Abstract
A large and diverse library of glycan-directed monoclonal antibodies (mAbs) was used to determine if plant cell walls are modified by low-gravity conditions encountered during spaceflight. This method called glycome profiling (glycomics) revealed global differences in non-cellulosic cell wall epitopes in Arabidopsis thaliana root extracts recovered from RNA purification columns between seedlings grown on the International Space Station-based Vegetable Production System and paired ground (1-g) controls. Immunohistochemistry on 11-day-old seedling primary root sections showed that ten of twenty-two mAbs that exhibited spaceflight-induced increases in binding through glycomics, labeled space-grown roots more intensely than those from the ground. The ten mAbs recognized xyloglucan, xylan, and arabinogalactan epitopes. Notably, three xylem-enriched unsubstituted xylan backbone epitopes were more intensely labeled in space-grown roots than in ground-grown roots, suggesting that the spaceflight environment accelerated root secondary cell wall formation. This study highlights the feasibility of glycomics for high-throughput evaluation of cell wall glycans using only root high alkaline extracts from RNA purification columns, and subsequent validation of these results by immunohistochemistry. This approach will benefit plant space biological studies because it extends the analyses possible from the limited amounts of samples returned from spaceflight and help uncover microgravity-induced tissue-specific changes in plant cell walls.
Similar content being viewed by others
Introduction
Land plants evolved under the continuous presence of the Earth’s gravitational field. As such, plant development on Earth is strongly influenced by gravity1. One of the most obvious manifestations of gravity’s effects on plants is the redirection of the growth of their major organs when they are turned on their sides. Whereas roots redirect their growth downward toward the gravity vector, most shoots grow upward, opposite the pull of gravity. This process called gravitropism is widely regarded as a major environmental signal that shapes the architecture of roots and shoots, enabling plants to more efficiently capture soil resources and light inputs required for optimal growth2.
In shoots, the initial growth redirection of the organ due to gravitropism is followed by a sustained and upward growth. The continuous growth of shoots opposite the direction of gravity requires mechanical strength provided by the rigid, polysaccharide-rich cell wall, which is a process referred to as gravity resistance and is independent of gravitropism3. Signals for the gravity resistance response are believed to be perceived by mechanoreceptors that transmit information directing cortical microtubules to reorient. These changes in cortical microtubule orientation then lead to modified cell wall properties in the plant4. Because of their importance in providing mechanical support for processes, such as gravity resistance, cell walls and the elaboration of the molecular machinery required for their construction and remodeling, played a major role in the transition of plants from an aquatic environment to life on land5.
As the US National Aeronautics and Space Administration (NASA) considers the return of humans to the Moon, and for astronauts to set foot on Mars, the development of technological platforms for safe and sustainable space transportation will be essential. A crucial component for the creation of such technology is a convenient and reliable source of nutrient-rich food and oxygen for humans as they embark on long duration spaceflight missions6. Plants fulfill this need as they not only provide food and oxygen, but are also known to help in recycling waste, removing CO2, and improving the psychological well-being of astronauts who have to spend months, and likely years living in a confined spacecraft environment7,8. However, before plants can be deployed as major components of advanced life support systems, understanding how their biology is affected by the space environment is essential9,10,11. Doing so will enable the development of plant cultivars that are better adapted to the unique environment of space, including reduced gravity (i.e., microgravity) and confined growth environments, both of which can influence gravitropism and gravity resistance.
The era of the Space Shuttle and the International Space Station (ISS) enabled a number of experiments that address how spaceflight influences plant growth from whole plant and cell/tissue responses, to changes in the expression of genes and proteins12,13,14,15,16,17,18. In transcriptomic studies of spaceflight-grown A. thaliana seedlings, genes that are often found to be differentially regulated by spaceflight are those encoding proteins related to cell wall remodeling and oxidative stress11,14,16,19. Changes in the expression of cell wall-related genes may be due to the reduced mechanical load encountered by plants in microgravity. In fact, studies that directly measure cell wall metabolites or microscopically examine cell walls from plants grown in space are consistent with spaceflight transcriptomics data. For example, cellulose microfibrils of soybean seedlings grown on the Foton-M2 capsule are more disorganized than those of seedlings grown on Earth. Disorganized cellulose microfibrils of space-grown seedlings leads to thinner xylem vessel walls20. Another study showed that expression of microtubule regulatory proteins and the percentage of cells with transverse cortical microtubules increases in A. thaliana seedlings grown in microgravity21. Cortical microtubules serve as tracks for cellulose synthesizing complexes22. Thus, reduced growth anisotropy of microgravity-grown seedlings, which had longer and thinner hypocotyls than those of Earth-grown seedlings, could be partly explained by cortical microtubule-influenced modifications in cellulose microfibril orientation21.
Plant cell walls consist primarily of cellulose, hemicellulose, and pectin, and in secondary walls, lignin23,24. The polysaccharide composition of each cell wall type varies among plant tissue and cell types, stage of plant development, and the environment, in which the plant is grown25,26,27. Glycome profiling is a powerful tool to study the complexity of plant cell walls. It involves the use of a large and diverse collection of monoclonal antibodies (mAbs) that recognize unique epitopes in most major non-cellulosic plant cell wall glycans28 . The binding of mAbs to specific cell wall glycan epitopes as determined by Enzyme-Linked Immunoabsorbent Assay (ELISA), provides a comprehensive picture about environmentally- or genetically-induced changes in cell wall composition and structure28,29,30. However, the power of glycome profiling is best realized when it is combined with immunohistochemistry, which enables the visualization of cell wall changes occurring in specific tissue and cell types29. Here, as part of the Advanced Plant EXperiments (APEX) 03-1 in space project, we demonstrate the feasibility of combining glycomics and immunohistochemistry to study changes in non-cellulosic cell wall composition of A. thaliana roots adapting to spaceflight. We show that plant biomass recovered from RNA extraction columns provides valuable information on spaceflight-induced global changes in cell walls, some of which are manifested as tissue/cell-type differences in cell wall epitope accumulation between ground- and space-grown seedling roots.
Results
Space- and Earth-grown seedling morphology in Veggie
Six and eleven days after experiment activation (Supplementary Fig. 1), seedlings from ground controls and space displayed robust growth on the ISS-based and ground Vegetable Production System (Veggie). Six-day-old seedlings had about two true leaves, in addition to fully expanded cotyledons (Fig. 1a, c). On the other hand, 11-day-old seedlings had 4–5 true leaves and root systems with extensive laterals (Fig. 1b, d). As reported previously, roots from seedlings grown in space skewed to one side, while those on the ground grew downwards in alignment with the gravity vector12,18,31. The directional skewing of primary roots was also observed in 6-day-old APEX 03-1 seedlings grown in space (Fig. 1c). In 11-day-old space-grown seedlings, many roots grew toward the shoot with some roots reaching the top of the plate (Fig. 1d). Unlike roots, there were no clear qualitative differences in growth characteristics between shoots of ground- and space-grown seedlings. Therefore, we focused on characterizing root responses. To maximize return from the limited samples from spaceflight, we took the RNAlater-fixed root debris left over from RNA extraction columns used in a separate transcriptomic study of these plants for glycome profiling. A separate set of seedling roots fixed in aldehydes were used for immunohistochemistry.
Representative images of six- (a and c) and 11-day-old (b and d) seedlings prior to harvest and fixation. Note that roots of seedlings on the ground grow vertically toward the direction of gravity (a, b). On the other hand, roots of 6-day-old seedlings in space skewed generally to the right (c). Some roots of 11-day-old seedlings reached the top of the plate (magenta arrows). Bar = 1 cm for all panels.
Glycome profiling of space- and Earth-grown seedling roots
Glycome profiling involves sequential extraction of cell wall samples, consisting of six steps with increasingly harsh reagents in the following order: ammonium oxalate, sodium carbonate, 1 M potassium hydroxide (KOH), 4 M KOH, acidic sodium chlorite, and 4 M KOH post chlorite32. A complete glycome profile analysis with six extraction steps normally requires 50–200 mg of cell wall material, which is roughly equivalent to 0.5–2 grams of plant biomass32. For the glycomics experiments described here, the amount of plant material was a limiting factor. The RNAlater-fixed seedlings were used for RNA-Seq studies, but we realized that the plant debris retained in RNA extraction columns was from the cell walls of the samples, and therefore could potentially be used for glycomics to assess spaceflight-induced global changes in cell walls. Seedling root and shoot debris from QIAGEN RNeasy Mini Kit RNA extraction columns yielded approximately 2 mg of cell wall material. The low amount of material allowed the use of only a single extraction step (4 M NaOH), rather than the six sequential fractionations noted above. The fourth 4 M KOH extraction step was chosen because it can yield the highest amount and diversity of alkaline soluble cell wall glycans, and therefore should provide the most information on cell wall composition differences between ground- and space-grown seedlings.
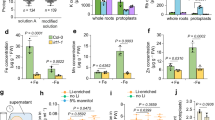
Glycome profiling with the 4 M KOH extract revealed that xylans and xyloglucans, which are major hemicelluloses in dicotyledonous plant cell walls23, were the most abundant carbohydrates detected in root extracts of ground controls and spaceflight samples. This conclusion was based on the higher binding intensities of xylan- and xyloglucan-directed mAbs than those of mAbs directed to other non-cellulosic polysaccharide epitopes (Fig. 2a; Supplementary Table 1). Signal intensity of mAb binding was also generally higher in extracts of roots from space-grown seedlings than from extracts obtained from Earth-grown seedlings (Fig. 2b, c). Xylan mAbs with higher binding intensity included those that recognized small degrees of polymerization (DP) in the unsubstituted xylan backbone (e.g., DP5 to 8; CCRC-M138, CCRC-M139, CCRC-M140, and CCRC-M148) and those with methylglucuronic acid (MeGlcA-Xylan; CCRC-M144) and arabinose (Ara) side chains (CCRC-M108) (Fig. 2b). Xyloglucan epitopes included those that were xylosylated (Xyl-XG; CCRC-M100), galactosylated (Gal-XG-2; CCRC-M55, CCRC-M96, and CCRC-M50), and fucosylated (Fuc-XG; CCRC-M1 and CCRC-M84). Signal intensity of mAb binding was generally higher in root extracts from 11-day-old seedlings than that of root extracts from 6-day-old seedlings (Fig. 2a). Furthermore, mAb binding was more consistent among three biological replicates in root extracts from 11-day-old seedlings than root extracts from 6-day-old seedlings, which prompted us to focus our immunohistochemical studies on the older seedlings.
a ELISA heat map of 4 M KOH root extracts from 6- and 11-day-old space-grown and ground control seedlings. b ELISA heat map of 11-day-old roots of 22 mAbs selected for immunohistochemistry. The mAbs selected bind to xylosylated xyloglucan (Xyl-XG), galactosylated xyloglucan (Gal-XG) fucosylated xyloglucans (Fuc-XG), and larger not yet defined xyloglucan epitopes, α-1,2-linked arabanosyl xylan (2-Ara-Xylan), 4-O-Me-GlcA xylan, unsubstituted xylan backbones (DP5-8), β-6-linked galactans, and arabinogalactan (AG) epitopes. c Bar graph of raw ELISA readout values of the 22 mAbs shown in panel (b). Values are means (n = 3) ± standard deviation. Heatmaps and bar graphs were generated from ELISA values in Supplementary Table 1. Galactose (Gal); Fucose (Fuc); Arabinose (Ara); Methyglucoronic acid (MeGlcA); DP (Degree of Polymerization); Xylose (Xyl).
As glycome profiling of the debris from the RNA isolation columns revealed global differences in xyloglucan levels between roots from ground- and space-grown seedlings (Fig. 2), procedural controls using plant debris from xyloglucan xylosyltransferase (xxt1/xxt2) double and mur3-3 single mutants were conducted. The xxt1/xxt2 mutant has no xyloglucan33 while the mur3-3 mutant, which is defective in a gene encoding a galactosyltransferase, contains xyloglucans that lack a galactose-fucose side chain34,35. Both mutants were stored in RNAlater following the same timeline as the APEX 03-1 pipeline (Supplementary Fig. 1), and were processed in a similar manner as seedlings returned from space. Results showed that the binding of mAbs to a range of xyloglucan epitopes was significantly lower or absent in extracts of the xxt1/xx2 mutants than that in extracts of wild-type seedlings. In mur3-3 plant extracts, mAb binding to fucosylated xyloglucan was lower than that of wild type (Supplementary Fig. 2). These results are consistent with previously published data on these mutants that were obtained from cell wall samples prepared according to standard six-step extraction glycome profiling protocols rather than using debris from RNA purification columns, thus validating our results from the space-grown plants.
Another procedural control was conducted on the ground. This involved processing whole 11-day old seedlings not fixed in RNAlater and without running plant material in RNA columns, and comparing the ELISA readout with RNA column debris from 11-day old whole seedlings fixed in RNAlater. Both sets were then subjected to the 4 M KOH extraction step described above. This procedural control was conducted to determine if certain cell wall components were depleted when using RNALater-fixed seedling extracts from RNA columns. It was found that some xyloglucan and xylan epitopes were depleted in two out of four RNALater-fixed whole seedling extracts from RNA columns (Supplementary Fig. 3).
Immunohistochemistry of space- and Earth-grown roots
Although ELISA readouts for glycome profiling revealed global differences in non-cellulosic cell wall components between root extracts of space- and Earth-grown seedlings, immunohistochemistry could provide more detailed information on where such changes are occuring. Twenty-two mAbs that exhibited higher binding to root cell wall extracts of space-grown seedlings than to extracts from ground controls were selected for immunohistochemistry based on the raw ELISA readout (Fig. 2b, c). These twenty-two mAbs recognized diverse epitopes of xylans, xyloglucans, and arabinogalactans (AGs) (Fig. 2b).
Two regions of 11-day-old roots were selected for immunohistochemistry (Fig. 3a). One region was located close to the root-hypocotyl junction, which represented mature root tissue (Fig. 3b). The other region was from the apical three millimeters of the primary root tip, which represented actively dividing and expanding cells of the meristem and elongation zone, respectively (Fig. 3c). Cross and longitudinal sections (0.25 μm) for mature root tissues and primary root tips, respectively, were obtained for immunohistochemistry.
Immunohistochemistry of root tip longitudinal sections revealed that labeling intensity of 10 of the 22 mAbs was higher in space-grown seedlings than that in ground controls. Among the ten mAbs, eight mAbs recognized xyloglucan epitopes, one was specific to a xylan epitope with arabinose side chains (2-Ara-Xylan), and one was directed to a β-6 Galactan-3 epitope (Fig. 4a–f; Supplementary Fig. 4). Seven of the 22 mAbs that recognize xylan and AG epitopes did not label root tips from space- and Earth-grown seedlings (i.e., sections showed no fluorescence signals). Furthermore, CCRC-M80 and CCRC-M100, which recognize a β-6 Galactan-3 and Xyl-XG epitope, respectively, had fluorescence signals that were similar in intensity between roots of space- and Earth-grown seedlings (Supplementary Fig. 4). Two mAbs that also recognized β-6 Galactan-3 epitopes (CCRC-M79 and CCRC-M123) and an AG-2 epitope (JIM19) showed lower root fluorescence signals in space than those in the ground controls (Fig. 5a–f).
M2-XG (a, b), Gal-XG-2 (c, d), and β-6 Galactan-3 epitopes (e, f), are labeled more intensely in roots of space-grown seedlings than in roots of ground controls. Values in box plots in b, d, and f are means of relative fluorescence intensity from randomly picked 10 × 10 μm regions that had positive fluorescence. Box limits indicate 25th and 75th percentiles, horizontal line is the median, and whiskers display minimum and maximum values. **P < 0.001 indicates statistical significance as determined by Student’s t-test. Each dot represents individual measurement from ten regions of three longitudinal root tip sections. Bar = 50 μm.
Epitopes labeled by CCRC-M79 (a, b), JIM19 (c, d), and CCRC-M123 (e, f) had lower fluorescence intensity in roots of 11-day-old seedlings grown in space than that in the ground controls. Values in box plots in b, d, and f are means of relative fluorescence intensity from randomly picked 10 × 10 μm regions that had positive fluorescence. Box limits indicate 25th and 75th percentiles, horizontal line is the median, and whiskers display minimum and maximum values. **P < 0.001 and *P < 0.01 indicates statistical significance as determined by Student’s t-test. Each dot represents individual measurement from 50 regions of three longitudinal root tip sections. Bar = 50 μm.
Immunohistochemistry of cross-sections of the root maturation zone also revealed spaceflight-induced changes in glycan epitope labeling intensities. Like space-grown root tip longitudinal sections, CCRC-M1 and CCRC-M50, which recognize a fucosylated and galactosylated xyloglucan, respectively, showed space-induced fluorescence increases in root cross-sections (Supplementary Fig. 5). Antibodies that recognized Gal-XG-2 (CCRC-M55), M2-XG (CCRC-M99), and 2-Ara-Xylan (CCRC-M108) had lower fluorescence signals in space root cross-section than those in ground controls. Four mAbs that bind to xyloglucans (CCRC-M58, CCRC-M96, CCRC-M84, and CCRC-M86) and three β-6 Galactan-3 epitopes (CCRC-M13; CCRC-M79, and CCRC-M123) had fluorescence signals that were similar in root cross-section of space- and Earth-grown seedlings (Supplementary Figs. 5 and 6; Fig. 6a).
a, b The β-6 Galactan-3 epitope recognized by CCRC-M79 labels root cell walls uniformly in space and on Earth. c, d The xylan backbone mAb, CCRC-M140, preferentially labels root xylem cells in space and on Earth (arrows). Note that xylem cells of roots from space-grown seedlings are more intensely labeled than those of the ground controls (arrows). e At low magnification, the AG-2 mAb, JIM19, appears to label root cells of space-grown seedlings in a similar manner as those of seedlings on the ground. f Enlarged image of the white boxes in the images in e. Note that wall spaces in roots of seedlings on the ground have AG-2 epitope fluorescence signals that fill the cell wall spaces (arrows). By contrast, cell wall spaces of roots of space-grown seedlings are less densely labeled (arrowheads). Quantification of fluorescence intensity in root cross-sections (b, d, g). Values in box plots are means of relative fluorescence intensity from randomly picked 10 × 10 μm regions that had positive fluorescence. Box limits indicate 25th and 75th percentiles, horizontal line is the median, and whiskers display minimum and maximum values. **P < 0.001 indicates statistical significance as determined by Student’s t-test. Not significant (ns). Each dot represents individual measurement from ten regions of three root cross-sections. Bar = 50 μm.
Some mAbs that did not label root tip longitudinal sections from space- and Earth-grown seedlings showed positive signals in root cross-sections of the maturation zone. Most notable were those that bind to the unsubstituted xylan backbone, such as, CCRC-M138, CCRC-M139, CCRC-M140, and CCRC-M148. Xylan backbone epitopes were more intensely labeled in xylem cells of root cross-sections from space-grown seedlings than in the equivalent tissues in ground controls (Fig. 6c, d; Supplementary Fig. 6a, b). CCRC-M148, which also recognizes the xylan backbone, preferentially labeled xylem cells in root cross-sections. However, unlike CCRC-M138, CCRC-M139, and CCRC-M140, the intensity of fluorescence signal in xylem cells of CCRC-M148-labeled root cross-sections was similar in space and the ground controls (Supplementary Fig. 6a).
Immunohistochemistry of root cross-sections also uncovered differences in the density of epitope labeling that were triggered by spaceflight. For instance, labeling of the AG-2-recognizing mAb, JIM1936,37,38, was less uniform in the cell wall spaces of roots from space-grown seedlings than that of the ground controls, leading to lower overall fluorescence signals (Fig. 6e–g).
Xyloglucan monoclonal antibodies label root post-Golgi bodies
Immunohistochemistry also revealed punctate bodies in root tip longitudinal sections labeled with mAbs against xyloglucans. In particular, CCRC-M1, which binds to the α-fucose-(1, 2)- β-galactose structure of xyloglucan39, labeled distinct bodies in roots of spaceflight- and Earth-grown seedlings (Fig. 7a, b). We cannot discount the possibility that the bodies labeled with anti-xyloglucan mAbs are an artifact of extended aldehyde fixation times. The standard aldehyde fixation time for immunohistochemistry with cell wall mAbs is typically 2 h40. On the other hand, seedlings in APEX03-1 used for immunohistochemistry remained in aldehyde fixatives for up to 28 days (Supplementary Fig. 1). We therefore fixed 11-day old A. thaliana seedlings for 2 h and compared CCRC-M1 root labeling patterns of sections from optimally fixed roots with those from APEX 03-1. We found that roots fixed for 2 h in aldehydes also displayed Fuc-XG-positive bodies, suggesting that immunohistochemistry results from roots fixed in aldehyde for extended periods during APEX-03-1 truly reflected the observed mAb labeling patterns (Fig. 7c).
Root sections from seedlings on the ground (a) and in space (b) labeled with CCRC-M1, which binds to fucosylated xyloglucans (Fuc-XG), have distinct bodies (arrowheads in the enlarged inset). c Roots from seedlings optimally fixed for 2 h in aldehydes also exhibit XG-positive bodies (arrowheads in inset). d Without xyloglucan-specific endoglucanase (XEG) pretreatment, CCRC-M1 labels fluorescent bodies in root tip longitudinal sections. e Root sections pretreated with XEG, which cleaves off xyloglucan epitopes, followed by CCRC-M1 immunofluorescence labeling, have no fluorescent bodies. f Glycome profiling of root microsomal fractions from 11-day-old seedling roots reveal binding of several XG mAbs. Bar in d = 10 μm for panels a to e.
The occurrence of fluorescent puncta in root sections labeled with some xyloglucan mAbs was validated further by enzymatic digestion by first treating root sections with a xyloglucan-specific endoglucanase (XEG), which cleaves xyloglucans to oligosaccharides that are released from the tissue sections, prior to immunolabeling. We found that XEG-pretreated roots labeled with CCRC-M1 had no fluorescence (Fig. 7d, e). The bodies labeled with CCRC-M1 are reminiscent of endomembrane-associated Golgi and post-Golgi organelles tagged with fluorescent proteins41. To further verify if xyloglucan mAbs recognize endomembrane components, root microsomal fractions were isolated from 11-day-old A. thaliana primary roots and subjected to glycome profiling. Results showed that several xyloglucan mAbs, including CCRC-M1, bound to root microsomal fractions (Fig. 7f).
Discussion
We demonstrate the use of glycome profiling and immunohistochemistry to better understand how spaceflight affects plant cell wall development in A. thaliana seedling roots. We were motivated to implement these methods as part of the APEX03-1 study because of accumulating transcriptomic studies showing that genes encoding cell wall remodeling proteins are among those that most significantly change expression during spaceflight14,16,19,42. Proteomic studies also reveal that proteins related to cell wall biosynthesis are upregulated in microgravity43. However, information about specific plant cell wall components that are modified by spaceflight is scarce. Unlike Earth-based laboratory experiments, biological spaceflight studies can be fraught with technical challenges because major steps in method implementation are not directly controlled by the investigator team44,45. Furthermore, there are studies indicating that the spaceflight hardware affects plant morphology, as well as gene and protein expression46,47. Adding to these problems is the limited amount of biological material that can be retrieved from spaceflight experiments. Given the technical challenges in conducting plant experiments in space48, it is important to implement procedural controls that can mitigate potential data interpretation errors from biological samples retrieved from space. One procedural control implemented here considered how seedlings were handled during APEX03-1. This control involved processing A. thaliana xyloglucan mutants on the ground in a similar manner as wild-type seedlings grown in space, and asking if glycome profiling still detected cell wall alterations in the mutants despite using only root debris from RNA columns, and subjecting these samples to one step extraction with 4 M KOH. We found that xyloglucan mAb binding to RNA column root debris of the mutants was lower than that of wild type (Supplementary Fig. 2), which was consistent with results obtained from whole plants using the six-step sequential glycome profiling extractions34,49. The procedural control with xyloglucan-defective mutants indicates that glycome profiling of plant debris from RNA purification columns using one extraction step can still reliably report environmentally-induced cell wall changes. Another procedural control performed on the ground was to compare ELISA heatmaps of whole seedlings fixed in RNALater and debris collected from RNA columns with whole seedlings processed directly using the 4 KOH step without RNALater fixation. It was observed that two RNA column-derived, RNALater-fixed whole seedling extracts had lower xylan and xyloglucan binding than that of controls (Supplementary Fig. 3). This result indicates that some non-cellulosic epitopes that are loosely bound might be depleted in RNA column-derived cell wall extracts, which indicates a limitation of this approach. Nonetheless, results from this procedural control does not change the validity of using plant debris from RNA extraction columns provided that comparisons are made between samples processed in a similar manner.
It is also important to note that the aldehydes in APEX-03-1 were stored for an extended period in Kennedy Fixation Tubes (KFTs) prior to seedling fixation, and samples remained in fixative for almost four weeks before they were turned over to the investigator team (Supplementary Fig. 1). Indeed, APEX03-1 science and payload verification tests (SVT and PVT) revealed that extended aldehyde fixation can adversely affect the quality of root sections50. The results obtained from SVT and PVT enabled us to implement methods for APEX03-1 that mitigated the extended fixative storage and seedling fixation times. The methods implemented included bubbling the aldehyde solution in nitrogen gas prior to loading in KFTs and storing aldehyde-containing KFTs in the dark and at 4 °C prior to seedling fixation. Furthermore, SVT and PVT indicated that paraformaldehyde and glutaraldehyde in combination could better preserve root morphology than glutaraldehyde alone when seedlings are fixed for long periods50.
Some of the immunohistochemical labeling results presented here were also validated by a post-flight procedural control, in which APEX03-1-processed roots were compared with roots fixed under standard ground laboratory conditions that involved short duration (i.e., 2 h) seedling fixation and glycome profiling of root membrane fractions (Fig. 7). This control coupled with pretreatment of root sections with xyloglucan digesting enzymes enabled us to show that bodies that were detected by the xyloglucan-directed mAb, CCRC-M1, were likely endomembrane structures. Our results are consistent with membrane fractionation studies demonstrating that CCRC-M1 distributed in the same fractions as proteins that are known to be associated with post-Golgi vesicles51. Moreover, the enrichment of xyloglucan epitopes in the Golgi and trans-Golgi Network revealed by glycome profiling of isolated vesicles is consistent with the punctate staining patterns in roots observed here52. It is notable that labeling intensity of xyloglucan-directed mAbs was generally higher in roots from spaceflight seedlings than in roots of the ground controls. It is possible that the spaceflight-induced increases in fluorescence intensity observed after labeling with xyloglucan mAbs is caused by altered biosynthesis and polysaccharide secretion in microgravity. In this regard, proteomic experiments with A. thaliana seedlings on the European Modular Cultivation System (EMCS) on-board the ISS uncovered alterations in the expression of membrane-associated proteins by microgravity53. Furthermore, some proteins of A. thaliana callus in microgravity were found to be involved in processes related to intracellular trafficking54.
This study highlights the need to validate glycome profiling with immunohistochemistry to gain more meaningful insights into spaceflight-induced modifications in plant cell walls. This is because glycome profiling is done at the whole organ level, while immunohistochemistry focuses on specific locations within the plant organ. Therefore, it is not surprising that results from glycome profiling will not always correlate with immunohistochemistry. Here, validation becomes even more important because only trace amounts of root debris and one extraction step was used for glycome profiling. It was shown previously that such an approach can yield very subtle differences between treatments, and are therefore difficult to interpret without corresponding immunohistochemical studies19. It is important to note, however, that glycome profiling and immunohistochemistry need to be assessed independently. Immunohistochemistry monitors actual epitope distribution per se. On the other hand, the extraction steps for glycome profiling renders cell wall epitopes freely available for antibody access. Immunohistochemistry is subject to effects of epitope masking in a complex cell wall matrix environments. Nonetheless, about 50% of the mAbs selected for immunohistochemistry mirrored results from glycome profiling (i.e., fluorescence signals in root sections increased in space), supporting the validity of our approach. To the best of our knowledge, there are no published reports on the extent by which glycome profiling mirrors immunohistochemistry.
The importance of combining glycome profiling and immunohistochemistry on different regions of the plant organ can be seen from results with xylan backbone-directed mAbs. Labeling of xylan backbone epitopes was absent in root tip longitunal sections because xylans are synthesized and deposited in mature tissue, particularly in secondary walls of developing xylem55. This is consistent with the enrichment of fluorescence signals in the xylem in mature root cross-sections labeled with xylan-directed CCRC-M138, CCRC-M139, and CCRC-M140 mAbs. By conducting immunohistochemistry in two root regions, one can conclude that spaceflight-induced increases in xylan backbone epitopes uncovered by glycomics are likely the result of localized changes in the xylem. The spaceflight-induced increases in labeling of xylan backbone epitopes in the root xylem with three different mAbs, along with the procedural controls noted earlier, further validates the results presented here. It is unclear why levels of xylan backbone epitopes in the root xylem of space-grown seedlings were higher than those of seedlings on the ground. One possibility is that spaceflight-associated stresses trigger activation of root developmental pathways that are accompanied by hemicellulose and lignin reinforcement of secondary cell walls56. In this regard, it is notable that transcriptome signature of spaceflight-grown A. thaliana seedlings overlap with those of cold, drought, and hypoxia14.
The spaceflight-induced root changes in xylans and xyloglucans revealed by glycome profiling and immunohistochemistry are consistent with results showing that the hemicellulose fraction of rice roots increased in space when compared with that of the ground controls57. By contrast, rice shoots on the ISS were shown to have reduced hemicellulose relative to their 1-g controls58. Differences in microgravity-triggered hemicellulose changes between roots and shoots noted in the above studies further underscore the need for coupling cell wall metabolic analyses with immunolocalizations in different organ- and/or tissue-types.
Our study also reinforces previous work showing a close relationship between spaceflight-induced changes in gene expression and plant phenotypes in space. For instance, shorter root hairs of A. thaliana seedlings grown in the Biological Research in Canisters 16 (BRIC-16) hardware onboard the space shuttle Discovery correlates with lower expression of genes encoding cell wall cross-linking class III peroxidases. Some loss-of-function peroxidase mutants grown on Earth have short and ruptured root hairs that are reminiscent of wild-type seedlings grown in space14. Consistent with these findings is the reduced formation of ferulate networks, and lower expression of genes encoding class III peroxidases in rice seedlings developing in microgravity59. Here, spaceflight-induced changes in xyloglucan epitopes uncovered by glycomics and immunohistochemistry could be the result of differential gene expression. Several transcriptomic studies show that genes encoding xyloglucan endotransglucosylases and glycosyl hydrolases are differentially regulated by spaceflight14,16,17,19,60,61. A RNA-Seq spaceflight study called APEX 03-2 that was conducted at the same time and with the same Veggie growth parameters as our APEX 03-1 is most relevant. Like APEX 03-1, A. thaliana roots on APEX 03-2 exhibited a skewing behavior42 (Fig. 1). Results of the APEX 03-2 study showed a number of genes that were differentially regulated in space, including those encoding xyloglucan endotransglucosylases and AG proteins62. Furthermore, several proteins associated with xylan biosynthesis, including xylose synthases, transferases, and hydrolases were upregulated during spaceflight43, which could explain differential xylan mAB binding between space- and ground-grown roots uncovered here. RNA-Seq analysis of APEX 03-1 seedling material from which root extracts for the glycomics work described here are ongoing to determine if transcripts differentially induced in space correlate with observed cell wall changes. Reproducing space-grown conditions on Earth can be a valuable tool to study microgravity-induced changes in plant growth and development. For example, clinostats, random positioning machines, and magnetic levitation devices, can eliminate the effects of constant gravity and used to simulate the low-gravity conditions experienced in space63. It would be interesting to see if these microgravity analogs can elicit similar cell wall changes revealed by glycome profiling of RNA column debris from space-grown seedlings.
In summary, the results presented here support previous studies showing that spaceflight triggers alterations in plant cell walls. These conclusions are based on global differences in binding intensities of mAbs to non-cellulosic glycans from glycome profiling and corresponding immunohistochemical studies of root sections. Given the technical challenges associated with conducting plant spaceflight experiments, this study further highlights the importance of implementing procedural controls that take into account potential data interpretation errors and sample processing artifacts.
Methods
Preflight, flight, and post-flight operations
The experimental timeline of APEX 03-1 is shown in Supplementary Fig. 1, and photographs of various stages involved in the process are shown in Supplementary Fig. 7. Processing of plant material for microgravity experiments was conducted at the Space Station Processing Facility (SSPF) of the NASA Kennedy Space Center (KSC) in Cape Canaveral, Florida in January 2015. Sterilization and planting methods of A. thaliana seeds are described in detail in Chai et al.64. Briefly, sterilized seeds of wild-type (ecotype Columbia-0) were planted on growth media (1.2% phyta-agar) consisting of 0.5X Murashige-Skoog (MS) salts and 1% (w/v) sucrose (pH of 5.7) layered onto 90 X 90 mm square Petri dishes (Simport Scientific Inc., Beloeil, Quebec, Canada) (Supplementary Fig. 7a).
Immediately after planting, Petri dishes containing the seeds were exposed to far-red (FR) light (3.2 μmol m-2 s-1) for 10 min using a specially constructed rectangular metal box as described in Nakashima et al.50. Such FR treatment promotes dormancy in A. thaliana seeds, preventing premature germination prior to arrival in orbit. A single light emitting diode (LED) (ER-R2, 4606, Norlux Corporation, Carol Stream, Illinois, USA) was used to provide FR illumination to the seeds inside the Petri dishes. FR treatment was carried out under dim light with the distance between the FR LED and Petri dish set at 70 mm. Petri dishes were immediately wrapped with a layer of aluminum foil after FR treatment to keep seeds in complete darkness during imbibition. Petri dishes were then stored in a 4 °C refrigerator prior to launch. The Falcon 9 rocket carrying the Dragon Spacecraft on the SpaceX Commercial Resupply Service (CRS)-5 mission was launched from the Cape Canaveral Air Force Station at 4:47 A.M. (EST) on January 10, 2015 (Supplementary Fig. 7b).
To activate the experiment and trigger germination while in orbit, the aluminum foil was removed, and the Petri dishes were installed in the Veggie plant growth hardware. One Veggie unit was located at the ISS Environmental Simulator (ISSES) located at the SSPF and used as the ground controls (Supplementary Fig. 7c). The other unit was located in the Columbus module on the ISS and used as the spaceflight set (Supplementary Fig. 7d). LEDs on Veggie were programmed to provide constant white light at an intensity of 120–140 μmol m-2 s-1. Temperature in the Veggie was maintained at 24 °C. After the Petri plates were installed in Veggie, seedlings were harvested at 6 and 11 days after experiment activation and transferred to Kennedy Fixation Tubes (KFTs), containing either RNAlater (Thermo Fisher Scientific, Waltham, MA) or 4% (v/v) paraformaldehyde and 2.5% (v/v) glutaraldehyde (both from Electron Microscopy Sciences, Hatfield, PA, USA) in phosphate buffered saline (PBS, pH 7.2). Harvesting and fixation on KFTs was performed by Expedition 42 commander Barry Wilmore who completed the process in 2 h (Supplementary Fig. 7e). KFTs containing the chemicals were kept in the dark and at 4 °C until fixation. Details on KFT actuation are described in Nakashima et al.50. Actuated KFTs with RNAlater were stored in a –80 oF laboratory freezer (ground controls), or in the Minus Eighty-Degree Laboratory Freezer for ISS (MELFI), whereas those with aldehydes were kept at 4 °C. Samples returned to Earth at 4:44 P.M. (PST) on February 11, 2015 (Supplementary Fig. 7f). Fixed seedlings were retrieved from the KFTs at KSC four days after the Dragon Spacecraft splash down and were turned over to the investigator team three days later. Individual seedlings fixed by paraformaldehyde and glutaraldehyde mixture were then carefully separated into shoots and roots, and photographed prior to processing for microscopy.
Glycome profiling
Seedlings from spaceflight and corresponding ground controls fixed in RNAlater were thawed, dried using Kimwipes, ground in liquid nitrogen, and stored in –80 oF prior to RNA isolation. Seedlings were divided into shoots and roots, and total RNA was isolated using Plant RNeasy Mini Kit (QIAGEN GmbH, Hilden, Germany). The pellet of cell-debris in the QIAGEN shredder spin columns were saved and processed for glycomics.
Glycome profiling of cell wall extracts was conducted using a single step extraction. Briefly, alcohol insoluble materials from root debris were first isolated and extracted with a 4 M KOH solution containing 1% (w/v) sodium borohydride as described in Pattathil et al.32. The extract was dialyzed exhaustively against water and lyophilized before subjecting to glycome profiling. The 4 M KOH extract was then screened by ELISA using a comprehensive suite of plant cell wall glycan-directed mAbs that recognized most of the major non-cellulosic glycan epitopes in plant cell walls32. ELISAs were performed by coating 0.3 µg glucose equivalents of carbohydrate materials onto the each well of a 384-well dish (Costar 3700, Corning Inc, Corning, NY, USA). ELISAs were conducted with a fully automated robotic system (Automated ELISA Workstation, Thermo Fisher Scientific Inc. Waltham, MA, USA). The ELISA response values are depicted as heatmaps to reflect the relative abundance of glycan epitopes recognized by the mAbs.
Immunohistochemistry
Immunohistochemistry was performed on roots of 11-day-old seedlings fixed in aldehydes. Roots were washed six times in PBS, dehydrated in a graded ethanol series, and embedded in LR White resin (London Resin Co. Ltd., Reading, Berkshire, UK) as described in Avci et al.29. The resin was then polymerized at 4 °C under UV light for 3 days using a PELCO UVC3 Cryo Chamber (Ted Pella, Redding, CA, USA). Serial semi-thin sections (0.25 μm thick) were cut with a diamond knife mounted on a Leica EM UC7 ultramicrotome (Leica Microsystems GmbH, Vienna, Austria). Longitudinal and cross-sections were obtained from root tips and the root maturation zone using the flat embedding method described in Avci & Nakashima40. The quality of sections was evaluated by staining in 1% (w/v) Toluidine Blue-O in 1% (w/v) sodium borate for 5 min and followed by observation under either a Nikon Microphot-2 microscope (Nikon Corporation, Tokyo, Japan) or a Carl Zeiss ApoTome.2 microscope (Carl Zeiss Microscopy GmbH, Oberkochen, Germany).
Immunohistochemistry was performed by applying and removing a series of 10 µL droplets of the appropriate reagents to the sections as follows: (1) Sections on glass slides were blocked with 3% (w/v) nonfat dry milk diluted in KPBS [0.01 M Potassium Phosphate (pH 7.1) containing 0.5 M sodium chloride and 2 mM Sodium Azide (Sigma-Aldrich, St. Louis, Missouri, USA) for 15 min; (2) Sections were then incubated with hybridoma supernatant of mAbs to 22 selected cell wall-derived glycans (CarboSource Services, Athens, Georgia, USA) diluted 1:5 in KPBS for 16 to 18 h at 4 °C; (3). After primary mAb treatment, sections were washed with KPBS three times for 5 min each, and incubated for 2 h at room temperature in the dark in goat anti-mouse IgG conjugated with Alexa-Fluor 488 or goat anti-rat IgG conjugated with Alexa-Fluor 488 (Molecular Probes, Life Technologies Corporation, Carlsbad, California, USA) diluted 1:100 in KPBS; (4). Sections were washed again with KPBS three times for 5 min and distilled water for an additional 5 min; (5). Finally, sections were mounted on coverslips using Citifluor antifade AF1 (Electron Microscopy Sciences, Hatfield, Pennsylvania, USA). The sections were imaged using a Leica TCS SP2 AOBS Confocal Laser Scanning Microscope (Leica Microsystems CMS GmbH, Mannheim, Germany) equipped with a 40x water immersion objective. Alexa-Fluor 488 was imaged by exciting the sections with the 488 nm line of an Argon laser and emission detected at 520 nm using a narrow band pass filter. Magnification and imaging parameters (i.e., laser power, pinhole size, and detector sensitivity) were kept constant for flight and ground control samples.
Immunohistochemistry was performed on three sections from the same embedding block that came from three roots for each mAb and treatment. Quantification of fluorescence intensity was conducted by drawing a 10 × 10 μm region of interest in areas of the sections with positive signal using Image J.
Root microsomal fractions and xyloglucan-specific endoglucanase treatment
Fresh roots (2 g) from 11-day-old seedlings were homogenized in 4 mL of a buffer containing 12% (w/w) sucrose, 100 mM Tris/HCl (pH 7.8), 1 mM EDTA, and protease inhibitor cocktails (Sigma-Aldrich, St. Louis, Missouri, USA) as described in Gomez and Chrispeels65. Cell walls were removed by centrifugation at 1000 × g for 5 min. Supernatants were layered over a two-step discontinuous gradient of 16% (w/v) sucrose (5 mL) on top of 48% (w/v) sucrose (1 mL) in 100 mM Tris/HCl (pH 7.8) with 1 mM EDTA. The gradient was centrifuged at 150,000 × g for 2 h and organelles were collected at the 16%/48% interface. Separation of organelles was then performed with an isopycnic 18–50% (w/v) sucrose gradient prepared in 100 mM Tris/HCl (pH 7.8) with 1 mM EDTA and centrifuged at 150,000 × g for 2 h. After centrifugation, 0.6 mL fractions were collected and aliquots were subjected to glycome profiling.
Prior to the immunohistochemistry, root tip longitudinal sections were treated with Xyloglucanase (GH5); E-XEGP (xyloglucan-specific endo-β-(1→4)-glucanase (Megazyme, Wicklow, Ireland) by adding 10 μL endoglucanase solution (0.4 U/μL, dissolved in 50 mM Ammonium formate, pH 4.5) as described in detail in Günl et al.66. Sections were incubated in the humid chamber for 16 h at 37 °C followed by washing with Ammonium formate buffer (pH 4.5) three times for 5 min. Subsequent immunofluorescence labeling was carried out as described above.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Data generated in this study are included in this article as a Supplemental Table and available from the authors on reasonable request. The raw RNA-Seq data for APEX 03-1, in which seedling debris for glycome profiling were collected, can be found at NASA GeneLab OSD-615 in the link with digital object identifier (DOI) OSD-615 https://doi.org/10.26030/2746-4n42.
References
Volkmann, D. & Baluska, F. Gravity: one of the driving forces for evolution. Protoplasma 229, 143–148 (2006).
Chin, S. & Blancaflor, E. B. Plant gravitropism: from mechanistic insights into plant function on earth to plants colonizing other worlds. Methods Mol. Biol. 2368, 1–41 (2022).
Hoson, T. & Wakabayashi, K. Role of the plant cell wall in gravity resistance. Phytochemistry 112, 84–90 (2015).
Matsumoto, S. et al. Gravity-induced modifications to development in hypocotyls of Arabidopsis tubulin mutants. Plant Physiol. 152, 918–926 (2010).
Del-Bem, L.-E. Xyloglucan evolution and the terrestrialization of green plants. N. Phytol. 219, 1150–1153 (2018).
Mortimer, J. C. & Gilliham, M. SpaceHort: redesigning plants to support space exploration and on-earth sustainability. Curr. Opin. Biotechnol. 73, 246–252 (2022).
Wheeler, R. Plants for human life support in space: from Myers to Mars. Gravit. Space Biol. 23, 25–36 (2010).
Barker, R. & Gilroy, S. Life in space isn’t easy, even if you are green. Biochem. (Lond.). 39, 10–13 (2017).
Poulet, L., Fontaine, J.-P. & Dussap, C.-G. Plant’s response to space environment: a comprehensive review including mechanistic modelling for future space gardeners. Bot. Lett. 163, 337–347 (2016).
Zabel, P., Bamsey, M., Schubert, D. & Tajmar, M. Review and analysis of over 40 years of space plant growth systems. Life Sci. Space Res. 10, 1–16 (2016).
Paul, A. L., Wheeler, R. M., Levine, H. G. & Fer, R. J. Fundamental plant biology enabled by the space shuttle. Am. J. Bot. 100, 226–234 (2013).
Millar, K. D. L., Johnson, C. M., Edelmann, R. E. & Kiss, J. Z. An endogenous growth pattern of roots is revealed in seedlings grown in microgravity. Astrobiology 11, 787–797 (2011).
Ferl, R. J., Koh, J., Denison, F. & Paul, A.-L. Spaceflight induces specific alterations in the proteomes of Arabidopsis. Astrobiology 15, 32–56 (2015).
Kwon, T. et al. Transcriptional response of Arabidopsis seedlings during spaceflight reveals peroxidase and cell wall remodeling genes associated with root hair development. Am. J. Bot. 102, 21–35 (2015).
Ferl, R. J. & Paul, A.-L. The effect of spaceflight on the gravity-sensing auxin gradient of roots: GFP reporter gene microscopy on orbit. npj Microgravity 2, 15023 (2016).
Choi, W.-G., Barker, R. J., Kim, S.-H., Swanson, S. J. & Gilroy, S. Variation in the transcriptome of different ecotypes of Arabidopsis thaliana reveals signatures of oxidative stress in plant responses to spaceflight. Am. J. Bot. 106, 123–136 (2019).
Paul, A.-L., Zupanska, A. K., Schultz, E. R. & Ferl, R. J. Organ-specific remodeling of the Arabidopsis transcriptome in response to spaceflight. BMC Plant Biol. 13, 112 (2013).
Nakashima, J., Liao, F., Sparks, J. A., Tang, Y. & Blancaflor, E. B. The actin cytoskeleton is a suppressor of the endogenous skewing behaviour of Arabidopsis primary roots in microgravity. Plant Biol. 16, 142–150 (2014).
Johnson, C. M., Subramanian, A., Pattathil, S., Correll, M. J. & Kiss, J. Z. Comparative transcriptomics indicate changes in cell wall organization and stress response in seedlings during spaceflight. Am. J. Bot. 104, 1219–1231 (2017).
Micco, V., de, Aronne, G., Joseleau, J.-P. & Ruel, K. Xylem development and cell wall changes of soybean seedlings grown in space. Ann. Bot. 101, 661–669 (2008).
Soga, K., Wakabayashi, K. & Hoson, T. Growth and cortical microtubule dynamics in shoot organs under microgravity and hypergravity conditions. Plant Signal. Behav. 13, e1422468 (2018).
Watanabe, Y. et al. Visualization of cellulose synthases in Arabidopsis secondary cell walls. Science 350, 198–203 (2015).
Cosgrove, D. J. Building an extensible cell wall. Plant Physiol. 189, 1246–1277 (2022).
Du, J., Anderson, C. T. & Xiao, C. Dynamics of pectic homogalacturonan in cellular morphogenesis and adhesion, wall integrity sensing and plant development. Nat. Plants 8, 332–340 (2022).
Keegstra, K. Plant cell walls. Plant Physiol. 154, 483–486 (2010).
Knox, J. P. Revealing the structural and functional diversity of plant cell walls. Curr. Opin. Plant Biol. 11, 308–313 (2008).
Popper, Z. A. Evolution and diversity of green plant cell walls. Curr. Opin. Plant Biol. 11, 286–292 (2008).
Pattathil, S. et al. A Comprehensive toolkit of plant cell wall glycan-directed monoclonal antibodies. Plant Physiol. 153, 514–525 (2010).
Avci, U., Pattathil, S. & Hahn, M. G. Immunological approaches to plant cell wall and biomass characterization: immunolocalization of glycan epitopes. Methods Mol. Biol. 908, 73–82 (2012).
Pattathil, S., Avci, U., Zhang, T., Cardenas, C. L. & Hahn, M. G. Immunological approaches to biomass characterization and utilization. Front. Bioeng. Biotechnol. 3, 1–14 (2015).
Schultz, E. R., Zupanska, A. K., Sng, N. J., Paul, A. L. & Ferl, R. J. Skewing in Arabidopsis roots involves disparate environmental signaling pathways. BMC Plant Biol. 17, 31 (2017).
Pattathil, S., Avci, U., Miller, J. S. & Hahn, M. G. Immunological approaches to plant cell wall and biomass characterization: glycome profiling. Methods Mol. Biol. 908, 61–72 (2012).
Cavalier, D. M. et al. Disrupting two Arabidopsis thaliana xylosyltransferase genes results in plants deficient in xyloglucan, a major primary cell wall component. Plant Cell 20, 1519–1537 (2008).
Kong, Y. et al. Galactose-depleted xyloglucan is dysfunctional and leads to dwarfism in Arabidopsis. Plant Physiol. 167, 1296–1306 (2015).
Madson, M. et al. The MUR3 gene of Arabidopsis encodes a xyloglucan galactosyltransferase that is evolutionarily related to animal exostosins. Plant Cell 15, 1662–1670 (2003).
Knox, J. P., Peart, J. & Neill, S. J. Identification of novel cell surface epitopes using a leaf epidermal-strip assay system. Planta 196, 266–270 (1995).
Wang, M., Heimovaara-Dijkstra, S., Van der Meulen, R. M., Knox, J. P. & Neill, S. He monoclonal antibody JIM19 modulates abscisic acid action in barley aleurone protoplasts. Planta 196, 271–276 (1995).
Smallwood, M. et al. Localization of cell wall proteins in relation to the developmental anatomy of the carrot root apex. Plant J. 5, 237–246 (1994).
Puhlmann, J. et al. Generation of monoclonal antibodies against plant cell-wall polysaccharides (I. characterization of a monoclonal antibody to a terminal [alpha]-(1->2)-linked fucosyl-containing epitope. Plant Physiol. 104, 699–710.
Avci, U. & Nakashima, J. A flat embedding method to orient gravistimulated root samples for sectioning. Methods Mol. Biol. 2368, 153–163 (2022).
Yoo, C. M. et al. Deletion analysis of AGD1 reveals domains crucial for plasma membrane recruitment and function in root hair polarity. J. Cell Sci. 131, jcs203828 (2018).
Califar, B., Sng, N. J., Zupanska, A., Paul, A. L. & Ferl, R. J. Root skewing-associated genes impact the spaceflight response of Arabidopsis thaliana. Front. Plant Sci. 11, 239 (2020).
Kruse, C. P. S. et al. Spaceflight induces novel regulatory responses in Arabidopsis seedling as revealed by combined proteomic and transcriptomic analyses. BMC Plant Biol. 20, 1–16 (2020).
Vandenbrink, J. P. & Kiss, J. Z. Space, the final frontier: a critical review of recent experiments performed in microgravity. Plant Sci. 243, 115–119 (2016).
Park, M.-R. & Hasenstein, K. H. Beware of fixation—it might affect your experiments. Gravit. Space Res 4, 47–57 (2016).
Basu, P., Kruse, C. P. S., Luesse, D. R. & Wyatt, S. E. Growth in spaceflight hardware results in alterations to the transcriptome and proteome. Life Sci. Space Res. 15, 88–96 (2017).
Johnson, C. M., Subramanian, A., Edelmann, R. E. & Kiss, J. Z. Morphometric analyses of petioles of seedlings grown in a spaceflight experiment. J. Plant Res. 128, 1007–1016 (2015).
Shymanovich, T. & Kiss, J. Z. Conducting plant experiments in space and on the moon. Methods Mol. Biol. 2368, 165–198 (2022).
Zabotina, O. A. et al. Mutations in multiple XXT genes of Arabidopsis reveal the complexity of xyloglucan biosynthesis. Plant Physiol. 159, 1367–1384 (2012).
Nakashima, J. et al. Delaying seed germination and improving seedling fixation: lessons learned during science and payload verification tests for Advanced Plant EXperiments (APEX) 02-1 in space. Gravit. Space Res 2, 54–67 (2014).
Kang, B. H., Nielsen, E., Preuss, M. L., Mastronarde, D. & Staehelin, L. A. Electron tomography of RabA4b- and PI-4Kβ1-labeled trans Golgi network compartments in Arabidopsis. Traffic 12, 313–329 (2011).
Wilkop, T. et al. A hybrid approach enabling large-scale glycomic analysis of Post-Golgi vesicles reveals a transport route for polysaccharides. Plant Cell 31, 627–644 (2019).
Mazars, C. et al. Microgravity induces changes in microsome-associated proteins of Arabidopsis seedlings grown on board the International Space Station. PLoS ONE 9, e91814 (2014).
Zhang, Y., Wang, L., Xie, J. & Zheng, H. Differential protein expression profiling of Arabidopsis thaliana callus under microgravity on board the Chinese SZ-8 spacecraft. Planta 241, 475–488 (2015).
Peralta, A. G., Venkatachalam, S., Stone, S. C. & Pattathil, S. Xylan epitope profiling: an enhanced approach to study organ development-dependent changes in xylan structure, biosynthesis, and deposition in plant cell walls. Biotechnol. Biofuels 10, 1–13 (2017).
Le Gall, H. et al. Cell wall metabolism in response to abiotic stress. Plants 4, 112–166 (2015).
Hoson, T., Soga, K., Wakabayashi, K., Kamisaka, S. & Tanimoto, E. Growth and cell wall changes in rice roots during spaceflight. Plant Soil 255, 19–26 (2003).
Wakabayashi, K. et al. Microgravity affects the level of matrix polysaccharide 1,3:1,4-β-glucans in cell walls of rice shoots by increasing the expression level of a gene involved in their breakdown. Astrobiology 20, 820–829 (2020).
Wakabayashi, K. et al. Suppression of hydroxycinnamate network formation in cell walls of rice shoots grown under microgravity conditions in space. PLoS ONE 10, e0137992 (2015).
Correll, M. J. et al. Transcriptome analyses of Arabidopsis thaliana seedlings grown in space: Implications for gravity-responsive genes. Planta 238, 519–533 (2013).
Sheppard, J., Land, E. S., Toennisson, T. A., Doherty, C. J. & Perera, I. Y. Uncovering transcriptional responses to fractional gravity in Arabidopsis roots. Life 11, 1010 (2021).
Zhou, M., Sng, N. J., Lefrois, C. E., Paul, A.-L. & Ferl, R. J. Epigenomics in an extraterrestrial environment: organ-specific alteration of DNA methylation and gene expression elicited by spaceflight in Arabidopsis thaliana. BMC Genomics 20, 205 (2019).
Kiss, J. Z., Wolverton, C., Wyatt, S. E., Hasenstein, K. H. & van Loon, J. J. W. A. Comparison of microgravity analogs to spaceflight in studies of plant growth and development. Front. Plant Sci. 10, 1–10 (2019).
Chai, C., Chin, S. & Blancaflor, E. B. Imaging the cytoskeleton in living plant roots. Methods Mol. Biol. 2364, 139–148 (2022).
Gomez, L. & Chrispeels, M. J. Complementation of an Arabidopsis thaliana mutant that lacks complex asparagine-linked glycans with the human cDNA encoding N-acetylglucosaminyltransferase I. Proc. Natl Acad. Sci. USA 91, 1829–1833 (1994).
Günl, M., Gille, S. & Pauly, M. OLIgo mass profiling (OLIMP) of extracellular polysaccharides. J. Vis. Exp. 40, 2046 (2010).
Acknowledgements
This work was supported by NASA grants NNX12AM94G and 80NSSC19K0129 (to E.B.B.), 80NSSC18K1462 and 80NSSC22K0024 (to S.G. and S.C.). Plant cell wall glycan-directed antibodies used here were generated with funsding from the National Science Foundation Plant Genome Program (DBI-0421683, to M.G.H.). Glycome profiling of samples described here was supported by a grant to MGH from the NSF Plant Genome Program (IOS-0923992). We thank Allison Mjoen, Howard Levine, Bryan Onate, Jose Camacho, Trent Smith, Gioia Massa, Colleen Huber, John Carver, Shawn Stephens, Gerald Newsham, David Reed, and Stacy Engel at NASA-Kennedy Space Center for technical support during ground and spaceflight operations.
Author information
Authors and Affiliations
Contributions
J.N., S.P., and U.A. conducted glycome profiling and immunohistochemistry. J.N., S.P., S.C., and E.B.B. analyzed the data, performed statistical analysis, and generated figures. J.N., J.A.S., and E.B.B. prepared seedlings for spaceflight and processed seedlings post-flight. S.G., M.G.H., and E.B.B. supervised the project and acquired funding support. All authors contributed to writing and editing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nakashima, J., Pattathil, S., Avci, U. et al. Glycome profiling and immunohistochemistry uncover changes in cell walls of Arabidopsis thaliana roots during spaceflight. npj Microgravity 9, 68 (2023). https://doi.org/10.1038/s41526-023-00312-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41526-023-00312-0