Abstract
The wide range of alloy composition controllability for multi-principal element alloys (MPEAs) may provide a great opportunity for discovering special forms of surface oxides to improve the corrosion and oxidation resistance in extreme environments. Changing the type and content of promoting passivation elements would not only change the microstructure of the alloy but also significantly affect the composition and structure of the surface passive film, resulting in a strong impact on the corrosion and oxidation resistance of the alloy. This article reviews recent research on the effects of alloying elements on the passivation properties, the contribution of each alloying element, and the synergistic effect between the elements on the passivation mechanisms and electrochemical dissolution characteristics of surface passive films that form on some MPEAs. In addition, the composition and structural characteristics of surface oxides relevant to the selective oxidation of elements are elaborated upon. Finally, several open questions and recommendations for research directions regarding the passivation and selective oxidation of MPEAs were provided to guide future exploration.
Similar content being viewed by others
Introduction
Multi-principal element alloys (MPEAs) have attracted the interest of materials scientists and engineers, who have aimed to investigate their mechanical properties, as well as wear, corrosion, and oxidation resistance. The high number of principal elements in MPEAs will increase the degree of freedom in the alloy composition, especially when non-equiatomic multi-principal elements are considered. Corrosion resistance is a performance index that must be met in the design and development of MPEAs as structural materials. Currently, research on the corrosion behavior of MPEAs has mainly focused on alloy systems composed of transition metal elements. The development of corrosion-resistant MPEAs has mainly considered the alloy composition design, manufacturing method, microstructure, corrosion mechanism, and surface passive film1,2,3,4,5. Most studies have focused on the effect of adding alloying elements, such as Cu, Al, Mn, Mo, Ti, Nb, V, and Si, on the corrosion resistance and corrosion mechanisms of CoCrFeNi-based MPEAs6. The addition of these alloying elements was found to affect the microstructure and phase structure evolution, thus affecting the corrosion performance of the MPEAs7,8,9,10. Based on previous research on the corrosion behavior of MPEAs, the addition of alloying elements Cr11,12,13, Mo8,14, Co15,16, Ni17,18, Ti19,20, and Nb21,22 was found to be generally beneficial, as long as no second phase formed, and they were usually present in the passive film. However, the addition of Cu10,23,24, Al25,26,27,28, and Mn7,29,30 was found to be oftentimes detrimental to corrosion resistance, which was mainly due to the phase transition that produced chemical and structural heterogeneity. The chemical composition of a bulk alloy will determine the composition of the passive film that forms on the alloy surface, especially the enrichment of beneficial elements in the film, thus influencing the initiation process of local corrosion behavior. The oxides that form on the surfaces of heterogeneities such as the phases of barren Cr element will be less protective and will break down or will undergo preferential dissolution at these regions. Thus, MPEAs with a single-phase structure and uniform distribution of alloying elements will be expected, contributing to enhancing the composition uniformity and structural stability of the passive films31,32,33. MPEAs with refractory elements, such as Ti, Zr, Nb, Ta, Mo, V, W, and Hf, namely refractory multi-principal element alloys (RMPEAs), have mostly sample BCC crystal structures and may also contain some intermetallic phases, mainly B2 and/or laves34,35,36,37,38,39. Some RMPEAs can maintain stability in the phase structure with increasing alloying elements and heat treatment temperature40,41,42,43, which will be conducive to maintaining high corrosion resistance. More importantly, these refractory elements will all easily form protective oxides that are thermodynamically stable across a broad range of potential and pH values44. Therefore, the characteristics of the passive film that govern the corrosion behavior of the MPEAs include the composition and structure uniformity, compactness, and electronic properties.
MPEAs may form passive films with superior protective properties against corrosion depending on either the presence of large amounts of passivating elements or the formation of specific oxides based on the unusual synergistic effects of alloying elements45,46. The high-entropy effect of MPEAs will contribute to achieving a single-phase solid solution, even with the addition of complex elements at a high concentration, which will decrease the occurrence of elemental segregation and favor the formation of a uniform surface passive film. The change in corrosion resistance and the corrosion mechanism of MPEAs can be extensively explored by adjusting the composition and content of alloying elements over a wide range. Passivity models constructed by single functional elements have been developed for pure metals and conventional single-principal element alloys (SPEAs) but may not be directly available for MPEAs47. Beneficial combinations of alloying elements or vacancies may be envisioned to produce synergistic corrosion benefits such as charge distribution48,49, passivity promotion and dissolution blocking50, the vacancy-mediated diffusion model51, and the point-defect model52,53. The chemical potential and activity of each alloying element may differ in an MPEA substrate, affecting the passivation and dissolution characteristics of the surface oxides. Thus, determining the contribution of each alloying element to the passivation and dissolution characteristics of MPEAs is essential for understanding the synergistic effect of the alloying elements and the corrosion resistance mechanism of MPEAs54,55,56.
The composition and structure of the surface oxide are also key factors that affect the corrosion resistance of the oxide film, and the presence of multi-elements at high concentrations may affect the formation of surface oxide structures in many ways. The Gibbs free energy of oxide formation of the elements and the transport rate of cations will determine the spatial distribution of elemental fractions in the bulk oxide film57. Uneven oxide films with multi-layer structures have been commonly reported in MPEAs composed of transition metal elements, such as NiCr20Mo1058, FeCr15Ni1559, CrMnFeCoNi60,61,62, CrFeCoNiMo63, Ni38Fe20Cr22Mn10Co1064,65, and Ni38Cr21Fe20Ru13Mo6W266 alloys after exposure to air or a solution environment, which was attributed to the selective oxidation behavior of each component at the metal/oxide interface. Selective oxidation can also lead to the enrichment or depletion of some beneficial elements in the oxide film, relative to their concentration in the alloy matrix. In addition to the formation of layered oxide film structures, complicated altered layers will likely exist underneath the oxide film, including modified alloy layers60,61 with void formation by both vacancy injection and Kirkendall voiding67. From the study of pure metals and conventional SPEAs, the surface oxide will generally form the most thermodynamically stable stoichiometric oxide form, based on the phase equilibrium expectations. In the case of MPEAs, superior corrosion or oxidation resistance can also be brought about by the formation of unexpected compositionally complex oxides68. More recent work utilizing three-dimensional atom-probe tomography (3D-APT) and transmission electron microscopy (TEM) has confirmed the non-stoichiometric ratios for any alloying elements and oxygen, suggesting that a metastable oxide formed on the MPEAs. The oxides could contain a dominant passivator affected by the other elements, with multiple oxide phases, and the formation of complex multi-cation oxides, which may result in the solid solution forming a highly protective oxide instead of phase-separated stoichiometric oxides. The formation of these multiple oxides was attributed to the occurrence of nonequilibrium solution capture at the interface between the metal and oxides during the growth of the oxide film69.
At present, research on the passivation and selective oxidation of MPEAs is in the preliminary exploration stage. Figure 1 illustrates the contributions of some research aspects in passivation and selective oxidation to the corrosion and oxide resistance of MPEAs. However, the role of each element in the formation and growth of a complex oxide, as well as the electrochemical dissolution characteristics, the relative mobility, and reactivity of cations in the oxide film, has remained elusive. Based on recent investigations, this article reviews passivation and selective oxidation and reveals the corrosion resistance of MPEAs associated with their passive film characteristics. The main contents include three aspects: (1) the effect of alloying elements on passivation properties, (2) the contribution of the alloying elements to the passivation mechanisms and electrochemical dissolution characteristics of MPEAs, (3) the composition and structural characteristics of surface oxides due to selective oxidation.
Effect of alloying elements on passivation properties
The changes in types and contents of alloying elements not only affect the microstructure of MPEAs but also significantly affect the composition and microstructure of the surface passive film, thereby influencing its passivation properties. Based on a thorough assessment of literature reports, the effect of alloying elements on the passivation properties was obtained for some MPEAs, as displayed in Table 1. The role of each alloying element in the stability of passive films on some MPEAs was elaborated as follows.
Chromium
Cr remains one of the most important alloying elements for improving the corrosion resistance of alloys. Because its passivating character is well-known for conventional SPEAs, it is reasonable to hypothesize that a similar effect could also be produced for MPEAs. A critical concentration of Cr is considered the key to enhancing the corrosion resistance of the alloy. It has been suggested that more than 12–13 at.% of Cr content in Fe-Cr binary alloys will be needed to achieve to complete primary passivation70,71. Cr can easily form Cr2O3 due to the lowest free energy of formation, with the content, stability, and distribution in the passive film remarkably influencing corrosion resistance in binary alloys as well as MPEAs72.
Shang et al.73 investigated the passivation properties of equal-atomic NiCo, NiCoCr, NiCoFe and NiCoCrFe alloys in NaCl and HCl solutions. When the Cr element was added to the alloys, the passive region in the polarization curves was considerably extended for NiCoCr and NiCoCrFe alloys in the NaCl solution. However, the passive region was not observed for NiCo and NiCoFe alloys in the HCl solution, indicating that the oxides/hydroxides of Ni, Co, and Fe were unstable and dissolved in the HCl solution. Thus, the introduction of Cr could inhibit the dissolution of the passive film for NiCoCr and NiCoCrFe alloys. Chai et al.11 found that the corrosion resistance of FeCoNiCrx (x = 0, 0.5, 1.0) MPEAs with an FCC structure was dependent on the Cr content in H2SO4 and NaCl solution. The passive film on equiatomic FeCoNi alloy was composed of Fe2O3, NiO, and CoO, which was less stable than the Cr-rich passive film on the FeCoNiCr0.5 alloy. A further increase in Cr content would increase the segregation of Cr elements in the interdendrites in the FeCoNiCr alloy, deteriorating the corrosion resistance, as a discontinuous state of the passive film on the alloy surface would result from the inhomogeneous distribution of the Cr element in the matrix. Lu et al.12 prepared Ni38Fe20CrxMn21–0.5xCo21–0.5x (x = 6, 10, 14, and 22) MPEAs and investigated the effect of Cr content on the corrosion resistance in NaCl solution. The microstructure maintained single-phase FCC with uniformly distributed compositions after solutionization. The pitting resistance of the passive film increased with increasing Cr content, which was confirmed by the increased passivation domain in the potentiodynamic polarization curves. Notably, the alloy with only 6 at.% Cr could still exhibit self-passivation at the open-circuit potential (OCP), despite the Cr content being below the typical reported thresholds.
Yan et al.74 found that the microstructure of Al0.3CrxFeCoNi (x = 0–2.0) alloys changed from single-phase FCC to FCC + BCC/B2 structures with increasing Cr content in the alloys. The increased Cr content induced a high concentration of Cr over 30 at.% in both the FCC and BCC/B2 phases, which was much higher than the other constituent elements, and the corrosion resistance increased with increased Cr content in the alloys. As shown in Fig. 2a, the alloys with higher Cr content at x = 1.5–2.0 exhibited no breakdown potential under anodic polarization up to 2.0 VSCE in the NaCl solution, which was attributed to the formation of passive films enriched in Cr2O3, based on X-ray photoelectron spectroscopy (XPS) analysis (Fig. 2b). Han et al.75 studied the pitting resistance of passive films on ultra-fine grained (UFG) and coarse-grained (CG) CoCrFeMnNi alloys and found that Cr-depleted regions formed in the UFG alloy, whereas a more homogeneous chromium element distribution was observed in the CG alloy. The enhanced diffusion of point defects through the passive film that formed in the Cr-depleted regions on the UFG alloy occurred easily, thus weakening the stability of the passive film. Huang et al.76 showed that the Co element could increase the stability of the passive film by inhibiting the hydrolysis of Cr3+, while Ni promoted the hydrolysis of Cr3+, weakening the stability of the passive film on CoCrNiAlTi alloy in H2SO4 solutions.
a Potentiodynamic polarization curves of the Al0.3CrxFeCoNi (x = 0–2.0) alloys in the 0.6 M NaCl solution, b XPS spectra of Cr 2p3/2 in the air-formed surface films for the Al0.3CrxFeCoNi (x = 0–2.0) alloys74. (Elsevier Copyright, Original Order Number: 501847115, https://s100.copyright.com/CustomerAdmin/PLF.jsp?ref=9f52b130-a732-4788-83ec-5fe3ca85d13b).
Thus, the change of microstructure due to the introduction of Cr, the content and distribution uniformity of Cr in the passive film, and the influence of other elements coupled with Cr determined the stability of passive films in MPEAs.
Molybdenum
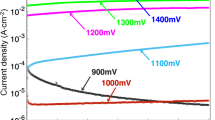
The enhanced corrosion resistance of stainless steel due to Mo addition has been extensively investigated. Mo-oxides or Mo-oxyhydroxides can inhibit the formation of active sites on passive films, thus impeding the pitting corrosion process77,78. Research has also shown that Mo can promote the formation of Cr2O3 or the enrichment of Cr content in the passive film because Mo can promote the deprotonation of OH− so as to provide O2− to enhance the formation of inner Cr oxides79,80,81,82,83. Li et al.84 confirmed the beneficial effects of Mo on the formation of passive films in Ni68Cr22Mo10 alloy through a comparison with an Ni78Cr22 alloy and pure Ni and Mo. Mo could facilitate the active-to-passive transition in the low potential domain by comparing the potentiodynamic polarization curves, which were attributed to the enhanced oxidation of Cr to Cr2O3 over Cr(OH)3, as promoted by Mo addition. Dai et al.8 studied the effect of Mo content on the corrosion behavior of FeCoCrNiMox (x = 0, 0.1, 0.3, and 0.6) MPEAs and found that the addition of Mo could decrease the point-defect density and enhance the Cr2O3/Cr(OH)3 ratio in the passive film on the FeCoCrNiMo0.1 alloy, which significantly improved the passivation ability compared to the Mo0 alloy. However, precipitates were observed in FeCoCrNiMo0.3 with an increase in the FeCoCrNiMo0.6 alloys, which induced preferentially localized corrosion at the regions around the precipitates depleted in Cr and Mo. Wang et al.14 found that the corrosion resistance of the (CoCrFeNi)100-xMox (x = 1, 2, 3) alloys gradually increased with increasing Mo content. However, the precipitated phases were observed in the Mo3 alloy aged at 900 °C, resulting in decreased corrosion resistance. The passive film of the (CoCrFeNi)100-xMox alloys mainly consisted of Cr2O3 and MoO3, and the interface between Cr2O3 and MoO3 was more susceptible to the cracking of Cl− ions. Chen et al.85 reported that Mo addition significantly improved the pitting resistance of Al-Mn-Mo alloys due to decreased n-type defect density in the passive film. Moreover, molecular dynamics simulations showed that the larger interatomic distance in Al-Mn-Mo compared to Al-Mn led to more Al-O bond formation, resulting in a more compact passive film. Wang et al.86 investigated the pitting resistance of the passive film on single FCC phase Cr-Fe-Co-Ni-Mo MPEAs with different Mo contents in H2SO4 with increasing Cl− ion concentration by potentiostatic analysis. As shown in Fig. 3, the alloys without Mo addition, such as Cr35Fe20Co5Ni40, 316 L SS, and the Cantor alloy, showed a stable pitting current peak at lower Cl− ion concentrations, which was indicative of the initiation of stable pitting once breakdown of the passive film occurred. Notably, no passivity breakdown was observed for the MPEAs containing Mo, even at high Cl− ion concentrations, confirming the beneficial effect of the combined alloying of Cr and Mo on the enhanced corrosion resistance of the passive film. Alloying of Cr mainly provided a role in passive film formation and protection against generalized dissolution, as indicated by the negative correlation of the passive current densities with Cr content in these alloys under the condition of potentiodynamic analysis in pure H2SO4 solution. The addition of Mo provided a tremendous beneficial effect on the corrosion properties with the formation of a passive film resistant to local passivity breakdown induced by Cl− ion attack.
a MPEA-Cr15Mo10 (Cr15Fe10Co5Ni60Mo10), b MPEA-Cr25Mo5 (Cr25Fe25Co5Ni40Mo5), c MPEA-Cr35 (Cr35Fe20Co5Ni40), d Ni71Cr23Mo6 (Cr35Fe20Co5Ni40), e 316 L SS, f Cantor alloy86. (Elsevier Copyright, Original Order Number: 501847644, https://s100.copyright.com/CustomerAdmin/PLF.jsp?ref=a5f27cf1-813d-4e3a-9705-5bb36c981d95).
Manganese
Manganese is one of the main alloying elements in Co-Cr-Fe-Ni-Mn MPEAs due to its effect on phase stability. Mn has an adverse effect on the corrosion resistance of MPEAs in H2SO4 and NaCl solutions. Hsu et al.30 elucidated the effect of Mn on the passivation behavior of FeCrNiCoMnx (x = 0, 0.3, 0.6, and 1.0) MPEAs in 0.5 M H2SO4 solution and found that a decrease in Mn addition could provide the improved corrosion resistance of FeCrNiCoMnx alloys. Increasing the Mn content will reduce Cr2O3 in the passive film due to the competitive oxidation of Mn with Cr, leading to deteriorated corrosion resistance. The effect of Mn on the electrochemical passivation behavior of equiatomic CoFeNiMnCr, (CoFeNi)80Cr20, (CoFeNiMn)75Cr25, and equiatomic CoFeNiCr MPEAs in 0.1 M H2SO4 solution was investigated, which showed that the addition of Mn mainly suppressed the passivation processes and increased the chemical dissolution rate of passive films, as determined by the EIS and potentiodynamic polarization7. The detrimental effects of Mn on passive film stability were caused by the suppression of oxygen or OH− adsorption during the passivation process. Mn was found to adsorb oxygen or OH− more easily than Cr, forming insoluble compounds87 or weak and porous oxides88, deteriorating the passivity. It has been found that the passive film formed on the equiatomic CrMnFeCoNi alloy was more defective than that formed on the equiatomic CrFeCoNi alloy, which meant that Mn addition facilitated the formation and migration of oxygen vacancies in the passive film29,89. However, for other MPEAs, such as Sn91Zn9 alloy, Mn alloying can markedly reduce the donor densities of the passive films and enhance their stability90.
Cobalt
Previous studies have shown that equiatomic CoCr and CoCrMo alloys showed more corrosion resistance than equiatomic NiCr and NiCrMo alloys, respectively, indicating that Co was a strong passivating agent91. Qiu et al.92 found that increasing the Co content resulted in the enhanced corrosion resistance of Al2CrFeCoxCuNiTi (x = 0, 0.5, 1.0, 1.5, 2.0) MPEAs in HCl solution. Zhao et al.16 showed that the corrosion resistance of the CoxCrCuFeMnNi (x = 0.5, 1.0, 1.5, 2.0) MPEAs in 0.6 M NaCl solution was enhanced with the increase of Co content, due to the increase in thickness and resistance of the passive films with the increase of Co content. In addition, Co enhanced the stability of Cr2O3 and Ni(OH)2 in the passive film through the formation of the Co(OH)2 adsorption layer on the alloy surface93. Wei et al.94 assessed the pitting resistance of Fe50Mn25Cr15Ni10-xCox (x = 0, 5, and 10) MPEAs in NaCl solution. The pitting potential decreased with the increase in Co content, indicating a decrease in pitting resistance due to the increase in grain boundary density in the microstructure. However, the passive film composition was not assessed in detail. Alqarni et al.95 showed that Co addition enhanced the resistance to uniform and pitting for Ni52Ti48-xCox (x = 0, 1.5, and 4.0) MPEAs in NaCl solution. The chemistry of the passive film was not dramatically influenced by Co addition and was primarily composed of TiO2. Moreover, the amount of Cl− ions that adsorbed on the passive film significantly decreased with Co addition, based on the XPS analysis results. The protective mechanism of the passive film that formed on the non-equiatomic Fe40Ni20Co20Cr20 MPEAs in 0.1 M H2SO4 was proposed by Wu et al.96, based on the XPS results. The study showed that Co cations tended to invade the lattice of (Cr,Fe)2O3 (Fe2O3 or Cr2O3) and replace trivalent Fe and Cr ions at the octahedral sites, forming some mixed CoCr2O4 and CoFe2O4 oxides with more dense structures and chemical stability, resulting in the formation of a more compact passive film than that formed on the Co-free MPEAs.
Aluminum
Al is also an element that is prone to forming compact oxide films that decrease the corrosion rate. However, for MPEAs, the introduction of Al can easily cause the formation of multiphase structures and aggravate element segregation in microstructures, thus influencing the stability of passive films27. The influence of Al on the microstructure and corrosion resistance of Alx(CoCrFeNi)100-x (x = 4.5–40) thin films was evaluated by Shi et al.97. The thin films with a uniform elemental distribution possessed outstanding corrosion resistance, but the resistance decreased with increasing Al content. It was found that increasing the Al content resulted in more Al oxide/hydroxide and less Cr oxide/hydroxide formation, weakening the compactness and protective effect of the passive films. Nascimento et al.98 compared the pitting resistance of equiatomic CoCrFeNi and CoCrFeNiAl alloys in 0.6 M NaCl solution. The introduction of Al decreased the relative fraction of Cr2O3 and Cr(OH)3 which are all belong to the p-type semiconducting compounds but enhanced the n-type semiconducting character of the passive film due to the increase in Al oxidized species. Al addition also yielded the formation of more defective oxides, thus hampering the pitting resistance of surface passive film on the CoCrFeNiAl MPEAs. Raza et al.99 investigated the pitting resistance of AlxCrFeMoV (x = 0, 0.2, 0.6, 1) MPEAs and found that the addition of Al did not have an adverse effect on the pitting resistance. This was attributed to the absence of the Cr-depleted and Al-rich BCC phase in the microstructure, favoring the formation of uniform composition distribution in the surface passive film. Recently, the effect of Al addition on the corrosion resistance of CoNiVAlx (x = 0, 0.1, 0.2, and 0.3) MPEAs was investigated100. which showed that Al addition decreased the defect density in the passive film, thus improving the corrosion resistance of the CoNiVAlx MPEAs. The increase in Al addition also led to the increase of metallic oxide in the film, which was confirmed by the enhanced ratio of Al/V and O2−/OH−. Fu et al.101 prepared homogenized FeCoCrNiAlx (x = 0.1, 0.3) MPEAs with a single-phase structure and uniform element distribution and evaluated their corrosion resistance in HCl and H2SO4 solutions. The increasing Al content enhanced the oxide/hydroxide ratios of Cr, Fe, and Al, which provided a more compact film to resist the dissolution of metal ions in the H2SO4 solution. However, increasing the Al content could enhance the combination of Cl− ions with the Al-containing metastable ion complexes, accelerating the dissolution of the MPEAs in the HCl solution.
Titanium
Wu et al.102 found that Ti addition raised the microstructural heterogeneity weakening of the local galvanic effect. Moreover, the strong passivation capability of Ti contributed to the formation of a more protective passive film, suppressing the pitting attack on the AlCrFeCoNi MPEAs in NaCl and HCl solutions. The contents of oxidized Cr3+ and Ti4+ in the passive film were the main contribution to the improved corrosion resistance of this MPEA system103. However, the unoxidized Ti atoms in the passive film were also a considerable factor that was responsible for the improved pitting resistance104. Ti addition promoted the formation of single-phase structures for FeCoNiCuTix (x = 0, 0.2, and 0.4) MPEAs, thus improving the stability of the passive film on the surface105. Wang et al.106 found that Ti addition produced the high energy barriers at the Cr2O3/TiO2 oxide interfaces in the passive film, which could increase the energy barriers of adsorption and diffusion for Cl− ions.
Niobium
The effect of Nb addition on the passivation behavior of CoCrNiNbx (x = 0–0.5) MPEAs in NaCl and HCl solutions were investigated by Wang et al.21. The addition of Nb effectively improved the repassivation ability and reduced pitting sensitivity, which was attributed to Nb addition promoting the production of Cr2O3 oxides in the passive film. Moreover, the increase in Nb alloying degree promoted the structural change in the passive film from a single layer to a bi-layer structure with a Cr-enriched outer layer and Nb-enriched inner layer. Wen et al.107 revealed that the improved passive film stability was mainly attributed to the reduced point-defect density in the passive film of Ni1.5CrCoFe0.5Mo0.1Nbx (x = 0.55, 0.68, and 0.8) MPEAs due to the increased content of Nb addition. Tanji et al.108 showed that increasing Nb content in the TiHfZrNbx (x = 0.2, 0.3, and 0.4) MPEAs resulted in a greater resistance of the passive film due to decreasing concentration of point defects. Nb had a smaller atomic radius (1.42 Å) than Ti, Hf, and Zr (1.46, 1.57, and 1.60 Å, respectively), which facilitated the mobility and diffusivity of the Nb cations, occupying the formed vacancies, reducing its concentration in the passive film, and maintaining the electrical neutrality of the passive film.
Tungsten
W addition could also improve the pitting resistance and passivation properties of the CoCrFeNiWx (x = 0, 0.2, 0.5) MPEAs in seawater solution109. W-added WxFeCoNiCrMn MPEAs (x = 0, 0.2, 0.4, 0.6, 0.8, and 1.0) had an improved corrosion resistance referring to the base MPEAs and W0.8 MPEAs had a best performance. However, for increasing the content of W to 1.0, the cohesionless phases with a large area could not be covered by the passive film, which destroyed the continuity of film and would be as initial sites of pits110.
Zirconium
The opposite effect of Zr addition on the passive film stability of CoCrFeNiZrx (x = 0, 0.25, 0.5, and 1) MPEAs was verified, which could be related to the content of beneficial oxide in the passive film and the reduced thickness of the passive film, according to XPS analysis111. Brito-Garcia et al. found that the pitting resistance of the passive film formed on CoCrFeMoNi MPEAs decreased with the addition of Zr due to the increased number of defect points in the passive film112. The corrosion properties of the AlCoCrFeNiZrx (x = 0, 0.1, 0.2, 0.3, and 0.5) MPEAs first increased and then decreased with the increase in the Zr contents in 0.5 M H2SO4113. A serious galvanic corrosion attack was found for the Al- and Ni-rich, Cr-depleted B2 phases in both the dendrite and interdendrite of the Zr0.3 alloys.
Passivation mechanisms of MPEAs
Most studies have mainly focused on the properties and compositions of passive films already formed on the MPEAs, with limited investigations on the formation process of passive films, i.e., the passivation mechanism of MPEAs. The passivity of MPEAs likely consists of a selective oxide process under certain conditions, which is dependent on the specific chemical and electrochemical properties of each alloying element, as well as the interactions with each other114. Atomic emission spectroelectrochemistry (AESEC) is an analytical technique that allows for the monitoring of the dissolution of a large number of elements simultaneously and in real-time, during the reaction of MPEAs with an aggressive electrolyte. The fate of each element, whether in metals, oxides, or a solution, during spontaneous dissolution, activation, and passivation could be ascertained by the AESEC technique.
Ni-Cr-Mo alloys
Lutton et al.115 studied the kinetics of passivation and dissolution of Ni78Cr22 and Ni72Cr22Mo6 MPEAs in acidic solutions using AESEC coupled with inductively coupled plasma-mass spectrometry (ICP-MS). For the passivation of binary NiCr alloys, initially, significant Ni-rich oxide formation occurred, followed by the continuous build-up of Cr-rich oxides and an increase in preferential Ni dissolution. The dissolutions of Cr and Mo were near their respective limits of detection during passivation, indicating their accumulation of oxides in the passive films of the Ni72Cr22Mo6 MPEAs. Ni-dissolution was found to almost dominate the dissolution rate of the passive film until the film reached a stable thickness. Henderson et al.55 found that the spontaneous passivation of non-equiatomic NiCrMo MPEAs was facilitated by the accumulation of mainly Mo-rich as well as Cr-rich oxides after the cathodic activation process in 1 M HCl solution. High Mo content in the alloys was conducive to spontaneous passive film formation, while all elemental dissolution became congruent and the rates increased with time for the alloy with low Mo content, which indicated that an appropriate ratio between the alloyed Cr and Mo must exist to promote the passivation stability. During the subsequent electrochemically assisted passivation processes, previously accumulated Mo species in the spontaneous passive film were found to be partially removed, while the accumulation of Cr species dominated the film formation process. Li et al.84 also noticed that the contributions of Cr and Mo changed markedly with the potentials in the active-to-passive transition domain (A1) and the passive domain (A2) during potentiodynamic polarization for the Ni68Cr22Mo10 MPEAs. The Mo(IV) oxides that formed in A1 facilitated the oxidation of Cr to Cr2O3, which was possibly considered a step in the nucleation of the passive film, as a high Cr2O3/Cr(OH)3 ratio has been associated with a more protective oxide. Mo was potentially conducive to stabilizing oxygen adsorption, thus rendering the adsorption process more thermodynamically favorable for Cr2O3 oxide formation116. The role of each alloying element during the transpassive dissolution and repassivation process of the non-equiatomic NiCrMo and NiCrMoFe alloys was explored using AESEC in NaCl solution by Henderson et al.117. As shown in Fig. 4, Ni was selectively removed from the oxides during transpassive dissolution, while other alloying elements Cr, Mo, and Fe were enriched at least to some extent. Immediately following repassivation, the Mo species that were enriched during transpassive dissolution were released from the surface oxide, while enriched Cr and Fe maintained stability. The researchers also found that the increasing Fe content of the NiCrMoFe alloys could enhance the degree of Mo enrichment and subsequent transpassive dissolution at the surface, which was attributed to the rapid hydrolysis of Fe3+, leading to increased local acidification and the promotion of Mo-species deposition.
a BC-1(Ni63Cr15Mo22), b C-22(Ni65Cr22Mo13), c G-35(Ni58.7Cr33.2Mo8.1), d G-30(Ni49.5Cr30Mo5.5Fe15)117. (Elsevier Copyright, Original Order Number: 501847117, https://s100.copyright.com/CustomerAdmin/PLF.jsp?ref=22d7a21a-03af-4d5c-b550-a89f307b015d).
Al-Ti-V-Cr alloys
Qiu et al.52 reported a single-phase Al-Ti-V-Cr MPEA, which exhibited excellent passivity and repassivation behavior in NaCl solution (with Epit > 1.27 VSCE). The dissolution and repassivation behavior of this MPEA were further investigated in NaCl solution using an AESEC cyclic polarization test by Choudhary et al.118. As shown in Fig. 5, Cr, followed by V, underwent preferential dissolution at a higher fraction during anodic polarization, resulting in a surface enriched with Al and Ti just prior to the final breakdown. Subsequently, the dissolution rate of Al increased significantly above +1.4 VSHE, leading to a surface that was predominantly enriched with Ti, which in turn could play a major role in repassivation. The dissolution rate of Ti decreased quickly during the reverse potential scan, reaching a negligibly low value and showing rapid repassivation on the alloy surface by the formation of the Ti oxide. This also suggested the highest repassivation ability of Ti among the alloying elements and a greater contribution from Ti in repassivation119. XPS analysis also confirmed that the fraction of TiO2 in the passive film increased during the transpassive dissolution of V and Cr. The dissolution of V2O3 and Cr2O3 would also increase the defect density in the passive film, thus providing an easy path for the oxygen to migrate in, and assist in the growth of stable TiO2. Moreover, the transpassive dissolution reactions of V2O3 and Cr2O3 led to the localized acidification of the adjacent electrolyte by releasing the protons, which could also increase the dissolution kinetics of Al2O3120. Qiu et al.119 also found that Al dissolution was 1–2 orders of magnitude greater than V, Cr, and Ti for unequiatomic Al1.5TiVCr MPEAs in NaCl solutions. The dissolution rate of Al was found to decrease with increasing fractions of Cr2O3, TiO2, and V2O3 oxides in the passive film of AlTiVCr.
AESEC cyclic polarization curve revealing incongruent dissolution behavior of AlTiVCr, during forward followed reverse potential scan118. (Elsevier Copyright, Original Order Number: 501847120, https://s100.copyright.com/CustomerAdmin/PLF.jsp?ref=9e393be0-5665-44c3-98fd-dfad797de871).
Co-Cr-Fe-Ni-Mn alloys
Luo et al.121 employed the AESEC approach to investigate the overall dissolution rate of the equiatomic CoCrFeMnNi MPEAs in 0.1 M H2SO4 solution. The researchers found that no obvious selective dissolution of Mn, Co, Ni, and Fe occurred during passivation on the MPEAs, resulting in Cr content in the passive film that was much lower than that in the 304 SS passive film. Han et al.54 focused on non-equiatomic Ni38Fe20Cr22Mn10Co10 MPEAs and tracked the fate of each alloying element during linear sweep voltammetry in a slightly acidified chloride solution. Ni was found to dissolve into the solution and only minor or trace amounts of Fe and Co were incorporated into the oxides or hydroxides at low concentrations. Both Cr and Mn enrichment occurred during the active-to-passive transition and the complete passive regions, reflecting that Mn contributed to the surface film. Potentiostatic polarization indicated that Mn dissolved congruently with Cr enrichment at a lower anodic passive potential, whereas significant enrichment of Mn was observed at a higher anodic passive potential. In addition, Cr enrichment occurred in the early stage of passivation, while Mn accumulation was much greater as a function of time compared to Cr64. Han et al.114 also found that the selective dissolution rate of Mn increased with time at an applied potential in the passive potential domain, while the other alloying elements showed typical passivation trends for Ni38Fe20Cr22Mn10Co10 MPEAs in the 2 M H2SO4 solution, showing a less stable and high dissolution rate of the formed Mn-based passive film.
Dworschak et al.122 studied the electrochemical passivity characteristics of a library of alloys, spanning from equiatomic binary alloys up to equiatomic NiCoCrFeMn MPEAs using normal pulse voltammetry (NPV) combined with ICP-MS in NaCl solution. As shown in Fig. 6, with higher Ni, the Fe-anodic dissolution peak from equiatomic NiCoFeMn MPEAs demonstrated difficulty establishing stable passivity at a high anodic potential, which showed that the Fe and Ni-induced oxide film was less pronounced, if no Cr was present. Fe showed a low anodic dissolution rate with time and a pronounced cathodic peak profile, indicating that its oxide could be deposited at applied anodic potentials and stripped at negative potentials in equiatomic NiCoCrFe MPEAs. No significant Cr anodic dissolution was observed, even if high anodic potential was applied. This slow current evolving profile at higher polarizations from anodic to cathodic transition was indicative of delayed Cr-dissolution after polarization switching, which corresponded to the initiation of cathodic Fe dissolution. This confirmed the synergistic stabilizing effect of Fe and Cr in the passive film, where moderated and delayed Fe dissolution resulted in a gradient of Fe with increased concentration at the oxide/liquid interface and which delayed Cr-dissolution, leading to enriched Cr at the metal/oxide interface. However, anodic dissolutions for Cr and Fe at high applied potentials were more significant for equiatomic NiCoCrFeMn MPEAs. Mn addition was found to effectively change the Fe dissolution pattern from cathodic to anodic transition, thus catalyzing the dissolution of the Fe/Cr layer and preventing the formation of stable oxides.
a NiCoFeMn, b NiCoCrFe, c NiCoCrFeMn stacked with selected step potentials. Anodic polarization (red shading) at rising potentials is followed by reconditioning potential at –0.5 VAg/AgCl (cathodic polarization)122. (Elsevier Copyright, Original Order Number: 501847128, https://s100.copyright.com/CustomerAdmin/PLF.jsp?ref=c47416c4-5442-4031-b5d6-0d8037fd3f7c).
Composition and structures of surface oxide
The inhomogeneous oxide structure in MPEAs, such as lamellar characteristics and embedded oxide structure, have been verified by scanning transmission electron microscopy (STEM), 3D-APT, and time-of-flight secondary ions mass spectrometry (TOF-SIMS). The occurrence of selective oxidation due to the significant differences in Gibbs free energy of oxide formation of the elements and the transport rate of cations can prompt the uneven distribution of elements in space, thus resulting in the inhomogeneous oxide structure. In addition, the solid solution of cations in other elemental oxides and cation dopants can lead to the formation of complex multi-element oxides. For the MPEAs, multi-element oxides containing many elements within one phase instead of phase-separated stoichiometric oxides were confirmed by STEM and 3D-APT. According to the driving force for oxidation of elements in the MPEAs, they are mainly divided into naturally formed oxide, electrochemically grown oxide, and high-temperature formed oxide.
Native oxide
Recently, Sasidhar et al.123 performed high-resolution structural and chemical characterization of the native oxide film that formed on a Fe87Cr13 alloy. This steady-state oxide film was enriched with Cr at the oxide/metal interface and depleted in Cr at the oxide/environment interface, which was attributed to the higher mobility of the Fe3+ cations compared to Cr3+ cations, leading to a greater extent of Fe3+ cation diffusion through the Cr-enriched oxides and accumulation at the oxide/environment interface. Zhang et al.59 observed the growth process of a native oxide film on a ternary FeCr15Ni15 alloy, where the initial oxide consisted of a layer of amorphous Cr-rich oxide. Subsequently, the dominant outward diffusion of Fe yielded a bi-layer structure with an inner Cr-rich layer and an outer Fe-rich layer. The native oxide layer was consistent with the early stages of oxidation of high-temperature oxides in the FeCr18Ni14 alloy124. With long-term aging in air, the amorphous oxide first turned to a crystalline NaCl-structured Cr2O3·FeO mixture, and then, Fe2+ at the outer layer was oxidized to higher valance Fe3+, causing the NaCl structure to partially transform into a spinel structure. Moreover, Cr2O3 further oxidized to the higher valence state of CrO3, forming the outermost amorphous oxide layer. Adjacent to the oxide film/matrix interface, a Ni enrichment layer would always exist. Wang et al.60 investigated the compositions of native oxides and passive films of equiatomic CoCrFeMnNi MPEAs using TOF-SIMS and XPS. A duplex passive film formed on the substrate, where Fe and Co were located preferentially in the outer layer, Mn was in the inner layer, and Cr was distributed in the entire film. A modified alloy layer, enriched in Ni, was observed under the oxide film.
The native oxide films of amorphous CuxZr1-x (x = 0.33, 0.5, 0.67, and 0.75) alloys were studied by Xu et al.125. A thin ZrO2 film formed on top of the amorphous alloys due to a higher affinity for oxygen, with a Cu-enriched alloy layer located below and tetragonal ZrO2 nucleates located between the oxide film and the alloy. For the alloys with dominant Cu content, the Cu atoms with a smaller atomic radius that diffused faster to the surface and reacted with oxygen gradually formed an amorphous Cu2O layer on top of the oxide film, with an amorphous ZrO2 oxide layer below. The researchers further studied the oxidation mechanism of Cux(Zr0.67Al0.33)100-x (25≤x ≤ 50) amorphous alloys at high temperatures126. Native surface oxides on Cu47Zr45Al8 metallic glassy formed under ambient conditions, which were observed by aberration-corrected STEM127, and consisted of dominant Al2O3 and ZrO2 amorphous oxides with homogenously embedded crystalline Cu2O nanoparticles that formed at the metallic glass/oxide interface and were attributed to the much lower formation Gibbs free energies of Cu2O (–110 kJ mol–1) than that of ZrO2 (–990 kJ mol–1) and Al2O3 (–1580 kJ mol–1). Single-phase amorphous Zr-Al-O films with a fixed stoichiometric composition of (Zr0.67Al0.33)O1.83 formed on the top of the alloys with relatively low Cu alloying content. However, for alloys with relatively high Cu content, the relatively strong chemical interactions of Cu with Al reduced their atomic mobilities compared to Zr, leading to immobilized Cu and Al by the nucleation of tiny intermetallic precipitates in the Cu-enriched zone directly behind the oxide growth front. Thus, only Zr was preferentially oxidized to form ZrO2 at the outer layer. In addition, Mendis et al.128 studied the oxidation of Ti100–xTax alloys (x = 10, 20, 30, 50, 60 and 75) alloys and found that the volume fraction of Ta measured in the underlying substrate was consistently higher than its nominal fraction in the bulk alloy. This suggested that the oxidation of Ta was inhibited due to the preferential oxidation of Ti. As more Ti was depleted due to preferential oxidation, the dealloying layer under the oxide film became Ta-rich, allowing for the formation of Ta-rich oxides129. As indicated by the above research, the lamellar characteristics of the oxide film components of the MPEAs were the result of selective oxidation of the constituent elements, which was related to the oxidation Gibbs free energy of the constituent elements, the migration rate of the cations, the interactions between the constituent elements, and the formation of special alloy phases due to the dealloying process.
Electrochemically grown oxide
Gerard et al.130 characterized the passive films formed on the non-equiatomic Ni38Fe20Cr10Mn17Co17 MPEA during potentiostatic hold experiments with 3D-APT, as shown in Fig. 7. An obvious enrichment of Cr and Mn was observed in the outer layer, and an accumulation of Fe and Co was observed within the inner oxide. Enrichment of Ni within the altered zone at the metal/oxide interface and altered zone could be found. Kim et al.131 found that the lower corrosion resistance of austenitic stainless steels (Fe64Cr18Mn18) compared to 304 and 316 stainless steels was due to their instability in the passive film structure. As shown in Fig. 8a, the STEM image and corresponding Energy Dispersive Spectrometer (EDS) maps showed that nanocrystalline MnO oxides were embedded in the passive film subjected to the potentiodynamic polarization test. The High Resolution Transmission Electron Microscope (HRTEM) image of MnO and the corresponding Fast Fourier Transform (FFT) pattern confirmed that the crystal structure of MnO had a hexagonal wurtzite structure (Fig. 8b, c). Cracks or pores were observed in the vicinity of MnO, which potentially deteriorated the passive film stability. While adding Mo and Ni, which could reduce the density of MnO by absorbing oxides, the presence of embedded nanocrystalline oxides possibly facilitated the local short-circuit diffusion of oxygen through the grain boundaries to the base alloy and the outward diffusion of cations, thus potentially influencing elemental redistribution, particularly Mn, as demonstrated in a previous study for Cantor alloys57. Zhang et al.132 showed that some nanocrystalline oxides were embedded in the amorphous passive film that formed on FeCr15Ni15 single-crystal alloys in chloride-containing media. The energy barriers for Cl− ion diffusion from one oxygen vacancy to its neighboring one were the lowest at the interfacial region than in the interior of the amorphous or nanocrystalline oxides.
a The concentration profile (the standard error σ in the concentration profiles is defined as: \(\sigma =\sqrt{{c}_{{\rm{i}}}\left(100-{c}_{{\rm{i}}}\right)/{N}_{T}}\), where ci is the local concentration of species i, and NT is the total number of atoms in the concentration measurement), b The 3D element distribution maps130. (Elsevier Copyright, Original Order Number: 501847135, https://s100.copyright.com/CustomerAdmin/PLF.jsp?ref=f6716687-7122-49d4-810f-151cbfeb59e9).
a TEM image of the passive film on the Fe64Cr18Mn18 subjected to potentiodynamic polarization test, and corresponding EDS maps of Mn and O, b TEM image of MnO, showing crack formation at the interface between the pre-existing MnO and surrounding oxide, c HRTEM image of MnO, corresponding FFT pattern and Fourier Transform fringes (bottom) under [010] zone axis, revealing hexagonal wurtzite MnO131. (Open access article, no permission is required to use this article, https://s100.copyright.com/AppDispatchServlet?publisherName=ELS&contentID=S1359646221003924&orderBeanReset=true).
Rost et al.133 confirmed the formation of single-phase structure oxides when five equiatomic binary oxides were mixed, such as MgO, CoO, NiO, CuO, and ZnO. Moreover, all cation distributions were proven to be random and homogeneous (Fig. 9), which showed that many elements could be substituted on a single sublattice and that a host structure could be retained even when adding elements that formed different crystal structures134,135. Yu et al.69 studied the oxide compositions and structures that formed on Ni72Cr22Mo6 MPEAs during both high-temperature oxidation and aqueous corrosion. The researchers verified the presence of rock salt (Cr1−xNixO1.5−x/2) or corundum (Cr2−xNixO3) structure oxides close to the alloy/oxide interface, and the chemical composition was far from equilibrium (Fig. 10a). The formation of non-stoichiometric oxides was the result of nonequilibrium solute capture at the moving oxidation fronts, as illustrated in Fig. 10b. The velocity of physical interfacial motion achieved by the cations and vacancies moving into the oxides was significantly larger than the interfacial diffusion coefficient of the cations, resulting in local equilibrium breakdown at the interface and the formation of metastable multi-element oxide solid solutions. The metastable oxide solid solutions were possible across the entire compositional range, which was a function of the interfacial velocity.
a High-angle annular dark field (HAADF) image shows the arrangement of atomic structure for the mixed oxide. The individual EDS maps show uniform spatial distributions for b Mg element, c Co element, d Ni element, e Cu element, and f Zn element133. (Open access article, no permission is required to use this article, https://s100.copyright.com/AppDispatchServlet?title=Entropy-stabilized%20oxides&author=Christina%20M.%20Rost%20et%20al&contentID=10.1038%2Fncomms9485©right=The%20Author%28s%29&publication=2041-1723&publicationDate=2015-09-29&publisherName=SpringerNature&orderBeanReset=true&oa=CC%20BY).
a A bright field image of Ni72Cr22Mo6 sample, showing fringes characteristic of the corundum structure oxide, A high-angle annular dark field image with the corresponding electron energy loss line scan, showing the corundum structure adjacent to the metal has a composition of approximately CrNiO3, b Diagram of an oxidation front with a rocksalt oxide moving into an alloy with a velocity v, with the different types of atoms color-coded, with a number of metal vacancies69. (The American Physical Society Copyright, Order identification number RNP/23/SEP/070493, https://powerxeditor.aptaracorp.com/sciprisaps/RnPRequest/ViewRequest?Collection=T48pzZLYz1s%3d).
The potentiostatically formed passive film on the Al0.3Cr0.5Fe2Mn0.25Mo0.15Ni1.5Ti0.3 MPEAs were found to be enriched in Ti, Cr, and Al using XPS analysis by Inman et al.136, as shown in Supplementary Fig. 1a, b. It was found that the existence of complex Al, Cr, and Ti containing solid solution oxides, such as Ti2CrO5 and TiAl2O5 that are suggested to potentially be stable from thermodynamic calculations, as illustrated by the convex-hull diagrams in Supplementary Fig. 1c, d. Quiambao et al.68 showed that an oxide containing all elements in the bulk Ni38Cr21Fe20Ru13Mo6W2 alloy in a non-equilibrium, non-stoichiometric solid solution formed without distinct phase separation into single-element oxide phases during potentiostatic potential passivation. Meanwhile, the oxide film showed significant enrichment in Cr and Ru, with Mo and W near their bulk composition, and the depletion of Fe and Ni due to selective dissolution at the solution/oxide interface. The enrichment of Cr and Ru in the oxides was due to the significant dissolution of Ni, Fe, Mo, and W at rates approximately proportional to their bulk concentrations.
This meant that the formation process of the multi-element oxide solid solutions on the MPEAs in the aqueous solutions was likely controlled by a combination of thermodynamics and kinetics. According to thermodynamic databases for multicomponent systems based on the methodology known as the CALculation of PHAse Diagram (CALPHAD), some of the oxide solid solution phases, i.e., corundum (Fe,Cr)2O3, spinel (Fe,Ni,Cr,Mn,Co)3O4, cubic (Co,Fe)2O3, and (Ni,Mn)2(Ni,Mn,O)3 phases, that formed on Ni38Fe20Cr22Mn10Co10 were found to be thermodynamically stable for the low Gibbs energy of formation of the compounds over large portions of the E-pH diagram, as shown in Supplementary Fig. 244. The enrichment or depletion of select elements was dependent on the details of the various rate-determining processes, such as selective oxidation at the metal/oxide interface, preferential dissolution of the cations and redeposition rate combined with the oxygen and oxyhydrogen ions at the solution/oxide interface, and the differential ionic transport rates across the oxides. Gerard et al.64 found that much greater enrichment of Cr in the oxide occurred to a greater extent in the Ni38Fe20Cr22Mn10Co10 alloy than in the Ni76Cr24 binary alloy, for which selective dissolution did not fully explain the observed Cr enrichment. The unusual combination of multiple vacancy sources came from the different rates of vacancy-mediated transport as a result of the dissolution of multiple elements, and the differences in the interfacial or surface reaction rates may have enhanced the transport of Cr to the metal/oxide interface, which could have contributed to the observed Cr enrichment.
High-temperature formed oxide
The oxide films formed at high temperatures have semiconductor characteristics similar to that of the electrochemically grown oxide films137. The semiconductor properties of oxide films are determined by their composition and structure, which is expected to be crucially important in studying the protective characteristics against corrosion. In addition, the surface oxide formed in the air at high temperatures presents great thickness, which is beneficial for characterizing its microstructure and can help in understanding the transport characteristics of cations and oxygen. Consequently, it is necessary to gain a detailed perception of the structural and compositional information of the oxide films formed at high temperatures for a comprehensive understanding of the growth mechanisms behind the electrochemically grown oxide films.
The oxide film of the ternary Ni76Cr16Fe8 alloy after oxidation at 350 °C consisted of a duplex structure: an inner Cr-rich layer and an outer layer that was rich in Ni and Fe, and the inner layer from pure Cr2O3 toward spinel (NiCr2O4)138. Li et al.60 analyzed the elements distribution throughout the oxide films on nanocrystalline CrMnFeCoNi thin films exposed to air at 500 °C for 5 min using 3D-APT. Mn and Cr oxides formed on the top of the oxide film, suggesting outward diffusion through the high-density grain boundaries and triple junctions supplying the shortest diffusion path. The resultant high concentration of Fe and Co, as well as reduced Ni content, led to decomposition at the oxide/alloy interface and the formation of the FeCo-rich B2 phase, which potentially accelerated the outward diffusion of Mn and Cr, as shown in Supplementary Fig. 3. From the above research results, it could be seen that the composition and elemental distribution of the oxide film would be affected by the composition ratio, microstructure and oxidation methods of MPEAs.
Yu et al.67 investigated the early-stage oxidation of an equiatomic CoCrFeNi alloy and found that the formation of multi-element oxide solid solutions was dependent on the competition between thermodynamics and kinetics involving the relative diffusion rate of the cations and oxygen. As shown in Supplementary Fig. 4, the oxide layers consisted of inner corundum and outer spinel structures, which were determined by electron diffractions. The EDS compositional line scan showed that Ni was abundant at the metal/oxide interface, while Cr and Fe were depleted. Cr dominated in the corundum layer, and Fe, Co, and Ni were rich in the spinel phase (Supplementary Fig. 4b). The dominant oxygen transport in the Cr-rich corundum led to inward growth of the corundum phase. The growth of the Fe-rich spinel oxides with Co and Ni dopants was dominated by outward cation diffusion. The diffusion of Fe and Co in the corundum solid solution was significantly faster than that of the host Cr cations, as the Gibbs energy of formation of Cr2O3 was significantly lower than that of Fe2O3 and Co2O3, meaning higher permeability of Fe and Co in the corundum phase. Yu et al.139 also found that the oxide films that grew on the surfaces of the grains with different crystal orientations in the NiCr binary alloy had markedly different structures and compositions. A solute that captured rock salt oxides formed with a cube-cube epitaxial orientation on the (100) oriented grains, while a solute that captured corundum oxides formed, with the (0001) basal plane parallel to the (111) oriented grain. For the (100) plane, the oxide composition at the alloy/oxide interface was controlled by how rapidly the cations crossed the interface versus exchanged with the alloy. For the (111) plane, it was controlled by cation addition at the interface versus exchange across the interface. The surface passive films formed by galvanostat experiments in 0.1 M deaerated NaCl solution on NiCr and NiCrMo alloys were studied by TEM140. Compared to the chemical composition of rock salt oxide on the top layer for NiCr alloy, the corundum in the external oxide layer for NiCrMo alloy contains obviously more Ni by EDS line scanning, as shown in Supplementary Fig. 5. The results confirmed that Mo addition promoted the formation of a metastable corundum phase as a consequence of nonequilibrium solute capture of Ni in corundum. The external corundum oxide layer could change the transport mechanism as well as the diffusivity of oxygen through the passive film and across the metal/oxide interface, thus changing the localized oxygen penetration and improving the passive film protectiveness. Under high-temperature oxidation conditions, compared to the NiCr alloy, Mo doping possibly stabilized the cation vacancies and inhibited Kirkendall void formation by promoting the nucleation of the metastable Ni2–xCrxO3 corundum structure phase at the metal/oxide interface for the NiCrMo alloy141. Therefore, nonequilibrium solute capture during oxide film growth controlled by diffusion dynamics was a general phenomenon independent of whether the oxides formed by dry oxidation under high temperature or in aqueous conditions. Studying the structure of oxide and the sequence of phase nucleation during the high-temperature oxidation could help to understand the migration in the mass transportation process, which was important to understand the morphological stability and breakdown mechanism of protective passive film on alloy surface.
Thus, the formation of multi-element oxides was related to the occurrence of nonequilibrium solute capture during oxide film growth. The nonequilibrium solute capture leads to the trapping of large dopant concentrations in the oxide, which could affect the protectiveness of passive film against breakdown in corrosive environments142. The dopant cation has a lower valence state, such as Al-doped TiO2143 and Ni-doped Cr2O3144, which has been shown to promote an increase in positively charged oxygen vacancies, in order to increase the electron charge carriers to maintain charge neutrality of the passive film. For the situation where Mo6+ is substituted onto a Ni2+ or Cr3+ lattice site142, and Nb5+ is substituted onto a Ti4+ lattice site145,146, the net positive charge would result in the annihilation of electron holes and attraction of negatively charged defects, such as cation vacancies. Shuang et al.147 found that the chemically complex multi-element oxide film containing all alloying elements that formed on the FeCrNiCoNb0.5 MPEAs was a less defective or a more compact amorphous structure, which contributed to high anti-corrosion performance. Thus, the composition and structure of the formed multi-element oxides were complex, as some factors were at play, such as the oxidation temperature, bulk alloy composition, grain orientation, selective oxidation at the metal/oxide interface, the diffusion coefficient of the cations in the oxide, and cation doping through substitution into a host oxide lattice.
Open questions
The passivation ability and stability of the formed passive film have significant effects on the corrosion resistance of the MPEAs. In-depth research on the passivation mechanism will be helpful in understanding the specific role of constituent elements in the passive film formation process. It is extremely challenging to select an appropriate composition to optimize the protectiveness of passive films for different corrosion environments by trial-and-error experiments. The composition and structure of surface oxides affected by alloy composition and selective oxidation are complex and play an important role in the corrosion resistance of MPEAs. Some key issues for exploration are summarized as follows.
(1) What are the contributions of individual alloying elements or synergistic effects between the elements on the improved protectiveness of the passive film on an MPEA substrate? The corrosion resistance of the passive film will be determined by the dissolution rate of surface oxides and the vulnerability to pitting corrosion. Therefore, it is necessary to determine whether the main contribution of specified alloying element addition will slow down the chemical dissolution of the passive film or improve the pitting resistance of the passive film.
(2) What is the critical concentration of a single passivating element or optimal combination of elements that needs to be applied, to a large degree, to optimize the corrosion resistance of a passive film on MPEAs under a condition where the alloy composition will be systematically altered without changing alloy phase structure?
(3) For the MPEAs, how does selective dissolution at active surfaces or oxide/electrolyte interfaces contribute to surface modification and multi-element oxide formation? How does each element contribute to the passivation mechanism and dissolution kinetics characteristic of the MPEAs?
(4) How does the formation of phase-separated or complex multi-principal oxides contribute to the corrosion and oxidation resistance of MPEAs?
(5) What are the composition and structure of initially formed complex multi-principal oxides, and how do they evolve with time? Will the cation substitution process occur, and whether it will affect the ionic and electronic defect concentrations in the oxide?
(6) How do changes in alloy composition affect the synergistic effect of multiple principal components, thereby altering the cation dissolution kinetics and microstructure of the passive film, which is crucial for revealing the uniform corrosion and pitting resistance mechanisms of MPEAs?
Summary and outlook
Emerging MPEAs open up a large compositional space, with the possibility of complex oxides with enhanced passivity properties, demonstrating excellent corrosion and oxidation resistance. The stability of passive film, passivation mechanism, and surface oxide film characteristics of the MPEAs, have been preliminarily studied. (1) The formation of stable passive film was found to be dependent on the microstructure of the MPEAs with a single-phase structure and uniform elemental distribution. It is necessary to use machine learning, phase diagram calculations, and high-throughput technology to comprehensively and efficiently optimize the microstructures of MPEAs, to improve the protectiveness of passive films. From the research results on the influence of alloying elements on the corrosion resistance of MPEAs, Cr was found to be the most important passivating element. The stability of the passive film on MPEAs was found to be significantly affected by the concentration and distribution uniformity of Cr in the passive film and by the influence of other elements, such as Al, Ni, Mo, Co, and Ti coupled with Cr, which influenced the occurrence of galvanic corrosion. In general, the addition of Mo and Co could enhance the stability of Cr2O3, improving the protectiveness of the passive film, while the introduction of Mn and Al could decrease the concentrations of Cr2O3, with a deleterious effect on passive film stability. The addition of Mo was found to provide a tremendous beneficial effect on the corrosion properties with the formation of a passive film resistant to pitting corrosion. (2) For the MPEAs, elements dissolved incongruently, modifying the surface chemistry and leading to the enrichment or accumulation of elements in an oxidized state on the surface at the open-circuit potential or a given potential, which could be determined by in situ AESEC. The preferential dissolution of Ni in the oxides of some MPEAs consisted of transition metal elements, such as NiCr, NiCrMo, and CoCrFeNiMn, which was confirmed by AESEC analysis, thus causing a significant lack of Ni in the oxide film. The preferential dissolution of some specific elements in the MPEAs could increase the defect density in the oxide, thus providing an easy path for the oxygen to migrate in and assist in the growth of oxides of other elements. Many important aspects of passivity including transpassive dissolution and repassivation behavior associated with elements’ selective dissolution and reprecipitation characteristics, should be studied on MPEAs. Further experiments using AESEC combined with the analysis of oxide composition using XPS and 3D-APT are needed to understand the correlation of preferential element release, the bonding environment, formation mechanisms, and passivity of these oxide films, as well as their impacts on the corrosion kinetics and material chemical durability. (3) The formation of phase-separated oxides, such as layered oxide structures and embedded oxide structures, on MPEAs was found to be the result of selective oxidation of elements at the metal/oxide interface, differential cation transport rates across the oxides, the interactions between constituent elements, and the formation of some alloy phases due to the dealloying process. (4) The oxides were found to grow too fast or the fluxes through the interface were too large for local interfacial equilibrium to exist, resulting in the occurrence of nonequilibrium solute capture. The formation of non-stoichiometric multi-element oxides was found to be related to nonequilibrium solute capture, which occurred at the interface of the alloy/film during oxide growth and was controlled by a combination of thermodynamics and kinetics. For the MPEAs, determining whether high-entropy oxides containing many elements with one phase are thermodynamically stable needs to be further studied. Multi-element oxide databases also need to be established based on the CALculation of PHAse Diagram method to understand the corrosion or oxidation of MPEAs. The stability of solid solution oxide compositions based on common crystal structures, such as corundum, spinels, and rock salt, will have to be further explored, through modern high-resolution microscopy techniques such as STEM and 3D-APT, to determine whether the cocktail effect of these multi-element oxides with a very wide range of compositions can lead to lower diffusion rates or influence the transport mechanism of the existing point defects, and this may present kinetic barriers to enhance the corrosion or oxidation resistance of MPEAs.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Code availability
Not applicable.
References
Shi, Y., Yang, B. & Liaw, P. K. Corrosion-resistant high-entropy alloys: a review. Metals 7, 43 (2017).
Qiu, Y., Thomas, S., Gibson, M. A., Fraser, H. L. & Birbilis, N. Corrosion of high entropy alloys. Npj Mater. Degrad. 1, 1–18 (2017).
Tang, Z., Huang, L., He, W. & Liaw, P. K. Alloying and processing effects on the aqueous corrosion behavior of high-entropy alloys. Entropy 16, 895–911 (2014).
Qiu, Y., Gibson, M., Fraser, H. & Birbilis, N. Corrosion characteristics of high entropy alloys. Mater. Sci. Technol. 31, 1235–1243 (2015).
Nascimento, C. B., Donatus, U., Ríos, C. T., Oliveir, a M.C.L.d. & Antunes, R. A. A review on corrosion of high entropy alloys: exploring the interplay between corrosion properties, alloy composition, passive film stability and materials selection. Mater. Res. 25, e20210442 (2022).
Fu, Y., Li, J., Luo, H., Du, C. & Li, X. Recent advances on environmental corrosion behavior and mechanism of high-entropy alloys. J. Mater. Sci. Technol. 80, 217–233 (2021).
Yang, J. et al. Effects of Mn on the electrochemical corrosion and passivation behavior of CoFeNiMnCr high-entropy alloy system in H2SO4 solution. J. Alloy. Compd. 819, 152943 (2020).
Dai, C., Zhao, T., Du, C., Liu, Z. & Zhang, D. Effect of molybdenum content on the microstructure and corrosion behavior of FeCoCrNiMox high-entropy alloys. J. Mater. Sci. Technol. 46, 64–73 (2020).
Shi, Y., Collins, L., Balke, N., Liaw, P. K. & Yang, B. In-situ electrochemical-AFM study of localized corrosion of AlxCoCrFeNi high-entropy alloys in chloride solution. Appl. Surf. Sci. 439, 533–544 (2018).
Muangtong, P., Rodchanarowan, A., Chaysuwan, D., Chanlek, N. & Goodall, R. The corrosion behaviour of CoCrFeNi-x (x= Cu, Al, Sn) high entropy alloy systems in chloride solution. Corros. Sci. 172, 108740 (2020).
Chai, W., Lu, T. & Pan, Y. Corrosion behaviors of FeCoNiCrx (x= 0, 0.5, 1.0) multi-principal element alloys: role of Cr-induced segregation. Intermetallics 116, 106654 (2020).
Lu, P. et al. Computational design and initial corrosion assessment of a series of non-equimolar high entropy alloys. Scr. Mater. 172, 12–16 (2019).
Yang, S. et al. Effect of Cr content on corrosion behavior of AlCrxFeNi2Cu1.6 high entropy alloys. Mater. Res. Express 6, 076501 (2019).
Wang, W. et al. Effect of Mo and aging temperature on corrosion behavior of (CoCrFeNi)100-xMox high-entropy alloys. J. Alloy. Compd. 812, 152139 (2020).
Godlewska, E. M. et al. Corrosion of Al(Co)CrFeNi high-entropy alloys. Front. Mater. 7, 566336 (2020).
Zhao, R. F. et al. Corrosion behavior of CoxCrCuFeMnNi high-entropy alloys prepared by hot pressing sintered in 3.5% NaCl solution. Results Phys. 15, 102667 (2019).
Qiu, X. W. & Liu, C. G. Microstructure and properties of Al2CrFeCoCuTiNix high-entropy alloys prepared by laser cladding. J. Alloy. Compd. 553, 216–220 (2013).
López Ríos, M. et al. Effects of nickel content on the microstructure, microhardness and corrosion behavior of high-entropy AlCoCrFeNix alloys. Sci. Rep. 10, 1–11 (2020).
Han, Z. et al. Structures and corrosion properties of the AlCrFeNiMo0.5Tix high entropy alloys. Mater. Corros. 69, 641–647 (2018).
Shi, H. et al. Influence of alloying elements (Cu, Ti, Nb) on the microstructure and corrosion behaviour of AlCrFeNi-based high entropy alloys exposed to oxygen-containing molten Pb. Corros. Sci. 190, 109659 (2021).
Wang, X. Z., Hu, Q., Zhang, L. & Cui, Z. The influence of Nb addition on the passivity of CoCrNiNbx multi-principal element alloys. J. Electroanal. Chem. 908, 116107 (2022).
Liu, C. et al. Effect of Nb content on the microstructure and corrosion resistance of FeCoCrNiNbx high-entropy alloys in chloride ion environment. J. Alloy. Compd. 935, 168013 (2023).
Hsu, Y. J., Chiang, W. C. & Wu, J. K. Corrosion behavior of FeCoNiCrCux high-entropy alloys in 3.5% sodium chloride solution. Mater. Chem. Phys. 92, 112–117 (2005).
Peng, Z. et al. A lightweight AlCrTiV0.5Cux high-entropy alloy with excellent corrosion resistance. Materials 16, 2922 (2023).
Lee, C., Chang, C., Chen, Y., Yeh, J. & Shih, H. Effect of the aluminium content of AlxCrFe1.5MnNi0.5 high-entropy alloys on the corrosion behaviour in aqueous environments. Corros. Sci. 50, 2053–2060 (2008).
Shi, Y. et al. Homogenization of AlxCoCrFeNi high-entropy alloys with improved corrosion resistance. Corros. Sci. 133, 120–131 (2018).
Shi, Y. et al. Corrosion of AlxCoCrFeNi high-entropy alloys: Al-content and potential scan-rate dependent pitting behavior. Corros. Sci. 119, 33–45 (2017).
Gu, X. H. et al. Microstructure characterization and corrosion behavior of Alx(CoCrFeNi)100−x (x= 0, 5, 10, 15, 20) high entropy alloys in 0.5 M H2SO4 solution. J. Alloy. Compd. 944, 169247 (2023).
Wang, C. et al. Microstructure and corrosion properties of laser remelted CrFeCoNi and CrMnFeCoNi high entropy alloys coatings. J. Mater. Res. Technol. 15, 5187–5196 (2021).
Hsu, K. M., Chen, S. H. & Lin, C. S. Microstructure and corrosion behavior of FeCrNiCoMnx(x= 1.0, 0.6, 0.3, 0) high entropy alloys in 0.5 M H2SO4. Corros. Sci. 190, 109694 (2021).
Li, T. et al. Localized corrosion behavior of a single-phase non-equimolar high entropy alloy. Electrochim. Acta 306, 71–84 (2019).
Zhang, P. et al. A high-corrosion-resistant high-entropy alloys (HEAs) coatings with single BCC solid solution structure by laser remelting. Mater. Lett. 324, 132728 (2022).
Marcus, P. et al. Enhanced passivity and resistance to pitting of new Cr-Fe-Co-Ni-Mo multi-principal element single-phase alloys. ECS Meet. Abstr. 242, 732–732 (2022).
Senkov, O. N., Miracle, D. B., Chaput, K. J. & Couzinie, J. P. Development and exploration of refractory high entropy alloys-A review. J. Mater. Res. 33, 3092–3128 (2018).
Zhou, J. L., Cheng, Y. H., Chen, Y. X. & Liang, X. B. Composition design and preparation process of refractory high-entropy alloys: a review. Int. J. Refract. Met. Hard Mater. 105, 105836 (2022).
Xiong, W. et al. Refractory high-entropy alloys: a focused review of preparation methods and properties. J. Mater. Sci. Technol. 142, 196–215 (2023).
Hua, X. J. et al. Development and property tuning of refractory high-entropy alloys: a review. Acta Metall. Sin. (Engl. Lett.). 35, 1231–1265 (2022).
Xie, X. et al. Research progress of refractory high entropy alloys: a review. Chin. J. Mech. Eng. 35, 142 (2022).
Srikanth, M., Annamalai, A. R., Muthuchamy, A. & Jen, C. P. A review of the latest developments in the field of refractory high-entropy alloys. Crystals 11, 612 (2021).
Yao, H. et al. NbTaV-(Ti, W) refractory high-entropy alloys: experiments and modeling. Mater. Sci. Eng. A. 674, 203–211 (2016).
Coury, F. G., Kaufman, M. & Clarke, A. J. Solid-solution strengthening in refractory high entropy alloys. Acta Mater. 175, 66–81 (2019).
Soni, V. et al. Phase stability as a function of temperature in a refractory high-entropy alloy. J. Mater. Res. 33, 3235–3246 (2018).
Yao, J. et al. Phase stability of a ductile single-phase BCC Hf0.5Nb0.5Ta0.5Ti1.5Zr refractory high-entropy alloy. Intermetallics 98, 79–88 (2018).
Wang, K., Han, J., Gerard, A. Y., Scully, J. R. & Zhou, B. C. Potential-pH diagrams considering complex oxide solution phases for understanding aqueous corrosion of multi-principal element alloys. Npj Mater. Degrad. 4, 1–11 (2020).
Lu, P. et al. Computational materials design of a corrosion resistant high entropy alloy for harsh environments. Scr. Mater. 153, 19–22 (2018).
Scully, J. R. et al. Controlling the corrosion resistance of multi-principal element alloys. Scr. Mater. 188, 96–101 (2020).
Birbilis, N., Choudhary, S., Scully, J. & Taheri, M. A perspective on corrosion of multi-principal element alloys. Npj Mater. Degrad. 5, 1–8 (2021).
Hurley, P. K. et al. The characterization and passivation of fixed oxide charges and interface states in the MOS system. IEEE Trans. Device Mater. Reliab. 13, 429–443 (2013).
Clayton, C. & Lu, Y. A bipolar model of the passivity of stainless steel: the role of Mo addition. J. Electrochem. Soc. 133, 2465 (1986).
Marcus, P. On some fundamental factors in the effect of alloying elements on passivation of alloys. Corros. Sci. 36, 2155–2158 (1994).
Urquidi-Macdonald, M. & Macdonald, D. D. Theoretical analysis of the effects of alloying elements on distribution functions of passivity breakdown. J. Electrochem. Soc. 136, 961 (1989).
Qiu, Y. et al. Microstructure and corrosion properties of the low-density single-phase compositionally complex alloy AlTiVCr. Corros. Sci. 133, 386–396 (2018).
Zheng, S., Cai, Z., Pu, J., Zeng, C. & Wang, L. Passivation behavior of VAlTiCrSi amorphous high-entropy alloy film with a high corrosion-resistance in artificial sea water. Appl. Surf. Sci. 542, 148520 (2021).
Han, J. et al. Potential dependent Mn oxidation and its role in passivation of Ni38Fe20Cr22Mn10Co10 multi-principal element alloy using multi-element resolved atomic emission spectroelectrochemistry. J. Electrochem. Soc. 168, 051508 (2021).
Henderson, J. D. et al. Investigating the role of Mo and Cr during the activation and passivation of Ni-based alloys in acidic chloride solution. J. Electrochem. Soc. 168, 021509 (2021).
Choudhary, S. et al. On the dynamic passivity and corrosion resistance of a low cost and low density multi-principal-element alloy produced via commodity metals. Electrochem. Commun. 125, 106989 (2021).
Laplanche, G., Volkert, U., Eggeler, G. & George, E. Oxidation behavior of the CrMnFeCoNi high-entropy alloy. Oxid. Met. 85, 629–645 (2016).
Seyeux, A. et al. ToF-SIMS investigation with 18O isotopic tracer of the ion transport mechanisms in surface oxides on nickel-chromium and nickel-chromium-molybdenum alloys. Electrochim. Acta 426, 140797 (2022).
Zhang, B. et al. Quasi-in-situ observing the growth of native oxide film on the FeCr15Ni15 austenitic alloy by TEM. Corros. Sci. 140, 1–7 (2018).
Li, Y., Kostka, A., Savan, A. & Ludwig, A. Atomic-scale investigation of fast oxidation kinetics of nanocrystalline CrMnFeCoNi thin films. J. Alloy. Compd. 766, 1080–1085 (2018).
Wang, L. et al. Study of the surface oxides and corrosion behaviour of an equiatomic CoCrFeMnNi high entropy alloy by XPS and ToF-SIMS. Corros. Sci. 167, 108507 (2020).
Wang, L. et al. Insight on passivity of high entropy alloys: Thermal stability and ion transport mechanisms in the passive oxide film on CoCrFeMnNi surfaces. Corros. Sci. 188, 109540 (2021).
Wang, X. et al. Origin of enhanced passivity of Cr-Fe-Co-Ni-Mo multi-principal element alloy surfaces. Npj Mater. Degrad. 7, 13 (2023).
Gerard, A. Y. et al. Aqueous passivation of multi-principal element alloy Ni38Fe20Cr22Mn10Co10: Unexpected high Cr enrichment within the passive film. Acta Mater. 198, 121–133 (2020).
Kautz, E. J. et al. Element redistributions during early stages of oxidation in a Ni38Cr22Fe20Mn10Co10 multi-principal element alloy. Scr. Mater. 194, 113609 (2021).
Schreiber, D. K. et al. Revealing the complexity of high temperature oxide formation in a 38Ni-21Cr-20Fe-13Ru-6Mo-2W (at.%) multi-principal element alloy. Scr. Mater. 210, 114419 (2022).
Yu, X. X., Taylor, M. A., Perepezko, J. H. & Marks, L. D. Competition between thermodynamics, kinetics and growth mode in the early-stage oxidation of an equimolar CoCrFeNi alloy. Acta Mater. 196, 651–659 (2020).
Quiambao, K. F. et al. Passivation of a corrosion resistant high entropy alloy in non-oxidizing sulfate solutions. Acta Mater. 164, 362–376 (2019).
Yu, X. X. et al. Nonequilibrium solute capture in passivating oxide films. Phys. Rev. Lett. 121, 145701 (2018).
Newman, R., Meng, F. T. & Sieradzki, K. Validation of a percolation model for passivation of Fe-Cr alloys: I current efficiency in the incompletely passivated state. Corros. Sci. 28, 523–527 (1988).
Uhlig, H. H. & Woodside, G. E. Anodic polarization of passive and non-passive chromium-iron alloys. J. Phys. Chem. 57, 280–283 (1953).
Ferrari, A. & Körmann, F. Surface segregation in Cr-Mn-Fe-Co-Ni high entropy alloys. Appl. Surf. Sci. 533, 147471 (2020).
Shang, X. et al. The intrinsic mechanism of corrosion resistance for FCC high entropy alloys. Sci. China Technol. Sci. 61, 189–196 (2018).
Yan, X. et al. Al0.3CrxFeCoNi high-entropy alloys with high corrosion resistance and good mechanical properties. J. Alloy. Compd. 860, 158436 (2021).
Han, Z. et al. The corrosion behavior of ultra-fine grained CoNiFeCrMn high-entropy alloys. J. Alloy. Compd. 816, 152583 (2020).
Huang, L., Wang, X., Zhao, X., Wang, C. & Yang, Y. Analysis on the key role in corrosion behavior of CoCrNiAlTi-based high entropy alloy. Mater. Chem. Phys. 259, 124007 (2021).
Pardo, A. et al. Pitting corrosion behaviour of austenitic stainless steels–combining effects of Mn and Mo additions. Corros. Sci. 50, 1796–1806 (2008).
Hashimoto, K., Asami, K. & Teramoto, K. An X-ray photo-electron spectroscopic study on the role of molybdenum in increasing the corrosion resistance of ferritic stainless steels in HC1. Corros. Sci. 19, 3–14 (1979).
Montemor, M., Simões, A., Ferreira, M. & Belo, M. D. C. The role of Mo in the chemical composition and semiconductive behaviour of oxide films formed on stainless steels. Corros. Sci. 41, 17–34 (1999).
Dou, Y., Han, S., Wang, L., Wang, X. & Cui, Z. Characterization of the passive properties of 254SMO stainless steel in simulated desulfurized flue gas condensates by electrochemical analysis, XPS and ToF-SIMS. Corros. Sci. 165, 108405 (2020).
Li, Y. et al. Effects of partially substituting cobalt for nickel on the corrosion resistance of a Ni-16Cr-15Mo alloy to aqueous hydrofluoric acid. Corros. Sci. 78, 101–110 (2014).
Hu, Q. et al. Mo content-depended competition between Cr2O3 enrichment and selective dissolution of CoCrFeNiMox high entropy alloys. Npj Mater. Degrad. 6, 97 (2022).
Shuang, S. et al. Unusually high corrosion resistance in MoxCrNiCo medium entropy alloy enhanced by acidity in aqueous solution. J. Mater. Sci. Technol. 139, 59–68 (2023).
Li, X. et al. The contribution of Cr and Mo to the passivation of Ni22Cr and Ni22Cr10Mo alloys in sulfuric acid. Corros. Sci. 176, 109015 (2020).
Chen, J. et al. Effects of alloying concentration on the aqueous corrosion and passivation of aluminum-manganese-molybdenum concentrated alloys. Corros. Sci. 198, 110137 (2022).
Wang, X. et al. Enhanced passivity of Cr-Fe-Co-Ni-Mo multi-component single-phase face-centred cubic alloys: design, production and corrosion behaviour. Corros. Sci. 200, 110233 (2022).
Pardo, A. et al. Effect of Mo and Mn additions on the corrosion behaviour of AISI 304 and 316 stainless steels in H2SO4. Corros. Sci. 50, 780–794 (2008).
Park, K. & Kwon, H. Effects of Mn on the localized corrosion behavior of Fe-18Cr alloys. Electrochim. Acta 55, 3421–3427 (2010).
Torbati-Sarraf, H., Shabani, M., Jablonski, P. D., Pataky, G. J. & Poursaee, A. The influence of incorporation of Mn on the pitting corrosion performance of CrFeCoNi High Entropy Alloy at different temperatures. Mater. Des. 184, 108170 (2019).
Liu, J. C. & Zhang, G. Effect of trace Mn modification on the microstructure and corrosion behavior of Sn-9Zn solder alloy. Mater. Corros. 69, 781–792 (2018).
Qiu, J. et al. Corrosion behaviour and surface analysis of a Co-Cr and two Ni-Cr dental alloys before and after simulated porcelain firing. Eur. J. oral. Sci. 119, 93–101 (2011).
Qiu, X. W., Wu, M. J., Liu, C. G., Zhang, Y. P. & Huang, C. X. Corrosion performance of Al2CrFeCoxCuNiTi high-entropy alloy coatings in acid liquids. J. Alloy. Compd. 708, 353–357 (2017).
Yen, C. C. et al. Corrosion mechanism of annealed equiatomic AlCoCrFeNi tri-phase high-entropy alloy in 0.5 M H2SO4 aerated aqueous solution. Corros. Sci. 157, 462–471 (2019).
Wei, R. et al. The effect of Co substitutions for Ni on microstructure, mechanical properties and corrosion resistance of Fe50Mn25Cr15Ni10 medium-entropy alloy. Intermetallics 149, 107654 (2022).
Alqarni, N. D. et al. Effect of cobalt addition on the corrosion behavior of near equiatomic NiTi shape memory alloy in normal saline solution: Electrochemical and XPS studies. RSC Adv. 8, 19289–19300 (2018).
Wu, P., Gan, K., Yan, D., Fu, Z. & Li, Z. A non-equiatomic FeNiCoCr high-entropy alloy with excellent anti-corrosion performance and strength-ductility synergy. Corros. Sci. 183, 109341 (2021).
Shi, Y. et al. High-throughput synthesis and corrosion behavior of sputter-deposited nanocrystalline Alx(CoCrFeNi)100-x combinatorial high-entropy alloys. Mater. Des. 195, 109018 (2020).
Nascimento, C. B., Donatus, U., Rios, C. T. & Antunes, R. A. Electronic properties of the passive films formed on CoCrFeNi and CoCrFeNiAl high entropy alloys in sodium chloride solution. J. Mater. Res. Technol. 9, 13879–13892 (2020).
Raza, A., Abdulahad, S., Kang, B., Ryu, H. J. & Hong, S. H. Corrosion resistance of weight reduced AlxCrFeMoV high entropy alloys. Appl. Surf. Sci. 485, 368–374 (2019).
Pan, Z. et al. Tailoring microstructure and corrosion behavior of CoNiVAlx medium entropy alloys via Al addition. Corros. Sci. 207, 110570 (2022).
Fu, Y. et al. The corrosion behavior and film properties of Al-containing high-entropy alloys in acidic solutions. Appl. Surf. Sci. 560, 149854 (2021).
Wu, M., Setiawan, R. C. & Li, D. Benefits of passive element Ti to the resistance of AlCrFeCoNi high-entropy alloy to corrosion and corrosive wear. Wear 492, 204231 (2022).
Gu, X., Zhuang, Y. & Huang, D. Corrosion behaviors related to the microstructural evolutions of as-cast Al0.3CoCrFeNi high entropy alloy with addition of Si and Ti elements. Intermetallics 147, 107600 (2022).
Zhao, Y. et al. Effects of Ti-to-Al ratios on the phases, microstructures, mechanical properties, and corrosion resistance of Al2-xCoCrFeNiTix high-entropy alloys. J. Alloy. Compd. 805, 585–596 (2019).
Yang, J. et al. Enhanced electromagnetic-wave absorbing performances and corrosion resistance via tuning Ti contents in FeCoNiCuTix high-entropy alloys. ACS Appl. Mater. Interfaces 14, 12375–12384 (2022).
Wang, X. et al. Effect of Ti content on the microstructure and corrosion resistance of CoCrFeNiTix high entropy alloys prepared by laser cladding. Materials 13, 2209 (2020).
Wen, X. et al. Corrosion and tribo-corrosion behaviors of nano-lamellar Ni1.5CrCoFe0.5Mo0.1Nbx eutectic high-entropy alloy coatings: The role of dual-phase microstructure. Corros. Sci. 201, 110305 (2022).
Tanji, A., Fan, X., Sakidja, R., Liaw, P. K. & Hermawan, H. Niobium addition improves the corrosion resistance of TiHfZrNbx high-entropy alloys in Hanks’ solution. Electrochim. Acta 424, 140651 (2022).
Niu, Z. et al. Microstructure, mechanical properties and corrosion resistance of CoCrFeNiWx(x= 0, 0.2, 0.5) high entropy alloys. Intermetallics 112, 106550 (2019).
Zhou, Z. et al. Effects of W addition on the corrosion behaviors of FeCoNiCrMn high entropy alloy composites in the 3.5 wt.% NaCl solution. Surf. Interfaces 23, 100956 (2021).
Qi, W. et al. Effect of Zr on phase separation, mechanical and corrosion behavior of heterogeneous CoCrFeNiZrx high-entropy alloy. J. Mater. Sci. Technol. 109, 76–85 (2022).
Brito-Garcia, S., Mirza-Rosca, J., Geanta, V. & Voiculescu, I. Mechanical and corrosion behavior of Zr-doped high-entropy alloy from CoCrFeMoNi system. Materials 16, 1832 (2023).
Yao, Y. et al. Corrosion behavior of AlFeCrCoNiZrx high-entropy alloys in 0.5 M sulfuric acid solution. Metals 11, 1471 (2021).
Han, J. et al. Elementally resolved dissolution kinetics of a Ni-Fe-Cr-Mn-Co multi-principal element alloy in sulfuric acid using AESEC-EIS. J. Electrochem. Soc. 169, 081507 (2022).
Lutton, K., Gusieva, K., Ott, N., Birbilis, N. & Scully, J. R. Understanding multi-element alloy passivation in acidic solutions using operando methods. Electrochem. Commun. 80, 44–47 (2017).
Samin, A. J. & Taylor, C. D. First-principles investigation of surface properties and adsorption of oxygen on Ni-22Cr and the role of molybdenum. Corros. Sci. 134, 103–111 (2018).
Henderson, J. D., Li, X., Shoesmith, D. W., Noël, J. J. & Ogle, K. Molybdenum surface enrichment and release during transpassive dissolution of Ni-based alloys. Corros. Sci. 147, 32–40 (2019).
Choudhary, S., Qiu, Y., Thomas, S. & Birbilis, N. Element-resolved electrochemical analysis of transpassive dissolution and repassivation behavior of the multi-principal element alloy AlTiVCr. Electrochim. Acta 362, 137104 (2020).
Qiu, Y. et al. Real-time dissolution of a compositionally complex alloy using inline ICP and correlation with XPS. Npj Mater. Degrad. 4, 1–6 (2020).
Choudhary, S., Thomas, S., Macdonald, D. & Birbilis, N. Growth kinetics of multi-oxide passive film formed upon the multi-principal element alloy AlTiVCr: effect of transpassive dissolution of V and Cr. J. Electrochem. Soc. 168, 051506 (2021).
Luo, H., Li, Z., Mingers, A. M. & Raabe, D. Corrosion behavior of an equiatomic CoCrFeMnNi high-entropy alloy compared with 304 stainless steel in sulfuric acid solution. Corros. Sci. 134, 131–139 (2018).
Dworschak, D., Tseng, K. K., Yeh, J. W., Cheng, H. W. & Valtiner, M. Bottom-up characterization of electrochemical passivity from simple binary alloys to high entropy alloys. Electrochim. Acta 405, 139804 (2022).
Sasidhar, K. et al. Understanding the protective ability of the native oxide on an Fe-13 at.% Cr alloy at the atomic scale: a combined atom probe and electron microscopy study. Corros. Sci. 211, 110848 (2018).
Devaraj, A. et al. Visualizing the nanoscale oxygen and cation transport mechanisms during the early stages of oxidation of Fe-Cr-Ni alloy using in situ atom probe tomography. Adv. Mater. Interfaces 9, 2200134 (2022).
Xu, Y. et al. Natural oxidation of amorphous CuxZr1-x alloys. Appl. Surf. Sci. 457, 396–402 (2018).
Xu, Y. et al. On the competition between synchronous oxidation and preferential oxidation in Cu-Zr-Al metallic glasses. Corros. Sci. 177, 108996 (2020).
Louzguine-Luzgin, D. et al. Bulk metallic glassy surface native oxide: Its atomic structure, growth rate and electrical properties. Acta Mater. 97, 282–290 (2015).
Mendis, S. et al. Characteristics of oxide films on Ti-(10-75) Ta alloys and their corrosion performance in an aerated Hank’s balanced salt solution. Appl. Surf. Sci. 506, 145013 (2020).
Backman, L., Gild, J., Luo, J. & Opila, E. J. Part I: Theoretical predictions of preferential oxidation in refractory high entropy materials. Acta Mater. 197, 20–27 (2020).
Gerard, A. Y. et al. The role of chromium content in aqueous passivation of a non-equiatomic Ni38Fe20CrxMn21-0.5xCo21-0.5x multi-principal element alloy (x= 22, 14, 10, 6 at%) in acidic chloride solution. Acta Mater. 245, 118607 (2023).
Kim, E. T. et al. Near atomic-scale comparison of passive film on a 17 wt% Cr-added 18 wt% Mn steel with those on typical austenitic stainless steels. Scr. Mater. 203, 114112 (2021).
Zhang, B. et al. Unmasking chloride attack on the passive film of metals. Nat. Commun. 9, 1–9 (2018).
Rost, C. M. et al. Entropy-stabilized oxides. Nat. Commun. 6, 1–8 (2015).
Miracle, D. High entropy alloys as a bold step forward in alloy development. Nat. Commun. 10(1), 3 (2019).
Hautier, G., Fischer, C., Ehrlacher, V., Jain, A. & Ceder, G. Data mined ionic substitutions for the discovery of new compounds. Inorg. Chem. 50, 656–663 (2011).
Inman, S. B., Sur, D., Han, J., Ogle, K. & Scully, J. R. Corrosion behavior of a compositionally complex alloy utilizing simultaneous Al, Cr, and Ti passivation. Corros. Sci. 217, 111138 (2023).
Hakiki, N. Comparative study of structural and semiconducting properties of passive films and thermally grown oxides on AISI 304 stainless steel. Corros. Sci. 53, 2688–2699 (2011).
Wu, X., Voyshnis, S., Seyeux, A., Chumlyakov, Y. & Marcus, P. ToF-SIMS study of oxide films thermally grown on nickel-base alloys. Corros. Sci. 141, 175–181 (2018).
Yu, X. X., Perepezko, J. H. & Marks, L. D. Crystallographic anisotropy of nonequilibrium solute capture. Acta Mater. 198, 223–229 (2020).
Yu, X. X., Han, J., Scully, J. R. & Marks, L. D. Oxygen injection during fast vs slow passivation in aqueous solution. Acta Mater. 213, 116898 (2021).
Yu, X. X. et al. In situ observations of early stage oxidation of Ni-Cr and Ni-Cr-Mo alloys. Corrosion 74, 939–946 (2018).
Cwalina, K. L., Demarest, C., Gerard, A. & Scully, J. Revisiting the effects of molybdenum and tungsten alloying on corrosion behavior of nickel-chromium alloys in aqueous corrosion. Curr. Opin. Solid State Mater. Sci. 23, 129–141 (2019).
Iwaszuk, A. & Nolan, M. Charge compensation in trivalent cation doped bulk rutile TiO2. J. Phys. Condens. Matter 23, 334207 (2011).
Carey, J. J., Legesse, M. & Nolan, M. Low valence cation doping of bulk Cr2O3: charge compensation and oxygen vacancy formation. J. Phys. Chem. C. 120, 19160–19174 (2016).
Zheng, L. et al. Investigation on the effect of Nb doping on the oxidation mechanism of Ti3SiC2. Corros. Sci. 140, 374–378 (2018).
Nikolay, T., Larina, L., Shevaleevskiy, O. & Ahn, B. T. Electronic structure study of lightly Nb-doped TiO2 electrode for dye-sensitized solar cells. Energy Environ. Sci. 4, 1480–1486 (2011).
Shuang, S., Ding, Z., Chung, D., Shi, S. & Yang, Y. Corrosion resistant nanostructured eutectic high entropy alloy. Corros. Sci. 164, 108315 (2020).
Acknowledgements
The authors would like to acknowledge the support of the National Natural Science Foundation of China under Grant No. 52371050.
Author information
Authors and Affiliations
Contributions
Z.W.: conceptualization, investigation, visualization, writing—original draft, writing—review & editing. Y.Y.: writing—review & editing, conceptualization, resources, funding acquisition, supervision. Y.W.: writing—review & editing. Y.Z.: writing—review & editing. X.Z.: writing—review & editing. Y.S.: investigation. L.Q.: resources, supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, Z., Yan, Y., Wu, Y. et al. Recent research progress on the passivation and selective oxidation for the 3d-transition-metal and refractory multi-principal element alloys. npj Mater Degrad 7, 86 (2023). https://doi.org/10.1038/s41529-023-00410-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41529-023-00410-0