Abstract
All dielectrics exhibit electrostriction, i.e., display a quadratic strain response to an electric field compared to the linear strain dependence of piezoelectrics. As such, there is significant interest in discovering new electrostrictors with enhanced electrostrictive coefficients, especially as electrostrictors can exhibit effective piezoelectricity when a bias electric field is applied. We present the results of a study combining data mining and first-principles computations that indicate that there exists a group of iodides, bromides, and chlorides that have electrostrictive coefficients exceeding 10 m4 C–2 which are substantially higher than typical oxide electrostrictive ceramics and polymers. The corresponding effective piezoelectric voltage coefficients are three orders of magnitude larger than lead zirconate titanate.
Similar content being viewed by others
Introduction
The development of novel materials exhibiting exceptional strain response in the presence of an external electric field is imperative to the development of tunable electromechanical devices1,2,3,4,5,6,7,8. Promising applications involve next-generation transducers9, ultra-efficient actuators1,2,10, sonars11, and low-power logic devices12 with application spaces in medicine, astronomy, and consumer electronics. Recent discoveries of ‘giant’ electrostrictors, for example, Gd-doped ceria8, and (Nb, Y) doping in bismuth oxide13 and lanthanum molybdenum oxide7, soft nano-composite based on carbon nanotubes (CNT)14 and liquid crystalline graphene6 have opened promising applications for superior electromechanical properties, and also as a replacement of lead-based Pb(Ti1–xZrx)O3 (PZT) solid solutions that are widely used in current applications. Of particular interest is the possibility to induce an effective piezoelectric behavior under a bias electric field, generating extraordinarily large piezoelectric coefficients (d33 ~ 2 × 105 pC N–1)15. These ‘giant’ electrostrictors have been reviewed recently16. There is a clear advantage in utilizing electrostrictive materials that exhibit a quadratic strain in response to an electric field. This may allow for a more significant strain response for a given applied external field, as well as take advantage of the anhysteretic nature of electrostriction for high-precision displacements.
This effect is mediated via an applied electric field (Ei) and the dielectric polarization (Pi) vectors as described by
where xij are the strain components, dijk and gijk are piezoelectric tensors, and Mijkl and Qijkl are fourth-rank tensors describing the electrostrictive response. In Eq. (1), the total strain (at constant stress) is expressed in terms of an applied electric field, whereas in Eq. (2) it is given as a function of the polarization. The M tensor is of particular interest for actuator applications whereas Q is used as a design criterion in sensing applications.
The search for novel electrostrictors has historically appeared bounded by the universal empirical relation connecting the electrostrictive coefficient to the ratio of the elastic compressibility over the permittivity5 until the discovery of ‘giant’ electrostrictors that are several orders of magnitude greater than the empirically expected values16. These include materials such as La2Mo2O9 (Mh ~ 10−18 m2 V–2 and Qh ~ 20 m4 C–2), (Nb,Y)-stabilized Bi2O3 (Mh ~ 4.7–12.5 × 10−18 m2 V–2 and Qh ~ 83-273 m4 C–2)13, CeO2–x (Mh ~ 300 × 10−18 m2 V–2)15, lead halide perovskites (Mh ~ 730–900 × 10−18 m2 V–2 and Qh ~ 1266–1417 m4 C–2)17, and Ce0.8Gd0.2O1.9 (Mh ~ 6.5 × 10−18 m2 V–2)6,7,8. The electrostriction coefficients of these materials are three orders larger than typical perovskites, including BaTiO3, PbTiO3, SrTiO3, and Pb(Ti1–xZrx)O3 for which Qh is in the range 0.02–0.07 m4 C–2 5.
Using large open materials databases composed of accurately determined materials properties, the programmatic search for materials exhibiting specific properties becomes possible. In line with data-driven methods, advanced modeling approaches across length scales, and the successful insertion of the concept of materials genomics that allows for rapid development and deployment of novel materials18,19, we employ here a combination of data mining and first-principles modeling in the discovery of materials exhibiting exceptional electrostrictive coefficients. Our results show several non-oxide inorganic compounds with electrostrictive properties one or two orders of magnitude higher than existing oxide ceramics and with comparable or higher electrostriction coefficients than polymers.
Results
Assessment of electrostrictive properties
The tensorial notation that has been employed in Eqs. (1) and (2) can be reduced to the following criteria to assess the magnitude of the electrostrictive properties of a wide variety of materials (see Supplementary Information), which is also defined as the Newnham’s proxy \(Q_{\rm {h}}^{{{{\mathrm{proxy}}}}}\), based on the approach presented in refs. 5,10. \(M_{\rm {h}}^{{{{\mathrm{proxy}}}}}\) can be derived from \(Q_{\rm {h}}^{{{{\mathrm{proxy}}}}}\) as below:
Here ε* is the average eigenvalue of the dielectric tensor ε with ε = ε0εr, εr the relative permittivity of the material, and ε0 the permittivity of vacuum. sR in Eq. (3) is the Reuss elastic compressibility given as20,21
The Supplementary Information provides a more detailed treatment to define constitutive relations for the electrostrictive coefficients Mijkl and Qijkl. Equations (3) and (4) follow from specific electrical and mechanical boundary conditions corresponding to hydrostatic pressure/expansion and a single electric field component (uniaxial applied electric field in a short-circuit configuration) as discussed in the Supplementary Information. We note that Eq. (3) describes the Qh criterion used by Newnham et al. in their landmark paper that classified electrostriction for a range of materials over an extensive response space5. We add here an additional criterion for Mh16,22. Although closely related, the quantities Mh and Qh are not interchangeable as converse effects (Supplementary Information). Subtle differences in how these are defined give rise to slightly different criteria for the proxy derivation used to approximate Mh and Qh. Mh is the readily accessible physical parameter in experiments.
In addition to the static empirical relations in Eqs. (3) and (4), it is advantageous to use the differential forms of Qh and Mh as described in the Supplementary Information, which is essentially the dielectric response with a change in external hydrostatic pressure (p). In the following, Qh and Mh derived from our density functional theory (DFT) calculations are defined as \(Q_{\rm {h}}^{{{{\mathrm{DFT}}}}}\) and \(M_{\rm {h}}^{{{{\mathrm{DFT}}}}}\), respectively. Thus,
It must be noted that Eqs. (6) and (7) capture the mechanical response as differential in pressure (p) and the equations are independent of Eq. (3) and Eq. (4).
DFT has been used to study the electrostriction properties of compounds having high structural symmetry23,24. Of particular interest in this regard is the study by Tanner et al. wherein the electrostrictive coefficients of cubic dielectric materials were computed using the differentials of the lattice parameters, which is similar to differentials of hydrostatic pressure for cubic crystal system25. We have adopted the latter method using Eqs. (6) and Eq. (7). In our computations, the trace of the external stress tensor, defined as pstress, is treated as the hydrostatic pressure with the conventional notation that positive and negative pstress values mean hydrostatic expansion and compression, respectively. This approach allows for uniform evaluation of electrostrictive coefficients over all seven crystal systems independent of their crystal structure.
Data mining
To assign proxies for selecting materials exhibiting high electrostriction, we utilize a set of relationships between the dielectric response, the elastic coefficients, the electric field-induced strain, and the electrostrictive properties of materials as described in the previous section. A similar methodology developed by Newnham et al. has shown a semi-empirical connection between the elastic compliance, the dielectric response, and the hydrostatic electrostrictive coefficient Qh4,5.
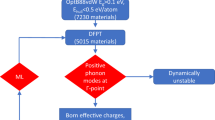
Existing databases of calculated functional properties provide easy access to data that can be used to shortlist materials with exceptional properties via a simple database query of related proxies26,27. The Materials Project (MP) serves as an ideal platform for novel materials discovery, given the large number of calculated material properties and optimized crystal structures contained in the database28. The database contains 124,515 inorganic compounds with properties calculated via medium/high accuracy DFT methods. The MP team has calculated the elastic tensors for 13,751 and the dielectric tensors for 4892 inorganic materials so far28 and made these publicly available20. In addition, there are currently 1974 materials for which MP has calculated both the dielectric and elastic properties. This set of materials is ideal for data mining and training machine learning models to predict electrostrictive response. Starting from this set, we down-select using a set of simple property filters to find new promising candidates for electrostriction applications. We limit the materials by considering non-elemental (>1 element) dielectrics with a bandgap >0.5 eV and seek compounds that should exhibit relative stability as determined via the energy above the hull (<0.1 eV). The schematic representation of the workflow is shown in Fig. 1.
These materials are then separated into centrosymmetric and non-centrosymmetric datasets based on their point group symmetries. This discretization serves the purpose of allowing us to consider materials lacking an inversion symmetry (non-centrosymmetric), which exhibit a spontaneous polarization via a piezoelectric response resulting from the dielectric and electrostrictive contributions separately from those that solely display an electrostrictive response (see Fig. 1). Taking these latter materials, it is then possible to consider the ratio of \((s_{\rm {R}}/\varepsilon ^ \ast )\) for each material and rank these according to the proxies described in Eqs. (3) and (4). To reduce the dielectric and elastic tensors to a scalar value for each data point, the inverse of the Reuss average bulk modulus was used in place of the elastic compressibility (\(s_{\rm {R}} \approx 1/K_{\rm {R}}\)) and the averaged dielectric tensor eigenvalue in place of ε*, thus \(Q_{\rm {h}} = \left( {K_{\rm {R}}\varepsilon ^ \ast } \right)^{ - 1}\) is considered a replacement for s/ε and a proxy for predicting Qh. Alternatively, \(M_{\rm {h}} = \left( {\varepsilon ^ \ast /K_{\rm {R}}} \right)\)is the expression used for querying for high Mh. These proxies are utilized throughout the rest of the study in combination with DFT-calculated values for shortlisting materials which should exhibit exceptional electrostrictive strain response to an applied external electric field.
First Principles (DFT) validation
The accuracy of DFT for materials properties that are determined at the electronic and atomic levels has been well tested; it provides a platform to bridge the gap between fundamental theory, high-throughput computational data science, and experiments29,30,31,32,33,34,35,36,37. Here, we perform first-principles calculations with DFT using the plane-wave pseudopotential method38. The structural units of the compounds are taken from the MP database. We have used the PBEsol exchange-correlation functional39 in place of the standard general gradient approximation (GGA) in the MP. PBEsol is a revised Perdew–Burke–Ernzerhof GGA chosen due to the enhanced prediction of equilibrium properties of densely packed solids, such as the dielectric and elastic tensors for insulating materials compared to GGA25. Geometric optimization was carried out to obtain the equilibrium lattice parameters and corresponding atomic positions. The k-points grid size was set to 9 × 9 × 9, but models with atoms larger than 20 were treated with 7 × 7 × 7 grid size. The kinetic energy cutoff for the plane waves is 800 eV. The symmetry tag was switched on for the calculations so that the system did not deviate from the initialized structures. Non-spin-polarized calculations were performed as most compounds were not expected to have a magnetic ground state. Geometrical optimization for the models was carried out with the tolerance for total energy convergence set to 10−5 eV and was performed in five cycles. The relaxed model from one cycle was then fed as the initial model for the next cycle to ensure a thorough relaxation. The calculations were performed with the Vienna ab initio simulation package (VASP)40,41,42.
The elastic compliance tensor was calculated by performing six finite distortions of the supercells and constructing the Hessian matrix from total energies obtained from density functional perturbation theory (DFPT)43. The static ion-clamped dielectric matrix was computed using DFPT via the linear Sternheimer equations44,45. This method bypasses the complexity of computing the dielectric function through relaxation under finite electric fields. Equations (6) and (7) rely on the change in dielectric response as a function of hydrostatic pressure46. The DFT computation of the dielectric function was done at 25 kbar pstress above and 25 kbar pstress below the equilibrium volume for which the pstress is zero. The mechanical perturbation of the order ±25 kbar is well within the harmonic approximation and provides minimal deviation of the unit cell symmetry. We found slight variations in the equilibrium lattice parameters when compared to the MP. This is due to differences in the pseudopotentials that were utilized in this study in the relaxation procedures.
Discussion
Applying the selection criteria developed in Section III has allowed us to shortlist a set of materials that may potentially exhibit a significant electrostrictive strain response. \(Q_{\rm {h}}^{{{{\mathrm{proxy}}}}}\) values estimated from the MP data using Eqs. (3) and (4) are compared with the experimental data taken from Refs. 3,4,5,47 in Fig. 2. It is observed that specialized polymers have generally larger Qh than ceramics or glasses. Comparing Qh of materials from the MP dataset, one finds larger Qh in several ceramics and covalent salts that surpass polymers by almost 400% if the data from ref. 3 corresponding to polyurethane elastomer is considered an outlier. The top electrostrictive materials from MP are explored in more detail using more accurate DFT computations. The \(Q_{\rm {h}}^{{{{\mathrm{DFT}}}}}\) values based on Eq. (6) display even more promising results with electrostrictive coefficients over 500% larger than typical electrostrictive polymers.
Magnitude of Qh values obtained from experiments (data taken from ref. 3) and compared to \(Q_{\rm {h}}^{{{{\mathrm{proxy}}}}}\) and \(Q_{\rm {h}}^{{{{\mathrm{DFT}}}}}\) from our study.
The top ten Qh (\(Q_{\rm {h}}^{{{{\mathrm{proxy}}}}}\) and \(Q_{\rm {h}}^{{{{\mathrm{DFT}}}}}\)) and Mh (\(M_{\rm {h}}^{{{{\mathrm{proxy}}}}}\) and \(M_{\rm {h}}^{{{{\mathrm{DFT}}}}}\)) candidates from MP and DFT calculations are listed in Tables 1 and 2, respectively. It is encouraging to find that five out of our top ten high Qh materials appear in both proxy and DFT data sets. These are: SiCl4, BCl3, PCl3, GaBr3, and WCl6. On the other hand, three out of the top ten Mh materials appear in both the proxy and DFT data sets. These are SbSI, SbCl2, and WCl6. Materials in the top ten list of \(Q_{\rm {h}}^{{{{\mathrm{proxy}}}}}\) and \(M_{\rm {h}}^{{{{\mathrm{proxy}}}}}\) include MgCl2, MgI2, SiCl4, WCl6, and PCl3. Likewise, materials common to the top ten list of \(Q_{\rm {h}}^{{{{\mathrm{DFT}}}}}\)and \(M_{\rm {h}}^{{{{\mathrm{DFT}}}}}\) are WCl6, GaBr3, and SbCl3. Overall, the data supports that the halide groups, especially the chlorides, the bromides, and the iodides are promising materials for electrostriction functionality.
There are important differences in the output value of Qh that should be noted. For example, our \(Q_{\rm {h}}^{{{{\mathrm{proxy}}}}}\) values for WCl6 and PBr3 are 22.83 m4 C–2 and 20.99 m4 C–2, respectively, while \(Q_{\rm {h}}^{{{{\mathrm{DFT}}}}}\) for these compounds is significantly larger: 560.53 m4 C–2 for WCl6 and 393.32 m4 C–2 for PBr3. Other materials such as GaCl3 have similar values from the empirical and DFT relations (20.97 m4 C–2 and 17.78 m4 C–2, respectively). We also plot a comparison of \(Q_{\rm {h}}^{{{{\mathrm{proxy}}}}}\) with \(Q_{\rm {h}}^{{{{\mathrm{DFT}}}}}\) and \(M_{\rm {h}}^{{{{\mathrm{proxy}}}}}\) with \(M_{\rm {h}}^{{{{\mathrm{DFT}}}}}\) in Fig. 3a, b, respectively. There are significant deviations from the linear correlation that follows from the Newnham semi-empirical correlation between \(s_{\rm {R}}/\varepsilon ^ \ast\) and Qh. The result ascertains that Newnham’s semi-empirical relation, \(Q_{\rm {h}}^{{{{\mathrm{proxy}}}}}\) is not a universal measure that is applicable across all materials.
Comparison of a \(Q_{\rm {h}}^{{{{\mathrm{DFT}}}}}\) versus \(Q_{\rm {h}}^{{{{\mathrm{proxy}}}}}\)and b \(M_{\rm {h}}^{{{{\mathrm{DFT}}}}}\) versus \(Q_{\rm {h}}^{{{{\mathrm{proxy}}}}}\) obtained from direct application of the empirical formula to the Materials Project data and our DFT computations.
The most striking aspect of the top compounds is that they are halides, chlorides, bromides, and iodides. We anticipated this due to recent observations of high electrostrictive properties in fluorides such as CaF2, BaF2, SrF2, LiF, and NaF with electrostrictive responses around 0.36–0.46 m4 C–2 3,4,5,47. It is interesting to note that none of these compounds made our top ten obtained either via data mining of MP or from the further refinement of MP results through high-precision DFT. The materials that are discovered through our approach are mainly binary non-cubic semi-metal halides. We note that three materials appear common to the top ten \(Q_{\rm {h}}^{D{{{\mathrm{FT}}}}}\) and top ten \(M_{\rm {h}}^{{{{\mathrm{DFT}}}}}\) datasets: GaBr3, WCl6, and SbCl3. These compounds have not yet been explored with respect to their electrostrictive properties. We certainly hope that our results will generate more experimental work to verify our predictions. We note that not all the compounds in Table 1 are viable materials, some are liquid at room temperature (e.g., SiCl4, PCl3, PBr3O), some are gases or volatile at room pressure (e.g., BCl3, SF6, or WCl6). But others such as GaBr3 with \(Q_{\rm {h}}^{{{{\mathrm{DFT}}}}}\) of 560.53 m4 C–2 show significant promise.
To explain these results and to identify the origin of the giant electrostrictive properties of materials displayed in Tables 1 and 2, we carried out a detailed analysis of bonding, charge distribution, and hybridization for selected compounds. For example, we have examined GaBr3, one of the top candidates with \(Q_{\rm {h}}^{{{{\mathrm{DFT}}}}}\) = 560.53 m4 C–2 and \(M_{\rm {h}}^{{{{\mathrm{DFT}}}}}\) = 2.58 × 10−19 m2 V–2. Its oxide counterpart Ga2O3 has a significantly lower electrostriction response factor, \(Q_{\rm {h}}^{{{{\mathrm{DFT}}}}}\) = 0.02 m4 C–2 and \(M_{\rm {h}}^{{{{\mathrm{DFT}}}}}\) = 1.38 × 10−22 m2 V–2. As such, Ga2O3 did not make it to our lists even within the top 50 materials. The crystal structure and the atomic charge densities of GaBr3 and Ga2O3 are shown in Fig. 4a, b, respectively. The atom-resolved density of states (DOS) of the respective materials is plotted in Fig. 4c, d. Despite each having 24 electrons per formula unit in their valence band, the DOS is quite different. The bandgap observed from the DOS of the two compounds is 3.36 eV for GaBr3 and 2.04 eV for Ga2O3, which is >0.5 eV, a criterion used for our data mining. This corroborates with the charge separation observed between the cation and anion sites shown in Fig. 4a, b for GaBr3 and Ga2O3, respectively. Owing to the large band gaps, the two materials have a similar order of magnitude permittivities (εr = 2.86 for GaBr3 and εr = 9.28 for Ga2O3). The overall energy spread of both the valence and conduction bands of GaBr3 is smaller than for Ga2O3. In addition, within these bands, the DOS is composed of narrow peaks for GaBr3 whereas Ga2O3 exhibits a more continuous dispersion of its DOS.
The consequence of DOS differences is fundamentally connected with differences in elastic properties and thus on the compressibility sR, which for GaBr3 is 22.67 GPa–1. This value is three orders of magnitude larger than sR of Ga2O3 (0.06 GPa–1). The plot of εr versus sR is shown to gain an insight into which physical factors play a more important role in the derived properties. Figure 5 is a four-dimensional plot with εr and sR as the abscissa and ordinate. The color coding differentiates the crystal system of the compounds, and the circle diameter scales according to the value of Qh. Note that the values of sR and εr are independently computed using DFT. The figure is divided into four quadrants. Quadrant-II covers the region of large sR but small εr, and has large Qh. Conversely, the quadrant-IV covers the region of small sR but large εr, and has smaller Qh. These seem consistent with the semi-empirical relation of Eq. (1). However, we observe that quadrant-III is filled with data points showing that there are several materials with low sR and low εr that make up the chemical space. On the other hand, quadrant-I is almost empty. The result does not reflect the same trend as shown in refs. 5,10, indicating that electrostrictive properties cannot be generalized using Newnham’s proxy defined through Eqs. (3) and (4). The εr and sR derived from the Materials Project data are shown in Fig. S1 for all the datamined compounds and are provided in the Supplementary Information.
Electrostrictive coefficient \(Q_{\rm {h}}^{{{{\mathrm{DFT}}}}}\) as in Eq. (3) falls along the quadrants I and III with larger \(Q_{\rm {h}}^{{{{\mathrm{DFT}}}}}\) values appearing is quadrant-III and small \(Q_{\rm {h}}^{{{{\mathrm{DFT}}}}}\) values in quadrant-I.
As a cross-check of the quality of data obtained from our study, we calculated the effective piezoelectric coefficients d and g from the derived Qh and Mh. The electrostriction coefficients are related to piezoelectric charge (d) and voltage (g) coefficients as
where Fb is the breakdown voltage. This quantity essentially determines the maximum amount of strain that can be obtained through an applied electric field which is also true for electrostrictors. For stress sensing, the piezoelectric charge coefficient (d) relates the applied stress (Xjk) to the dielectric displacement generated (\(D_i = d_{ijk} \cdot X_{jk}\)) in short-circuit conditions whereas the piezoelectric voltage coefficient (g) relates the applied stress to the generated electric field (\(E_i = - g_{ijk} \cdot X_{jk}\)).
There is no direct way of computing Fb. We used the breakdown voltage expression from refs. 48,49:
where ωmax is the maximum phonon frequency at the Γ-point that was calculated from the linear response approach and Eg is the DFT obtained band gap. Although the band gap is underestimated in DFT, we do not expect it to affect the results of Fb because Eq. (10) is a machine-learned relationship that already accounts for the underestimated band gaps.
Table 3 lists the top ten materials for Fb, d, and g. The magnitudes of d for these materials are smaller than what generally is accepted as promising in the field of linear dielectrics, i.e., few hundreds of 10−12 m V–1. The values of g, on the other hand, are three orders of magnitude larger than those commonly appear in linear dielectrics applications. This complements the extremely large effective piezoelectric coefficients obtained on ceria (d33 ~ 2 × 105 pC N–1), a known ‘giant’ electrostrictor, and underlines the potential of electrostrictors as an alternative to piezoelectrics. As for the top candidates, we find 70% of the materials common in the top ten list of d and g. The data signifies further that fluorides, chlorides, bromides, and iodides are promising material systems for both linear and quadratic dielectric response.
We have used a data mining backed by first-principles calculations approach to down-select materials for the discovery of novel electrostrictive materials. Our analysis shows that the halides—fluorides, chlorides, bromides, and iodides, are promising materials systems for high electrostriction response. However, unlike linear dielectrics and ferroelectric glasses, they do not follow Newnham’s relation. We note that some of these materials may have significantly larger electrostrictive coefficients (Qh) than even electroactive polymers. The discovery of electrostrictors with electrostrictive coefficients comparable to the ‘giant’ electrostrictors, where the physics is governed by active defects, presents the prospect of applications that are not limited to low-frequency response as is the case for ‘giant’ electrostrictors. The corresponding effective piezoelectric voltage coefficients obtained under a bias electric field surpass classical piezoelectrics by three orders of magnitude and constitute an appealing alternative to piezoelectrics. We believe that this combination of computational and informatics-based approaches will provide the necessary guidance for future experimental work, resulting in a new generation of electrostrictors with exceptionally high property coefficients.
Methods
The Materials Project database was used for sourcing, compiling, and curating the crystal structure data and the existing elastic compliance and permittivity. The electrostriction coefficient was computed based on Newnham’s proxy. It provided early-stage learning for shortlisting a list of compounds for carrying out more detailed first-principles calculations. A set of top candidates was selected for first-principles calculations. A set of randomly selected compounds was also included in the list. Detailed density functional theory (DFT) calculations using the Vienna Ab initio Simulation Package (VASP) were carried out with generalized gradient approximation (GGA) as the exchange-correlation functional and a high-density k-points mesh. The electrostatic coefficient was calculated as a differential of pressure, where DFT calculations were applied with pressure constraint with the pstress tag.
Data availability
The data supporting the results are available upon reasonable request to the authors.
References
Fanson, J. L. & Ealey, M. A. Articulating fold mirror for the Wide-Field/Planetary Camera II. In Proc. SPIE 1920, Publ. SPIE. https://doi.org/10.1117/12.152675 (1993).
Uchino, K. Electrostrictive actuators: materials and applications. Am. Ceram. Soc. Bull. 65, 647–652 (1986).
Li, F., Jin, L., Xu, Z. & Zhang, S. Electrostrictive effect in ferroelectrics: an alternative approach to improve piezoelectricity. Appl. Phys. Rev. 1, 011103 (2014).
Uchino, K., Nomura, S., Cross, L. E., Newnham, R. E. & Jang, S. J. Electrostrictive effect in perovskites and its transducer applications. J. Mater. Sci. 16, 569–578 (1981).
Newnham, R. E., Sundar, V., Yimnirun, R., Su, J. & Zhang, Q. M. Electrostriction: nonlinear electromechanical coupling in solid dielectrics. J. Phys. Chem. B 101, 10141–10150 (1997).
Yuan, J. et al. Giant electrostriction of soft nanocomposites based on liquid crystalline graphene. ACS Nano 12, 1688–1695 (2018).
Li, Q. et al. Giant thermally-enhanced electrostriction and polar surface phase in La2Mo2O9 oxygen ion conductors. Phys. Rev. Mater. 2, 41403 (2018).
Korobko, R. et al. Giant electrostriction in Gd-doped ceria. Adv. Mater. 24, 5857–5861 (2012).
Chen, J., Shurland, A., Perry, J., Ossmann, B. & Gururaja, T. R. Electrostrictive transducers for medical ultrasonic applications. In Proc. 10th IEEE International Symposium on Applied Ferroelectrics (ISAF ’96) Vol. 1, 27–30 (1996).
Damjanovic, D. & Newnham, R. E. Electrostrictive and piezoelectric materials for actuator applications. J. Intell. Mater. Syst. Struct. 3, 190–208 (1992).
Pilgrim, S. M & Revathi, S. B., Electrostrictive Ceramics for Sonar Projectors, in Reference Module in Materials Science and Materials Engineering. https://doi.org/10.1016/B978-0-12-803581-8.01716-1 (2016).
Anam, M. K., Gopalakrishnan, P., Sebastian, A. & Ahn, E. C. Proposal for an electrostrictive logic device with the epitaxial oxide heterostructure. Sci. Rep. 10, 14636 (2020).
Yavo, N. et al. Large nonclassical electrostriction in (Y, Nb)-stabilized δ-Bi2O3. Adv. Funct. Mater. 26, 1138–1142 (2016).
Luna, A. et al. Giant electrostrictive response and piezoresistivity of emulsion templated nanocomposites. Langmuir 33, 4528–4536 (2017).
Park, D.-S. et al. Induced giant piezoelectricity in centrosymmetric oxides. Science (80-.) 375, 653–657 (2022).
Yu, J. & Janolin, P.-E. Defining ‘giant’ electrostriction. J. Appl. Phys. 131, 170701 (2022).
Chen, B. et al. Large electrostrictive response in lead halide perovskites. Nat. Mater. 17, 1020–1026 (2018).
Tanaka, I., Rajan, K. & Wolverton, C. Data-centric science for materials innovation. MRS Bull. 43, 659–663 (2018).
Suh, C., Fare, C., Warren, J. A. & Pyzer-Knapp, E. O. Evolving the materials genome: how machine learning is fueling the next generation of materials discovery. Annu. Rev. Mater. Res. 50, 1–25 (2020).
Toonder, J. M. J., den, Dommelen, J. A. Wvan & Baaijens, F. P. T. The relation between single crystal elasticity and the effective elastic behaviour of polycrystalline materials: theory, measurement and computation. Model. Simul. Mater. Sci. Eng. 7, 909–928 (1999).
De Jong, M. et al. Charting the complete elastic properties of inorganic crystalline compounds. Sci. Data 2, 1–13 (2015).
Makagon, E. et al. All-solid-state electro-chemo-mechanical actuator operating at room temperature. Adv. Funct. Mater. 31, 2006712 (2021).
Jiang, Z. et al. Electrostriction coefficient of ferroelectric materials from ab initio computation. AIP Adv. 6, 065122 (2016).
Kornev, I., Willatzen, M., Lassen, B. & Lew Yan Voon, L. C. Electrostriction coefficients of GaN, AlN, MgO and ZnO in the Wurtzite structure from First‐Principles. AIP Conf. Proc. 1199, 71–72 (2010).
Tanner, D. S. P., Bousquet, E. & Janolin, P. E. Optimized methodology for the calculation of electrostriction from First-Principles. Small 17, 2103419 (2021).
Sahoo, T., Anene, U. A., Nayak, S. K. & Alpay, S. P. Electronic and optical properties of zinc based hybrid organic–inorganic compounds. Mater. Res. Express 7, 35701 (2020).
Nayak, S. K. et al. Insight into point defects and impurities in titanium from first principles. npj Comput. Mater. 4, 11 (2018).
Jain, A. et al. The Materials Project: a materials genome approach to accelerating materials innovation. APL Mater. 1, 11002 (2013).
Trujillo, D., Ghosh, A., Nakhmanson, S. M., Sahoo, S. & Alpay, S. P. Surface structure and energetics of low index facets of bismuth ferrite. Phys. Chem. Chem. Phys. 22, 16400–16406 (2020).
Sahoo, S., Dekel, D. R., Maric, R. & Alpay, S. P. Atomistic insights into the hydrogen oxidation reaction of palladium-ceria bifunctional catalysts for anion-exchange membrane fuel cells. ACS Catal. 11, 2561–2571 (2021).
Adeagbo, W. A. et al. Theoretical investigation of iron incorporation in hexagonal barium titanate. Phys. Rev. B 100, 184108 (2019).
Sahoo, S., Rollmann, G. & Entel, P. Segregation and ordering in binary transition metal clusters. Phase Transit. 79, 693–700 (2006).
Esquinazi, P. D. et al. Defect-induced magnetism in nonmagnetic oxides: basic principles, experimental evidence, and possible devices with ZnO and TiO2. Phys. Status Solidi (b). 257, 1900623 (2020).
Sahoo, S., Alpay, S. P. & Hebert, R. J. Surface phase diagrams of titanium in oxygen, nitrogen and hydrogen environments: a first principles analysis. Surf. Sci. 677, 18–25 (2018).
Nayak, S. K. et al. Chromium point defects in hexagonal BaTiO3: a comparative study of first-principles calculations and experiments. Phys. Rev. B 91, 155105 (2015).
Sahoo, S. et al. Magnetic properties of small Pt-capped Fe, Co, and Ni clusters: a density functional theory study. Phys. Rev. B 82, 54418 (2010).
Curry, E. B. et al. Optical response of nickel-based superalloy Inconel-718 for applications in additive manufacturing. J. Appl. Phys. 127, 245111 (2020).
Jones, R. O. & Gunnarsson, O. The density functional formalism, its applications and prospects. Rev. Mod. Phys. 61, 689–746 (1989).
Csonka, G. I. et al. Assessing the performance of recent density functionals for bulk solids. Phys. Rev. B 79, 155107 (2009).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Baroni, S., Giannozzi, P. & Testa, A. Green’s-function approach to linear response in solids. Phys. Rev. Lett. 58, 1861–1864 (1987).
Gajdoš, M., Hummer, K., Kresse, G., Furthmüller, J. & Bechstedt, F. Linear optical properties in the projector-augmented wave methodology. Phys. Rev. B 73, 45112 (2006).
Baroni, S. & Resta, R. Ab initio calculation of the macroscopic dielectric constant in silicon. Phys. Rev. B 33, 7017–7021 (1986).
Francis, G. P. & Payne, M. C. Finite basis set corrections to total energy pseudopotential calculations. J. Phys. Condens. Matter 2, 4395–4404 (1990).
Uchino, K. & Cross, L. E. Electrostriction and its interrelation with other anharmonic properties of materials. Jpn. J. Appl. Phys. 19, L171–L173 (1980).
Kim, C., Pilania, G. & Ramprasad, R. Machine learning assisted predictions of intrinsic dielectric breakdown strength of ABX3 Perovskites. J. Phys. Chem. C 120, 14575–14580 (2016).
Kim, C., Pilania, G. & Ramprasad, R. From organized high-throughput data to phenomenological theory using machine learning: the example of dielectric breakdown. Chem. Mater. 28, 1304–1311 (2016).
Acknowledgements
Authors A.G., S.K.N., and S.P.A. thank the Air Force Research Laboratory, Materials and Manufacturing Directorate (AFRL/RXMS) for support via Contract No. FA8650-18-C-5700. J.Y. and P.E.J. have been supported by the ANR-19-ASTR-0024-01 and ANR-20-CE08-0012-1 grants.
Author information
Authors and Affiliations
Contributions
The concepts presented herein were conceived by P.E.J. and S.P.A. D.T. performed the data mining process and provided the initial analysis. A.G. carried out DFT calculations under the supervision of S.K.N. All authors analyzed the data and prepared the manuscript for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Trujillo, D.P., Gurung, A., Yu, J. et al. Data-driven methods for discovery of next-generation electrostrictive materials. npj Comput Mater 8, 251 (2022). https://doi.org/10.1038/s41524-022-00941-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41524-022-00941-1
This article is cited by
-
Lead-free Zr-doped ceria ceramics with low permittivity displaying giant electrostriction
Nature Communications (2023)