Abstract
To evaluate the role of chemotherapy in stage IA triple-negative breast cancer, we conducted a retrospective population-based study including 8601 patients. The use of chemotherapy significantly increased from 2010 to 2019 in patients with T1b and T1c tumors (p = 0.001 and p < 0.001, respectively). Receipt of chemotherapy was associated with improved breast cancer-specific survival (BCSS, adjusted hazard ratio = 0.70; p = 0.006), particularly in patients with T1c tumors (5-year BCSS 94.5% vs. 91.2%).
Similar content being viewed by others
Patients with early-stage triple-negative breast cancer (TNBC) have a higher risk of distant recurrence and death compared to patients with other breast cancer subtypes, including patients with small tumors1. Currently, adjuvant chemotherapy is the mainstay of systemic therapy for patients with stage I TNBC, who represent approximately one-third of TNBC patients.2 Among patients with stage IA TNBC (i.e., tumors ≤2 cm and node-negative), the National Comprehensive Cancer Network guidelines recommend adjuvant chemotherapy for tumors 1.1–2 cm (T1c), with consideration of adjuvant chemotherapy for tumors 0.6–1 cm (T1b). Patients with tumors ≤0.5 cm (T1a) are not recommended to receive adjuvant chemotherapy2,3. However, the utilization and benefit of chemotherapy for this population in the modern era remain poorly defined.
We conducted a population-based study using Surveillance, Epidemiology, and End Results (SEER) to investigate adjuvant chemotherapy treatment patterns and survival outcomes among patients diagnosed with stage IA TNBC between 2010 and 2019. We included 8601 women diagnosed with stage IA TNBC between 2010 and 2019. Patient demographics and disease information are included in Table 1. The median age at diagnosis was 62 years old. Most patients (92.79%) had invasive ductal carcinomas, T1c disease (60.85%) and grade 3 (i.e., high-grade) tumors (70.14%). Adjuvant chemotherapy was administered to 5295 patients (61.6%). Median follow-up for survival analyses was 48 months (interquartile range: 20–83 months).
The rate of chemotherapy use among patients with T1mic and T1a tumors did not change significantly between 2010 and 2019 (<20% and <30% across years, respectively). However, chemotherapy use increased significantly from 2010-2019 among patients with T1b (p = 0.001) and T1c tumors (p < 0.0001), reaching ≥60% in patients with T1b and ≥70% in patients with T1c tumors across most years (Supplementary Table 1, Supplementary Fig. 1).
In multivariable analyses, variables significantly associated with chemotherapy use (all p < 0.02) were younger age (age <50 vs. >64, odds ratio [OR] = 5.19), married status (married vs. single, OR = 1.28), high tumor grade (high grade [grade III] vs. low grade [grade I], OR = 4.89), and tumor size (Reference T1mic: T1a, OR = 2.91; T1b, OR = 19.16; T1c, OR = 31.49), among others (Supplementary Table 2).
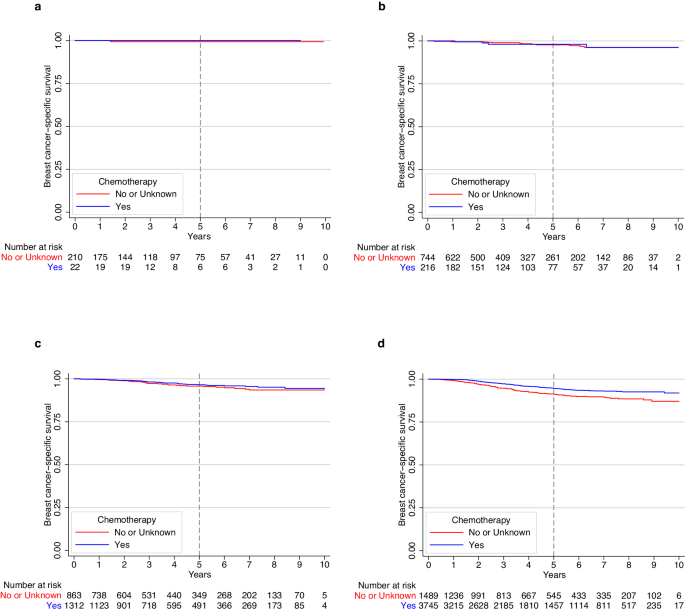
Multivariable analyses revealed that chemotherapy receipt (vs. no/unknown receipt) was associated with improved BCSS among patients with stage IA TNBC. The 5-year BCSS for patients receiving chemotherapy vs. no/unknown was 95.2% vs. 94.4% (Adjusted hazard ratio [HR] = 0.70; 95% confidence interval [CI]: 0.55–0.90; Cox p value = 0.006) (Supplementary Fig. 2). When BCSS was evaluated by tumor size, patients with T1mic (Fig. 1a) and T1a (Fig. 1b) had excellent outcomes regardless of chemotherapy administration, with finding of only 1 (0.4%) and 17 (1.8%) breast cancer deaths, respectively (Supplementary Table 3). The small number of events in these two groups prevented adjusted comparisons. For those with T1b cancers, there was no significant association between chemotherapy and BCSS, with a total of 63 (2.9%) breast-cancer deaths observed (Fig. 1c). In the setting of T1c disease, a total of 245 (4.7%) breast-cancer deaths were observed; in this subgroup, chemotherapy was associated with improved BCSS, with a 5-year BCSS of 94.5% for patients receiving chemotherapy vs. 91.2% in the no/unknown chemotherapy group (Adjusted HR = 0.64; 95% CI: 0.48–0.85; Cox p value = 0.002) (Fig. 1d).
Our large population-based study revealed that women with stage IA TNBC have excellent 5-year BCSS outcomes, and that associations of chemotherapy receipt with survival were primarily observed in patients with T1c disease. Our findings are relatively consistent with similar studies conducted in this space, despite different sample sizes and methodologic differences. Vaz-Luis et al.4 analyzed outcomes of patients with T1a-T1bN0 TNBC in the NCCN database (n = 363), highlighting favorable 5-year BCSS ( ≥ 95%) in both T1a and T1b, irrespective of chemotherapy receipt. Studies conducted in SEER by refs. 5,6,7 showed improved outcomes among patients with T1c TNBC who received chemotherapy. In contrast to our study, the above studies included patients treated in the neoadjuvant setting, potentially including patients with occult nodal disease; we opted for excluding this population, so that we could ensure inclusion of patients with node-negative disease and provide more reliable estimates. Additionally, our study evaluated outcomes adjusted for histology, race, ethnicity, marital status, income, and rurality, which are relevant prognostic factors. A recent study by Carbajal-Ochoa evaluated only T1b and T1c TNBC and showed improved BCSS in T1c tumors8.
Our study adds to a body of evidence that suggests a benefit of adjuvant chemotherapy among stage IA TNBC patients with T1c tumors. Additionally, we observed an increase in chemotherapy use over time among patients with T1b and T1c tumors, possibly associated with increased reporting on recurrence rates for these tumors. Our study also provides important information on the favorable outcomes of patients with tumors ≤1 cm (T1mic, T1a, and T1b) that can inform discussions with patients. Indeed, the limited data available to treat small TNBCs has commonly led to extrapolation from studies of larger TNBCs for treating patients in clinical practice, with relevant risk for overtreatment and unnecessary toxicity. Important limitations of our study include its retrospective nature, with potential prognostic imbalances between subgroups, the absence of recurrence data in SEER and the lack of information on patient/clinician preferences and type of chemotherapies administered. Of note, the chemotherapy variable is coded in SEER as “no/unknown” when there was no evidence of chemotherapy administration, which prevents us from separating “no” from “unknown”.
In conclusion, in a large population-based study we observed excellent long-term outcomes for patients with stage IA TNBC and identified a progressive increase in the use of adjuvant chemotherapy for this population. An association between BCSS and use of adjuvant chemotherapy was identified for patients with T1c tumors, but not for T1b. Integration of additional prognostic factors and shared decision-making for treatment choices in this low-risk setting are warranted.
Methods
Data source and study design
We obtained data from SEER, using the 17 registries database (Nov. 2021 Submission). We extracted all cases of women diagnosed with stage IA TNBC (T1mic,a,b,c,N0,M0) from 2010 to 2019. To be included, patients must have had only one primary malignancy in their lifetime, had known vital status and cause of death (n = 10,048). We excluded patients who did not undergo definitive breast surgery (n = 314), those who received neoadjuvant chemotherapy (n = 1116) or neoadjuvant radiation therapy (n = 17) (Supplementary Fig. 3). The following variables were collected for analysis: age at diagnosis, race and ethnicity, marital status, median household income, rurality, year of diagnosis, histology, tumor grade, stage and size (T), type of surgery, receipt of adjuvant chemotherapy (coded as yes vs. no/unknown), receipt of adjuvant radiation therapy, vital status, and cause of death.
Statistical analyses
We examined the cohort characteristics and the frequency of chemotherapy use over time by tumor size. Variables associated with receipt of chemotherapy were evaluated using multivariate logistic regression. Nonparametric test for trend was used to evaluate the trend in chemotherapy use over time. We also examined breast cancer-specific survival (BCSS), defined as the interval from initial breast cancer diagnosis to death from breast cancer or last follow-up for censored patients. We used multivariable cox models to evaluate the association of adjuvant chemotherapy with BCSS stratified by tumor size, adjusted for age, race, ethnicity, tumor grade, histology, receipt of radiation therapy, marital status, income, and rurality. All p values were two-tailed and values <0.05 were considered statistically significant.
Ethical considerations
This study used de-identified, publicly available data. As such, it was exempt from review by the Dana-Farber Office for Human Research Studies.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Jose P. Leone had full access to all of the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. The data that support the findings of this study are publicly available at SEER: https://seer.cancer.gov/.
References
Lin, N. U. et al. Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer 118, 5463–5472, https://doi.org/10.1002/cncr.27581 (2012).
Dent, R. et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin. Cancer Res. 13, 4429–4434, https://doi.org/10.1158/1078-0432.CCR-06-3045 (2007).
NCCN Clinical Practice Guidelines in Oncology. Breast Cancer Guidelines Version 5.2023. Available at: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
Vaz-Luis, I. et al. Outcomes by tumor subtype and treatment pattern in women with small, node-negative breast cancer: a multi-institutional study. J. Clin. Oncol. 32, 2142–2150, https://doi.org/10.1200/JCO.2013.53.1608 (2014).
Du, Z. L. et al. Evaluation of a beneficial effect of adjuvant chemotherapy in patients with stage I triple-negative breast cancer: a population-based study using the SEER 18 database. Breast Cancer Res. Treat. 183, 429–438, https://doi.org/10.1007/s10549-020-05776-2 (2020).
Zhai, Z. et al. Evaluation of adjuvant treatments for T1 N0 M0 triple-negative breast cancer. JAMA Netw. Open 3, e2021881, https://doi.org/10.1001/jamanetworkopen.2020.21881 (2020).
Shen, K. et al. Impact of adjuvant chemotherapy on T1N0M0 breast cancer patients: a propensity score matching study based on SEER database and external cohort. BMC Cancer 22, 863, https://doi.org/10.1186/s12885-022-09952-z (2022).
Carbajal-Ochoa, W., Bravo-Solarte, D. C., Bernal, A. M. & Anampa, J. D. Benefit of adjuvant chemotherapy in lymph node-negative, T1b and T1c triple-negative breast cancer. Breast Cancer Res. Treat. 203, 257–269, https://doi.org/10.1007/s10549-023-07132-6 (2023).
Acknowledgements
This study received no funding. The authors acknowledge Timothy K. Erick for assistance in medical writing and Valerie Hope Goldstein for editorial assistance in the preparation of this manuscript. Both are full-time employees of Dana-Farber Cancer Institute.
Author information
Authors and Affiliations
Contributions
P.T. helped with conceptualization, investigation, methodology, project administration, resources, software, supervision, validation, visualization, and writing—original draft, review and editing. J.L. helped with conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, software, validation, visualization, and writing—review and editing. C.T.V. helped with conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, software, validation, visualization, and writing-review and editing. R.A.F., A.G.W., O.M.-S., A.G.-C., F.L., N.U.L., and S.M.T. helped with investigation, methodology, project administration, resources, software, validation, visualization, and writing—review and editing. N.T. helped with data curation, formal analysis, investigation, methodology, project administration, resources, software, validation, visualization, and writing—review and editing. J.P.L. helped with conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, software, supervision, validation, visualization, and writing-original draft, review and editing.
Corresponding author
Ethics declarations
Competing interests
P.T. reports serving as advisor/consultant for AstraZeneca, Daiichi-Sankyo, Gilead and Lilly. O.M.S. reports travel expenses and consulting fees from Roche and Reveal Genomics and speaker fees from Eisai, Daiichi and Novartis. OMS is a SEOM visiting fellow 2022. A.G.C. reports research funding (to the Institution) from AstraZeneca, Daiichi-Sankyo, Merck, Gilead Sciences, Zenith Epigenetics, Bristol-Myers Squibb, Novartis, Biovica International AB, and Foundation Medicine; and travel/accommodations (to scientific meeting) from Roche/Genentech. R.A.F. reports institutional funding from Puma Biotechnology. F.L. reports consulting/advisory role for AstraZeneca, Pfizer, Merck and Daiichi-Sankyo; and institutional research funding from Eisai, AstraZeneca, CytomX and Gilead Sciences. N.U.L. reports institutional research support from Genentech, Pfizer, Merck, Seattle Genetics (now Pfizer), Zion Pharmaceuticals (as part of GNE), Olema Pharmaceuticals, and AstraZeneca; consulting honoraria from Puma, Seattle Genetics, Daiichi-Sankyo, AstraZeneca, Olema Pharmaceuticals, Janssen, Blueprint Medicines, Stemline/Menarini, and Artera Inc., and Eisai; royalties from UpToDate (book); and travel support from Olema Pharmaceuticals. SMT reports a consulting or advisory role for Novartis, Pfizer, Merck, Eli Lilly, AstraZeneca, Genentech/Roche, Eisai, Sanofi, Bristol Myers Squibb, Seattle Genetics, CytomX Therapeutics, Daiichi-Sankyo, Gilead, OncXerna, Zymeworks, Zentalis, Blueprint Medicines, Reveal Genomics, ARC Therapeutics, Infinity Therapeutics, Sumitovant Biopharma, Umoja Biopharma, Artios Pharma, Menarini/Stemline, Aadi Biopharma, Bayer, Incyte Corp, Jazz Pharmaceuticals, Natera, Tango Therapeutics, Systimmune, eFFECTOR, and Hengrui USA. S.M.T. receives research funding from Genentech/Roche, Merck, Exelixis, Pfizer, Lilly, Novartis, Bristol Myers Squibb, Eisai, AstraZeneca, Gilead, NanoString Technologies, Seattle Genetics, and OncoPep, and receives travel support from Eli Lilly, Sanofi, Gilead, and Pfizer. J.P.L. reports research funding from Kazia Therapeutics and consulting from Minerva Biotechnologies. The remaining authors declare no conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tarantino, P., Leone, J., Vallejo, C.T. et al. Prognosis and treatment outcomes for patients with stage IA triple-negative breast cancer. npj Breast Cancer 10, 26 (2024). https://doi.org/10.1038/s41523-024-00634-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41523-024-00634-6