflect Parkinson’s disease progression. Mov. Disord. 31, 1535–1542 (2016).
Stewart, T. et al. Cerebrospinal fluid α-synuclein predicts cognitive decline in Parkinson disease progression in the DATATOP cohort. Am. J. Pathol. 184, 966–975 (2014).
Pagano, G. et al. REM behavior disorder predicts motor progression and cognitive decline in Parkinson disease. Neurology 91, e894–e905 (2018).
Førland, M. G. et al. Evolution of cerebrospinal fluid total α-synuclein in Parkinson’s disease. Parkinson. Relat. Disord. 49, 4–8 (2018).
Parnetti, L. et al. CSF and blood biomarkers for Parkinson’s disease. Lancet Neurol. 18, 573–586 (2019).
Barba, L. et al. Cerebrospinal fluid β-synuclein as a synaptic biomarker for preclinical Alzheimer’s disease. Neurol. Neurosurg. Psychiatry 94, 83–86 (2023).
Oeckl, P. et al. Alpha-, beta-, and gamma-synuclein quantification in cerebrospinal fluid by multiple reaction monitoring reveals increased concentrations in Alzheimer’s and Creutzfeldt-Jakob disease but no alteration in synucleinopathies. Mol. Cell Proteom. 15, 3126–3138 (2016).
Sako, W., Murakami, N., Izumi, Y. & Kaji, R. Reduced alpha-synuclein in cerebrospinal fluid in synucleinopathies: evidence from a meta-analysis. Mov. Disord. 29, 1599–1605 (2014).
Mastrangelo, A. et al. Diagnostic and prognostic performance of CSF α-synuclein in prion disease in the context of rapidly progressive dementia. Alzheimers Dement. (Amst.). 13, e12214 (2021).
Barba, L. et al. Alpha and beta synucleins: from pathophysiology to clinical application as biomarkers. Mov. Disord. 37, 669–683 (2022).
Wellington, H. et al. Increased CSF neurogranin concentration is specific to Alzheimer disease. Neurology 86, 829–835 (2016).
Portelius, E. et al. Cerebrospinal fluid neurogranin concentration in neurodegeneration: relation to clinical phenotypes and neuropathology. Acta Neuropathol. 136, 363–376 (2018).
Nilsson, J., Constantinescu, J., Nellgård, B., Jakobsson, P. & Brum, W. Cerebrospinal fluid biomarkers of synaptic dysfunction are altered in Parkinson’s disease and related disorders. Mov. Disord. 38, 267–277 (2023).
Selnes, P. et al. Impaired synaptic function is linked to cognition in Parkinson’s disease. Ann. Clin. Transl. Neurol. 4, 700–713 (2017).
Hall, S. et al. Cerebrospinal fluid levels of neurogranin in Parkinsonian disorders. Mov. Disord. 35, 513–518 (2020).
Sancesario, G. M. et al. Amyloid-β42/neurogranin ratio as a potential index for cognitive impairment in Parkinson’s disease. Alzheimers Dis. 76, 1171–1178 (2020).
Casaletto, K., Elahi, F., Bettcher, B., Neuhaus, J. & Bendlin, B. Neurogranin, a synaptic protein, is associated with memory independent of Alzheimer biomarkers. Neurology 89, 1782–1788 (2017).
Bereczki, E. et al. Synaptic proteins in CSF relate to Parkinson’s disease stage markers. NPJ Parkinsons Dis. 3, 7 (2017).
Wilson, E. N. et al. Soluble TREM2 is elevated in Parkinson’s disease subgroups with increased CSF tau. Brain 143, 932–943 (2020).
Qin, Q., Wan, H., Wang, D., Li, J. & Qu, Y. The association of CSF sTREM2 with cognitive decline and its dynamic change in Parkinson’s disease: analysis of the PPMI Cohort. Front. Aging Neurosci. 14, 892493 (2022).
Suárez-Calvet, M. et al. sTREM2 cerebrospinal fluid levels are a potential biomarker for microglia activity in early-stage Alzheimer’s disease and associate with neuronal injury markers. EMBO Mol. Med. 8, 466–476 (2016). May 2.
Pedersen, K. F., Larsen, J. P., Tysnes, O.-B. & Alves, G. Natural course of mild cognitive impairment in Parkinson disease: A 5-year population-based study. Neurology 88, 767–774 (2017).
Pedersen, K. F., Larsen, J. P., Tysnes, O. & Alves, G. Prognosis of mild cognitive impairment in early Parkinson disease: the Norwegian ParkWest study. JAMA Neurol. 70, 580–586 (2013).
Postuma, R. B. et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 30, 1591–1601 (2015).
Goetz, C. G. et al. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations. Mov. Disod. 19, 1020–1028 (2004).
Tomlinson, C. L. et al. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov. Disord. 25, 2649–2653 (2010).
Goetz, C. G. et al. Movement Disorder Society-Sponsored Revision of the Unified Parkinson‟s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov. Disord. 23, 2129–2170 (2008).
Lessig, S., Nie, D., Xu, R. & Corey-Bloom, J. Changes on brief cognitive instruments over time in Parkinson’s disease. Mov. Disord. 27, 1125–1128 (2012).
Biundo, R. et al. MMSE and MoCA in Parkinson’s disease and dementia with Lewy bodies: a multicenter 1-year follow-up study. J. Neural Transm. (Vienna). 123, 431–438 (2016).
Faust-Socher, A. et al. Responsiveness to change of the Montreal cognitive assessment, mini-mental state examination, and SCOPA-Cog in non-demented patients with Parkinson’s disease. Dement. Geriatr. Cogn. Disord. 47, 187–197 (2019).
Teunissen, C. E. et al. A consensus protocol for the standardization of cerebrospinal fluid collection and biobanking. Neurology 73, 1914–1922 (2009).
Benjamini, Y. & Hochberg, Y. Controlling the False Discovery Rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodol.) 57, 289–300 (1995).
Cutter, G. R. et al. Development of a multiple sclerosis functional composite as a clinical trial outcome measure. Brain 122, 871–882 (1999).
Acknowledgements
L.P. was supported by the Marlene and Paolo Fresco Institute for Parkinson’s and Movement Disorders, Fresco Network of Excellence Italy Sites. This organization did not have direct input in any aspect of this work. G.B. is supported by the Postdoctoral Fellowship for Basic Scientists grant of the Parkinson’s Foundation (Award ID: PF-PRF-934916). We are thankful to Euroimmun for providing the ELISA kits and to Silvia Paciotti for her help in biomarker measurements at the Laboratory of Clinical Neurochemistry, University of Perugia.
Author information
Authors and Affiliations
Contributions
F.P.P. and L.G. made the first draft of the manuscript and contributed to the interpretation of the data. G.B. carried out the statistical analysis, helped in the interpretation of data, and prepared the figures. E.C., N.S., C.M., A.M., M.F., P.N., S.S., N.T. contributed to the acquisition of the data. M.D.F. contributed to the revision of the manuscript. L.P. conceived the study, coordinated the analysis and interpretation of data, and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Paolini Paoletti, F., Gaetani, L., Bellomo, G. et al. CSF neurochemical profile and cognitive changes in Parkinson’s disease with mild cognitive impairment. npj Parkinsons Dis. 9, 68 (2023). https://doi.org/10.1038/s41531-023-00509-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41531-023-00509-w